Abstract
Chlorinated pesticides (CP) are environmentally persistent pollutants that (prenatally through the placenta and post-natally via breastfeeding) are transferred from mother to child. Considering the significant bleeding tendency noted in infants of CP-intoxicated mothers in Egypt, this study aimed to investigate any correlation between levels of these xenobiotics in mothers’ milk and bleeding tendencies of their infants, as well as a possible role of any related immunosuppression in this phenomenon. This study examined 180 newborns presenting with altered bleeding tendencies and their mothers, and 180 normal newborns and their mothers (serving as a controls), selected from the Breastfeeding Unit, Center for Social and Preventive Medicine at the Cairo University Pediatric Hospital. Chlorinated pesticides (e.g., hexachlorocyclohexane, DDT, hepta-chloroepoxide, α- and β-endosulfan, aldrin, endrin, dieldrin) levels and their derivatives were measured in mothers’ milk as well as in serum of neonates using gas chromatography/high resolution mass spectrometry. To link bleeding tendency with lactational intoxication of neonates by CP, newborns’ blood was assessed for: platelet count, bleeding and prothrombin time, liver enzymes, Vitamin K, TNFα, and IL-10. Breast milk CP levels were associated with a higher incidence of bleeding in infants. Interference with the coagulation cascade was supported by changes in prothrombin time (prolonged), platelet counts (decreased), liver enzymes (increased), and serum Vitamin K concentrations (decreased). Moreover, the significant decrease in WBC count and lymphocytes added to depressed cytokine secretion, i.e., TNFα and IL-10, suggested an organochlorine-induced immunotoxicity in infants developmentally exposed to the agents. We conclude that maternal transfer of CP, via breastfeeding or across the placenta, was sufficient to achieve similar CP levels in the serum of their infants; this correlated with a manifesting of altered bleeding tendencies and perturbed cytokine biology in these infants.
| Abbreviations | ||
| Ah, | = | aryl hydrocarbon; |
| ALT, | = | alanine aminotransferase; |
| AST, | = | aspartate aminotransferase; |
| CBMC, | = | cord blood mononuclear cells; |
| CP, | = | chlorinated pesticide; |
| DDT, | = | dichlorodiphenyltrichloroethane; |
| HAH, | = | hydrogenated aromatic hydrocarbon; |
| HCH, | = | hexachlorocyclohexane; |
| HCB, | = | hexachlorobenzene; |
| HDN, | = | hemorrhagic disease in newborn; |
| IFNγ, | = | interferon gamma; |
| NF-κB, | = | nuclear factor-κB; |
| OHC, | = | organohalogen compounds; |
| PCB, | = | polychlorobiphenyl; |
| PCDD, | = | polychlorodibenzo-p-dioxin; |
| PCDD, | = | 2,3,7,8-chlorosubstituted polychlorodibenzo-p-dioxin; |
| PCDF, | = | polychlorodibenzofuran; |
| PCP | = | pentachlorophenol; |
| TCDD, | = | 2,3,7,8-tetrachlorodibenzo-p-dioxin; |
| TGF-β, | = | transforming growth factor-β; |
| TH1, | = | T-helper-1 cell; |
| TH2, | = | T-helper-2 cell; |
| TNF-α, | = | tumor necrosis factor-α. |
Introduction
During the past several decades, concerns have been raised regarding the effects of environmental toxins on public health, especially lactating women and their children. Organochlorine (OC) are a group of chemicals that are widespread environmental pollutants that can be detected in almost every ecosystem and in many industrial products. The main reasons for the environmental contamination by OC compounds include their great production, uncontrolled use, inadequate discharge, and persistence in the environment (Ross, Citation2004). The predominant mode of environmental transport of chlorinated pesticides is the atmosphere. Subsequently, the more highly-chlorinated pesticides (CP) that are virtually insoluble remain associated with the soil, while the lower chlorinated congeners have a low solubility in water. Traces of these substances leach out into the water, where they cling to sediment and are washed downstream.
Organochlorine pesticides were widely used worldwide until restrictions (initially for DDT) were introduced in the late 1970s in both Europe and the United States (Fontcuberta et al., Citation2008). Some of these pesticides are still widely used by farmers (especially in [sub]-tropical countries) because of their effectiveness and their broad spectrum activity in malaria control programs and against agricultural pests (Amoah et al., Citation2006). Though the use of most OC pesticides in Egypt was restricted, they are present in the environment. Recent studies have documented their presence both in human blood (Mokhles et al., Citation2006) and low levels in foods, suggesting that most of the levels found in humans related either to historical use or past human exposure (Falco et al., Citation2004). In general, foods provide the major source (i.e., >90%) of human exposure to CP (Huisman et al., Citation1995). Among the various food classes, dairy products are the major contributors to daily human intake of CP (Darko and Acquaah, Citation2008; Fontcuberta et al., Citation2008). The recent Egyptian study of Abou Donia et al. (Citation2010) detected OC pesticides in both buffalo’s and cow’s milk at levels exceeding the tolerance levels established by the United Nation’s Food and Agricultural Organization (FAO) and the World Health Organization (WHO).
Once ingested, the high lipophilic nature, chemical stability, and resistance to biodegradation make CP difficult to excrete, leading to their accumulation in fatty tissues, especially in association with lipoproteins found in cell membranes; these associations, in turn, result in changes to cell membrane structure and permeability (Chowdhury et al., Citation1990; Antunes-Madeira et al., Citation1993). The different levels of CP that are found in body organs are likely to be a result of their high affinity for select constituents within those organs. It is the affinity of highly apolar CP for similarly highly apolar (storage) lipids – notably triglycerides and cholesterol esters, and their lesser (moderate) affinity for structural amphipathic lipids (i.e., phospholipids and cholesterol), that provides for a basis for organ-based differences in content of these CP, i.e., variations in the levels of these various biomolecules will ultimately affect the degree of storage of any given CP ingested (Antunes-Madeira et al., Citation1993).
Previous research studies with pesticide applicators have revealed a wide range of neuromuscular disorders that affect the sensory and motor functions (Smit et al., Citation2003; Kamel et al., Citation2007) as well as deterioration of the hematological, hepatic and renal functions that are associated with the exposure to pesticides (Patil et al., 2003; Abu Mourad, Citation2005). Two studies have examined the sensory and motor functions and biochemical parameters in pesticide applicators in cotton crops in Egypt (Amr, Citation1999; Farahat et al., Citation2003). Recent concerns about the impact of pesticides on children came from the increased recognition of their vulnerability due to anatomical and physiological differences from adults. Abdel Rasoul et al. (Citation2008) and Ismail et al. (Citation2010) reported neurobehavioral deficits and neurological/neuromuscular signs/symptoms that might be added to the aforementioned list of induced derangements in host hematopoietic, renal, and hepatic functions.
While it is accepted that exposure to these various agents likely occurs throughout the “consumptive life” of a host, in fact, exposure to these substances can and does start even at the point of conception. Chlorinated pesticides, which can be found in all fat compartments of the human body (including adipose tissue and blood lipids), readily crosses the placenta, subsequently equilibrate among lipid compartments, and are transferred into breast milk (Antunes-Madeira et al., Citation1993). As such, developing organs in an exposed fetus is subjected to these substances (with the level varying in relation to content of select biomolecules; see above) during critical or the ‘sensitive’ periods of rapid growth and development wherein establishment of various tissues/organs are achieved.
Breast milk contains relatively large quantities of these compounds, and almost complete absorption takes place in the baby (Abraham et al., Citation1994; Dahl et al., Citation1995). Exposure of children to chlorinated pesticides/metabolites (including polychlorinated biphenyls [PCB], dichlorodiphenyltrichloroethane [DDT], polychlorodibenzofurans-p-dioxins [PCDD], pentachlorophenol [PCP], and polychlorodibenzofurans [PCDF]) via breastfeeding is well documented (Koppe et al., Citation1992); in several instances, these intoxications have been linked with hemorrhagic disease in newborn (HDN). Other studies have indicated a possible link between development of various health effects in children and exposure to CP via maternal milk. These effects often manifested as changes in endocrine, immunological, reproductive, bone, and internal organ morphology markers in the body (Neal, Citation1985). Several studies of the acute effects of PCBs in rats have associated these compounds with hepatotoxicity. Specifically in the liver, acute organo-halogen compound toxicity is mediated through subcellular toxicity that leads to impaired ATP and protein synthesis (Parkinson, Citation1996); chronic exposure also may affect endocrine homeostasis via up-regulation of the cytochrome P450 isozymes CYP1A and 1B (Lin and Chang, Citation2003). With respect to effects on blood parameters, the biological event leading to the hepatic porphyria appears to be an inhibition of uroporphyrinogen decarboxylase, the enzyme responsible for stepwise decarboxylation of uroporphyrinogen to co-pro-porphyrinogen (Elder, Citation1978).
Several CP are immunotoxic to animals and humans, especially TCDD and structurally related compounds (PCBs, PCDDs, PCDFs) (Safe, Citation1990, Citation1994). Studies in the laboratory and in wildlife have shown that the maturation of the immune system is especially vulnerable to the adverse effects of dioxin-like compounds and other OC (Holladay et al., Citation1991; Holladay and Blaylock, Citation2002). Both lymphoid and thymic involution, as well as thymic atrophy, is among the most consistent in vivo effects of TCDD so far noted (Lin and Chang, Citation2003). Among humans, prenatal exposures to high doses of PCBs and PCDFs have induced immunosuppression and increased susceptibility to infectious diseases in Taiwanese infants born to Yu-cheng mothers (Rogan et al., Citation1988); these subjects were among about 2000 people in central Taiwan were intoxicated in 1979, via rice oil that was contaminated with PCBs. Alterations in immune system function and a high incidence of infections have also been encountered in infants of populations environmentally exposed to other organochlorinated agents (see Weisglas-Kuperus et al., Citation2000).
Immune system cells, i.e., macrophages, monocytes, lymphocytes, granulocytes, as well as non-immune cells (i.e., epithelial and endothelial cells) produce pro- and anti-inflammatory cytokines that are key to initiation, progression, mitigation, or termination of immune responses (Elenkov and Chrousos, Citation1999). Luster et al. (Citation2005) recently summarized current efforts to identify and implement tests of immune function (e.g. cytokine profiles) in children with various diseases of the immune system. In a review by Duramad et al. (Citation2007), examples of literature and recent data from two studies of childhood leukemia (Ma et al., Citation2002; Buffler et al., 2005) and the CHAMACOS birth cohort of Latino mothers and children from agricultural communities (Eskenazi et al., Citation2003) were used to illustrate how cytokines markers may link environmental exposures to cytokine profiles and how these immunologic biomarkers can be applied in the study of adverse health outcomes in children. This has led to recognition of cytokine expression and secretion as a useful biomarker to assess the status and progression of a host immune response. Since then, numerous studies have used immune markers that include cytokine profiles to evaluate the effect of environmental toxicants such as volatile organic compounds, PCBs, and organochlorines on the immunity of children (Lehmann et al., Citation2002; Bilrha et al., Citation2003; ten Tusscher et al., Citation2003).
In general, pro-inflammatory cytokines, such as interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ (among lymphocytes, produced by T-helper-1 [TH1] cells) serve to initiate the acute inflammatory response (Janeway, Citation2001). In contrast, the immune response can be down-regulated by the production/release of anti-inflammatory cytokines IL-4, -5, -10, and -13 (produced by TH2 cells) (Elenkov and Chrousos, Citation1999; Kidd, Citation2003). A third class of T-helper cells (i.e., TH3) is the source of transforming growth factor (TGF)-β, a cytokine with strong anti-inflammatory characteristics (Mills, Citation2004). A correlation between different pollutant (i.e., PCB) burdens and cytokine expression has been previously detected in vitro (in cell cultures), in various fish species, and occupationally among coke-oven users; this correlation has been accepted as a tool for use in environmental stress monitoring (Xiao et al., Citation2002; Mukhopadhyay et al., Citation2003).
To our knowledge, the effect of CP on some Egyptian mothers and their infants has not yet been investigated. Among the clinical signs of CP toxicity in infants in the current study is a bleeding tendency with prolonged prothrombin time, altered hepatic function, and immu-nosuppression. Thus, the aim of this study was to relate concentrations of CP in breast milk to those in infant blood and to discern a possible link between these concentrations and effects on bleeding tendency, serum liver enzymes, and any potential contributions to altered immune responses in the infants (as manifest by changes in their ability to produce basal [unstimulated] TNFα and IL-10).
Materials and Methods
Study Subjects
This study was conducted on 360 infants (10–24 months-of-age) and their lactating mothers (20–40 years-of-age) selected from the Breastfeeding Unit at the Center for Social and Preventive Medicine, Cairo University Pediatric Hospital, Cairo, Egypt. The research was carried out in accordance with the Ethical Committee of Cairo University Pediatric Hospital. All mothers provided informed consent to participate in this work. All cases were subject to full history of the infants (age, sex, and birth weight; ), as well as to a full history taken from the mothers (age, smoking status, lactational period; ).
Table 1A. Clinical data for the CP-intoxicated infants and controls.
Table 1B. Clinical data for the CP-intoxicated mothers and controls.
For these studies, the 180 normal infants (no bleeding tendency, normal serum CP levels) and their non-CP-intoxicated lactating mothers were designated as Group I (Control group). For criteria purposes, threshold CP serum and breast milk levels for exclusion were set at 0.1 mg/L and 2 mg/kg fat, respectively. A population of 180 infants presenting with bleeding tendencies and having high CP serum levels (i.e., >0.3 mg/L) and their CP-intoxicated lactating mothers (with breast milk CP levels >6 mg/kg fat), were designated as Group II (i.e., CP-intoxicated infants/mothers). The initial criteria for placement of an infant in Group II was that the infant displayed: A – signs of abnormal bleeding tendency, such as petechiae, excessive bruising, prolonged bleeding from puncture sites, umbilical oozing, gastrointestinal bleeding, hematuria, pulmonary/intracranial hemorrhage, pallor, weak pulse, tachycardia, hypotension, metabolic acidosis, and/or signs of hypovolemia when blood loss is large, and B – laboratory findings of bleeding time > 6 min and prothrombin time (PT) >14 min. If an infant met this first criteria, but did not possess the threshold (or greater) level of CP in its serum – or if its mother did not have the threshold (or greater) level of CP in her milk – they were excluded from this Group.
Finally, for the benefit of the reader, we would be remiss if we did not note that in Egypt (and many other developing nations), it is not uncommon that until ≈ 7 mo-of-age, infants are near-exclusively breastfed. Thereafter, very small amounts of diluted yogurt, cow/buffalo milk, vegetables, or chicken meat might be added to supplement the breastfeeding; this remains the regimen until infants reach 24 mo-of-age. Of course, as a result of these supplementations, some diet-induced CP intoxication is possible (see Abou Donia et al., Citation2010); however, without accurate dietary histories covering the lifespan of every infant in this study, the impact from this potential confounder is unmeasurable. Nevertheless, it would not be incorrect to assert that the lactation-induced source of CP is predominant over the 24-mo period (much more so than the food-induced possibility).
Laboratory investigations of the infants
After testing for bleeding times, 3 ml of whole-blood random morning (8–10 AM) sample was collected from each infant by venipuncture and divided into three aliquots. The first aliquot was used for serum isolation and estimation of levels of liver enzymes (activities), Vitamin K, various CP, as well as TNFα and IL-10; the second was treated with EDTA to permit platelet count and CBC analyses; and, the third was citrated to generate plasma for determinations of PT.
Liver function analyses, i.e., measures of serum albumin (Alb), and of alanine (ALT), and aspartate aminotransferase (AST) activities were determined using commercial colorimetric kits purchased from Bio-Merieux (L’Etoile Charbonnierers, Les Bains, France). Serum vitamin K levels were assayed via a commercial kit from Incstar Corp. (Stillwater, MN). Hematological indices, i.e., RBC and WBC counts, hemoglobin (Hb), lymphocyte and eosinophil differentials, were assessed using an Auto Counter AC 920 (Swelab Instrument, Stockholm, Sweden).
Serum TNFα and IL-10 levels were assessed using ELISA kits from R&D Systems (Minneapolis, MN). For these studies, TNFα was selected as the representative pro-inflammatory, and IL-10 as the anti-inflammatory, cytokine for analysis in that each were previously monitored by Bilrha et al. (Citation2003) to investigate their association with prenatal exposure to some OC. Several other studies have monitored the impact of different pesticides on levels of these cytokines as well (see Duramad et al., Citation2007), but our study is the first performed on Egyptian infants. Moreover, altered production of TNFα in these children could cause a major imbalance in their immune response that, in turn, would give rise to health problems (autoimmune diseases and increased susceptibility to infections). Il-10, as an anti-inflammatory cytokine, inhibits synthesis of TNFα and other pro-inflammatory cytokines and promotes the development of regulatory T-cells. As such, dysregulation of the formation of this agent could shift the immune system balance to more of an inflammatory state that, again, would lead to health problems going forward in the affected child.
Extraction of PCBs from infant blood samples
The extraction of polychlorinated biphenyls (PCBs) from infant sample was performed according to Prapamonatal and Stevenson (Citation1991). An aliquot (0.5 ml) of sample serum was mixed with 0.5 ml water:n-propanol (85:15, v/v) solution and centrifuged (3000 x g, 37°C, 30 min). After the initial solvent extraction, solid-phase extraction cartridges were used to further clean up and concentrate the samples. The C18-cartridges (Macherey-Nagel, Duren, Germany) were pre-conditioned with 4 ml distilled water:n-propanol (85:15, v/v) solution and the samples then slowly pressed through the column. The cartridges were then washed with 1 ml water:n-propanol solution. All PCBs present were then eluted from the cartridge with 2 ml dichloromethane. Finally, 0.5 ml of the sample was injected into an Agilent 7890A gas-liquid chromatography (GLC) system (Agilent Technologies, Foster City, CA). The prepared samples were chromatographed under the following conditions: the column oven was programmed for an initial temperature of 70°C for 1 min, then increased by 30°C/min until it reached 190°C, followed by cooling for a 10 min period. In all studies, the inlet pressure was maintained at 4.3 PSI.
Extraction of DDT pesticide from infants’ serum
Extraction of DDT pesticide from the infant serum samples was performed according to the method of Trotter (Citation1975). Briefly, an aliquot (0.5 ml) of infant serum sample was mixed with 0.5 ml of 0.1 M HCl and centrifuged (3000 x g, 37°C, 60 min). After the initial solvent extraction, solid phase extraction cartridges were used to further clean up and concentrate the samples. The C18-cartridges used (Macherey-Nagel) were pre-conditioned with 1 ml 0.1 M HCl and the samples then slowly pressed through the column. The cartridges were then washed with 1 ml 0.1 M HCl and then with 3 ml of methanol:water (1:9 v/v) solution. The CP/other OC agents present were then eluted from cartridges with 2 ml of a 1:1 (v/v) methanol/water solution. Ultimately, 1 ml of the sample was injected into the Agilent GLC system. The prepared samples were chromatographed under the following conditions: the column oven was programmed for an initial temperature of 70°C for 1 min, then increased by 30°C/min until it reached 190°C, followed by cooling for a 10 min period. In all studies, the inlet pressure was maintained at 4.3 PSI.
Analyses of mothers’ breast milk
Extraction of CP/other OC agents from breast milk samples was conducted according to the method of Prapamonatal and Stevenson (Citation1991). Briefly, 20 ml samples of breast milk were collected from each mother in the study. Thereafter, 10 ml of the sample was extracted with 10 ml ethyl acetate:methanol:acetone (2:4:4, v/v/v) solution, vortexed for 1 min, and then centrifuged at 2000 rpm for 15 min at 20°C. The total supernatant generated was then collected. After the initial solvent extraction, solid phase extraction cartridges were used to further clean up and concentrate the samples. Here, C18-cartridges (Macherey-Nagel) were pre-conditioned with 2 ml of ethyl acetate:methanol:acetone (2:4:4, v/v/v) solution. The supernatants isolated above were diluted with 13 ml distilled water and then passed through the cartridges at a flow rate of 6-8 ml/min. Each cartridge was then washed with 2 ml of 25% acetonitrile (in water) solution and then dried by pulling air through the column for 3 min. All CP/other OC agents present were ultimately eluted from each cartridge with 1 ml isooctane.
Statistical analysis
All values were expressed as mean (± SE) of the number of samples (n). The data obtained from each study was statistically analyzed using a Student’s t-test as part of an SPSS Program (IBM Corporation, Somers, NY). The correlation analysis used in this study was assessed by a Pearson correlation at p < 0.01 and a 99% CI using the SPSS program.
Results
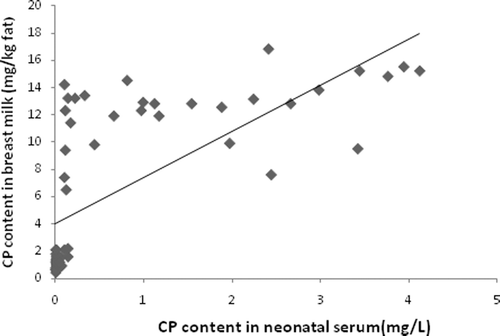
Assessment of CP concentrations in the breast milk of CP-intoxicated mothers revealed a 9.63-fold elevation in their levels as compared to in the milk of control mothers. These changes were subsequently reflected as ≈25.5-fold significant increases in the levels of the toxins in the serum of their infants (with bleeding tendency) as compared to that with control infants (). The correlation assessment in indicates a positive correlation (r = + 0.69, p < 0.01, 99% CI) between CP content in the serum of infants with a bleeding tendency and their concentration in their mothers’ breast milk. Further correlation assessments () reveal a positive correlation between CP content in the mothers’ milk and both PT (r = + 0.662, p < 0.01, 99% CI) and bleeding time (r = + 0.711, p < 0.01, 99% CI) of infants with a bleeding tendency. Residue levels of different CP (including hexachlorocyclohexane [HCH], DDT, heptachloro-epoxide, α- and β-endosulfan, aldrin, endrin, and dieldrin [each as mg/kg fat]) in breast milk of the CP-intoxicated and control mothers, as well as the residue levels in the serum of offspring with bleeding tendency, are indicated in . Levels of each were all significantly higher in the CP-intoxicated mother’s milk and in the serum of their infants, compared with their reference control counterparts.
Table 2. Chlorinated pesticide (CP) concentrations in breast milk of CP-intoxicated mothers and serum of their infants.
Table 3. Correlation between CP content in breast milk and infant PT and bleeding time.
Table 4. Levels of different CP in breast milk of intoxicated mothers and serum of their infants.
Figure 1. Correlation between breast milk CP concentrations (in mg/kg fat) from CP-intoxicated mothers and in the serum of their infants (in mg/L). The results illustrate positive correlation (r = + 0.69, p < 0.01, 99% CI) between CP content in serum of infants and its content in breast milk.
Comparing the effect of CP residue level in normal infants and the organocholorinated agent residue level in those with bleeding tendency, there was a significant increase reaching in bleeding time (2.18-times normal) and in PT (≈1.3-times normal), as well as in ALT and AST activities (2.63- and 1.35-times normal, respectively). Furthermore, significant decreases in albumin (29.8%), Vitamin K (46.5%), as well as platelet count (≈28.8%) were among the other prominent findings in this study (). All the aforementioned outcomes reflect the extent of hepatic dysfunction that was likely induced by the prenatal exposure to CP.
Table 5. Effect of CP on biochemical parameters in infant blood.
Lastly, most of the hematologic indices that were measured in these studies were significantly altered in intoxicated infants when compared to their control counterparts. Specifically, RBC counts were decreased from ≈ 4.3 (± 0.8) to ≈ 3.8 (± 0.4), Hb concentration decreased from 12.4 (± 0.4) to 10.1 (± 0.5), WBC counts were reduced from 6.3 (± 0.5) to 3.6 (± 0.3), and the percentage (%) lymphocytes dropped from 52 (± 3)% to 27 (± 4)%; in contrast, in these intoxicated infants, the % eosinophils increased from 8 (± 2)% to 13 (± 3)% ().
Table 6. Effect of CP on hematological parameters in infant blood.
With respect to the impact of these xenobiotics on the immune system, in situ formation of the cytokines TNFα and IL-10 were significantly lower in children of CP-exposed mothers as compared to that in infants in the control group (). Results of correlation analyses () revealed that there were negative correlations between both TNFα and IL-10 levels and the serum content of CP in these infants (r =−0.6142, r =−0.5878, respectively, each p < 0.01, 99% CI).
Table 7. Effect of CP on TNFα and IL-10 levels in neonatal serum.
Table 8. Correlation between serum CP in neonates and their serum TNFα and IL-10.
Discussion
The present study shows that the mean plasma residue levels of CP in breast-fed infants with a bleeding tendency were significantly higher than in normal children. Further, these measures positively correlated with the mother’s milk CP content. These findings suggest that lactation is one major source for CP body burden in infants.
Among the clinical findings in CP-intoxicated infants – other the bleeding tendency – was a decrease in birth weight, an increase in bleeding time and in PT, and decreases in Vitamin K concentrations and platelet count, relative to values in normal infants. Moreover, a significant deterioration in liver enzymatic and synthetic functions was evidenced by significant elevations of AST and ALT activity and decreases in albumin concentration. These findings are in line with those of several studies that reported deterioration of liver enzymes and protein synthesis as a result of pesticide exposure (Ilahi et al., 1986; Parron et al., Citation1996; Patil et al., 2003). Ismail et al. (Citation2010) provided further confirmation of the previous findings in noting a significant correlation between duration of exposure of children to pesticides with the extent of liver dysfunction (i.e., increase in ALT and AST and decrease in albumin). Decreases in Vitamin K levels, platelet count, and albumin levels, along with increases in ALT and AST levels, can be correlated to a prolonged PT (and so, the characteristic bleeding tendency). This then suggests the possibility of CP-induced liver toxicity causing hemolysis in the exposed infants. Many hydroxychlorobiphenyls are effective hemolytic agents; in contrast, the parent chlorobiphenyls are generally quite ineffective at inducing hemolysis. The hemolytic potencies of hydroxychlorobiphenyls vary with degree of chlorination and, more importantly, with the position of their chloro- and hydroxy-substituents (Bhanchet et al., Citation1977).
The findings here of disturbed hematologic profile, characterized by the reduced RBC counts and Hb indices, reflected the impact of pesticides on the hematopoietic system; these results coincide with those of Parron et al. (Citation1996). In rats, pesticides have been found to affect blood-forming organs wherein many steps in hematopoiesis were inhibited by pesticide residues. Our findings are also in line with those of Abu Mourad (Citation2005) who found that Hb and hematocrit levels were significantly decreased after exposure to pesticides, a phenomenon that may be attributed to microcytosis or impaired heme synthesis in the bone marrow and, possibly, through the binding of organophosphate pesticides to iron in Hb (Worthing, 1987).
This hemorrhage phenomenon described in our study resembled that of the Late Onset Hemorrhagic Disease of the Newborn (HDN) first reported in 1977 (Darlow, Citation1995). Hemorrhagic Disease of the Newborn mainly occurred in breast-fed infants and up to 75% of cases had an underlying liver disorder or malabsorption syndrome rather than insufficient dietary intake of Vitamin K. Hara (Citation1985) published data on children nursed with CP-contaminated milk and described similar clinical manifestations. Moreover, the Scientific Review Committee of the American Academy of Clinical Toxicology (Motz, Citation1992) described clinical signs of hemorrhagic disease in infants from mothers intoxicated with CP, results that again were in line with our findings.
Most reported cases of late-onset HDN had hepatitis, liver malfunction, or liver enzyme deficiencies. Vitamin K, necessary for normal clotting of blood, is required for the liver to synthesize factors necessary for proper clot formation (i.e., coagulate). These include Factors II (prothrombin), VII (pro-convertin), IX (thromboplastin component), and X (Stuart factor). Other Vitamin K-dependent clotting factors are protein C, protein S, and protein Z. A lack of Vitamin K or disturbed liver function may lead to deficiencies of clotting factors and excessive bleeding potential (Raulf and Konig, Citation1991). This means either that in our study subjects, it is plausible that their livers may not have been able to adequately synthesize blood clotting factors or store adequate amounts of Vitamin K. Birkbeck (Citation1988) believed there were two phenomena at work – low levels of prothrombin and of Vitamin K-dependent clotting Factors VII, IX, and X.
A discussion paper from the University of Amsterdam raised the idea that by-products of our industrial society, such as PCBs, PCDDs, and PCDFs, were a cause of late-onset HDN. These chemicals can induce enzymes in the liver that prolong prothrombin time (Belles-Isles et al., Citation2002). Although overseas studies have reported contamination of breast milk by these pollutants, a New Zealand Department of Health study on breast milk reported that levels of these contaminants were at the lower end of the international scale (Imanishi et al., Citation1980). To this end, there is a consensus that a causal relationship between PCs, Vitamin K deficiency, decreased PT, and the current bleeding tendency in some Egyptian infants is plausible.
Exposure to these substances could have possibly started at conception, a phenomenon termed prenatal toxicity. In this scenario, the developing organs in the fetus are subjected to varying degrees of the substances during critical (‘sensitive’) periods of rapid growth and development in which establishment of tissues and organs are achieved. Theoretically, exposure to CP and dioxins during early life can influence long-term outcome in three ways: (I) direct damage; (II) induction, deletion, or impaired development of a somatic structure resulting from exposure during a critical period; or, (III) physiological ‘setting’ by exposure at a critical period, with long-term consequences for function. Different levels of CP in the organs are likely to be caused by differences in the polarity of the fats in each organ. More specifically, it indicates the affinity of the highly apolar CP for the highly apolar (storage) lipids – notably triglycerides and cholesterol esters, and their moderate affinity for (structural) amphipathic lipids – notably phospholipids and cholesterol. The high degree of correlation between CP levels in maternal and fetal fat suggests that PCBs readily cross the placenta, and subsequently equilibrate among lipid compartments in accordance with their preferred affinities (Antunes-Madeira et al., Citation1993). Though measures of placental levels of OC/CP were not performed in the present study, apart from the breast milk or the very small amounts of any dietary supplements (e.g., yogurt, chicken meat, buffalo/cow mile, etc.) that the children may have received (starting at 8-mo-of-age or so), it is logical to presume that exposure via the transplacental is likely one major source of OC/CP in the children in this study.
The potential immunomodulatory properties of hydrogenated aromatic hydrocarbons (HAH) have been subject of extensive investigations, as the immune system is a sensitive target for HAH. The unique susceptibility of children to environmental toxicants has become an important focus in immunotoxicology (Duramad et al., Citation2007). Previous data of Raulf and Konig (Citation1991) emphasized that CP affect platelet function in vitro at concentrations that correlate with PCB blood levels obtained after exposure in vivo. Due to their lipophilic nature, CP will interact with membranes and initiate/modulate changes in human platelets (Ahne and Jarre, Citation2002).
Knowledge of the impact of pollutants on the immune system is important as chronic exposure to these agents could decrease host resistance to infections (O’Neill, Citation2002). This has led to the incorporation of the cytokine profile endpoint to the relatively few number of immune function assays available (e.g., CBC, lymphocyte counts, etc.) to evaluate whether an immune response is compromised/deviated from normal. Our present findings revealed a significant reduction in serum TNFα and IL-10 levels in conjunction with decreases in WBC and lymphocyte levels, outcomes that each confirms OC-induced immunopathology in exposed infants. Abu Mourad (Citation2005) and Parron et al. (Citation1996), in contradiction to our results, found that pesticide applicators had significantly higher WBC count; still, many researchers believe that pesticides can stimulate the immune system to induce more WBC activity (Ismail et al., Citation2010).
It was previously reported that prenatal exposure to OC was associated with a decrease in naive T-helper (TH) cells in umbilical blood samples collected from infants; this change blunted cellular activation and cytokine (TNFα) release (Beach and Whalen, Citation2006). In line with our findings here, a negative correlation was noted between plasma concentrations of the major OC (PCBs and DDT) and the in vitro secretion of TNFα by activated monocytes. Further evidence that PCBs cause reduced TNFα release by activated immune cells was provided by Ahne and Jarre (Citation2002); in these studies, human blood samples were exposed in vitro to PCB-77 or PCB-126, two non-ortho PCB congeners that display dioxin-like activity. After mitogenic stimulation, treated blood samples secreted less TNFα than did untreated samples. These results suggest that the negative association observed between some OC and TNFα secretion by activated cord blood mononuclear cells (CBMC) could be caused by dioxin-like PCB congeners (Devos et al., Citation2004). Furthermore, O’Neill (Citation2002) suggested that binding of dioxin-like compounds to the aryl hydrocarbon receptor (a transcription factor) can antagonize the effects of another transcription factor, nuclear factor (NF)-κB, an important regulator of immune and inflammatory gene expression (Sonne et al., Citation2006). Bilrha et al. (Citation2003) found that newborns exposed to OC in utero had significantly decreased TNFα production, suggesting that these children may be more susceptible to infection. These data indicate further that early exposures to environmental toxicants can directly impact the development and function of a child’s immunity.
Moreover, Reed et al. (Citation2004) reported that OC pesticides decreased the tumor-lytic function of natural killer (NK) cells, with PCP being the most effective one. These results provide evidence of the toxic potential of these compounds and their immunomodulatory on other mononuclear cells (such as T-cells, B-cells, and monocytes) as well as NK lymphocyte function. Beach and Whalen (Citation2006) provided an explanation for the retained capacity of PCP and oxychlordane to decrease the NK lytic function due to their ability to regulate the effect of T-cell-secreted interleukins that will influence the T/NK cells. PCP decreased the secretion of NK-stimulatory interleukins (i.e., IL-2, IL-10, and IL-12) and increased secretion of NK-inhibiting IL-4. In another study, a decrease in IL-10 production was seen in Lewis rats exposed to hexachlorobenzene (HCB) (Devos et al., Citation2004); this change is believed to contribute to the increased inflammatory responses seen with HCB in several experimental systems (Heilmann et al., Citation2006). In vitro studies by Kidd (Citation2003) and by Mills (Citation2004) using cultured lymph node cells showed that exposure to PCBs led to increased IL-4 and IL-6 expression, whereas IL-1, IL-2, and IFNγ expression were decreased, supporting an immunomodulating effect on the TH1/TH2 balance (naive CD4+CD8+ T-cells) and indicating a shift toward TH2 immune response (and thus, potential immunosuppression). Moreover, Sonne et al. (Citation2006) reported impairment of T-cell-mediated cellular immunity (as measured by intradermal testing) in West Greenland sledge dogs after dietary exposure to OHC-polluted Minke whale blubber for up to 52 weeks. Similar intradermal studies of guinea pigs fed a 50-ppm PCB diet, and of rabbits fed DDT and Aroclor 1254 (at 150 ppm), indicated significantly decreased cell-mediated immunity (Sonne et al., Citation2006). To this end, developmental exposure to PCBs has been implicated as a possible cause of deficient immune function in children, as increased perinatal PCB exposure may adversely impact on immune responses to childhood vaccinations (Heilmann et al., Citation2006).
Our interpretation of the findings here, including indications of immunosuppression (as reflected, in part, by lower basal TNFα and IL-10 levels) and of altered blood parameters (reflected by changes in bleeding tendency, etc.) leads us to suspect that the flux of maternal CP, primarily through breast feeding, is of sufficient magnitude during the first years of these children’s lives that CP levels are achieved in their bodies that give rise to these pathologic outcomes. Whether or not in utero exposures to the CP were sufficient to bring about these same outcomes (incidence/magnitude) remains to be determined; however, identification of populations of pregnant mothers who then cross a ‘bright line’ into a scenario in which they are no longer exposed to any CP from the moment they give birth onward (and assuming that no CP will be in their breast milk before/thereafter) is unlikely to be achieved in any study. Hence, animal models will need to be utilized to specifically address this issue.
Declaration of interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Abdel Rasoul, G. M., Abou Salem, M. E., Mechael, A. A., Hendy, O. M., Rohlman, D. S., and Ismail, A. A. 2008. Effects of occupational pesticide exposure on children applying pesticides. Neurotoxicology 29:833–838.
- Abou Donia, M.A., Abou Arab, A.A., Enb, A., El-Senaity, M. H., and Abd Rabou N. S. 2010. Chemical composition of raw milk and the accumulation of pesticide residues in milk products. Global Vet. 4:6–14.
- Abraham, K., Hille, A., Ende, M., and Helge, H. 1994. Intake and fecal excretion of PCDDs, PCDFs, HCB and PCBs (138, 153, 180) in a breast-fed and a formula-fed infant. Chemosphere 29:2279–2286.
- Abu Mourad, T. 2005. Adverse impact of insecticides on the health of Palestinian farm workers in the Gaza Strip: A hematologic biomarker study. Int. J. Occup. Environ. Health 11:144–149.
- Ahne, W., and Jarre, T. 2002. Environmental toxicology: Polychlorinated biphenyls impair TNFα release in vitro. J. Vet. Med. A Physiol. Pathol. Clin. Med. 49:105–106.
- Amoah, P., Drechsel, R. C., Abaidoo, R.C., and Ntow, W. J., 2006. Pesticides and pathogen contamination of vegetables in Ghana’s urban markets. Arch. Environ. Contam. Toxicol. 50:1–6.
- Amr, M. M. 1999. Pesticide monitoring and its health problems in Egypt, a Third World country. Toxicol. Lett. 107:1–13
- Antunes-Madeira, M. C., Almeida, L. M., and Madeira, V. M. 1993. Depth-dependent effects of DDT and lindane on the fluidity of native membranes and extracted lipids implication for mechanisms of toxicity. Bull. Environ. Contam. Toxicol. 5:787–794.
- Beach, T. M., and Whalen, M. M. 2006. Effects of organochlorine pesticides on interleukin secretion from lymphocytes. Hum. Exp. Toxicol. 25:651–659.
- Belles-Isles, M., Ayotte, P., Dewailly, É., Weber, J. P., and Roy, R. 2002. Cord blood lymphocyte function in infants from remote maritime population exposed to organochlorines and methylmercury. J. Toxicol. Environ. Health 65:165–182.
- Bhanchet, P., Tuchinda, S., Hathirat, P., Visudhiphan, P., Bhamaraphavati, N., and Bukkavesa, S. 1977. A bleeding syndrome in infants due to acquired prothrombin complex deficiency. Clin. Pediatr.16:992–998.
- Bilrha, H., Roy, R., Moreau, B., Belles-Isles, M., Dewailly, E., and Ayotte, P., 2003. In vitro activation of cord blood mononuclear cells and cytokine production in a remote coastal population exposed to organochlorines and methylmercury. Environ. Health Perspect. 111:1952–1957.
- Birkbeck, J. 1988. Vitamin K prophylaxis in the newborn: A position statement of the Nutrition Committee of the Pediatric Society of New Zealand. New Z. J. Med. 101:421–422.
- Chowdhury, A. P., Gauta, A. K., and Bhatnagar, V. K. 1990. Lindane-induced changes in morphology and lipids profile of testes in rats. Biomed. Biochim. Acta 49:1059–1065.
- Curtis, C. F. 1994. Should DDT continue to be recommended for malaria vector control? Med. Vet. Entomol. 8:101–112.
- Dahl, P., Lindstrom, G., Wiberg, K., and Rappe, C. 1995. Absorption of polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans by breast-fed infants. Chemosphere 30:2297–2306.
- Darko, J., and Acquaah, S. O., 2008. Levels of organochlorine pesticide residues in dairy products in Kumasi, Ghana. Chemosphere 71:294–298.
- Darlow, B.1995. Vitamin K deficiency hemorrhage: Dilemmas over prophylaxis continue. New Z. Practice Nurse February:35–37.
- Devos, S., van Den Heuvel, R., Hooghe, R., and Hooghe-Peters, E. L. 2004. Limited effect of selected organic pollutants on cytokine production by peripheral blood leukocytes. Eur. Cytokine Netw. 2:145–151.
- Duramad, P., Tager, I. B., and Holland, N. T. 2007. Cytokine and other immunological biomarkers in children’s environmental health studies. Toxicol. Lett. 172:48–59.
- Elder, G. H. 1978. Porphyria caused by hexachlorobenzene and other poly-halogenated aromatic hydrocarbons. In: Heme and Hemoproteins (De Matteis F., and Aldridge, W. N., Eds.), Berlin: Springer-Verlag, pp.157–200.
- Elenkov, I. J., and Chrousos, G. P. 1999. Stress hormones, TH1/TH2 patterns, pro-/anti-inflammatory cytokines, and susceptibility to disease. Trends Endocrinol. Metab. 10:359–368.
- Eskenazi, B., Bradman, A., Gladstone, E., Jaramillo, S., Birch, K., and Holland, N. 2003. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J. Child. Health 1:3–27.
- Falco, G., Bocio, A., Llobet, J. M., Domingo, J. L., Casas, C., and Teixido, A. 2004 Dietary intake of hexachlorobenzene in Catalonia, Spain. Sci. Total Environ. 322:63–70.
- Farahat, T. M., Abdelrasoul, G. M., Amr, M. M., Shebl, M. M., Farahat, F. M., and Anger, W. 2003. Neurobehavioral effects among workers occupationally exposed to organophos-phorous pesticides. Occup. Environ. Med. 60:279–286.
- Fontcuberta, M., Arques, J. F., Villaalbi, J. R., Martinez, M., Centrich, F., Serrahima, E., Pineda, L., Duran, J., and Casas, C. 2008. Chlorinated organic pesticides and marketed food; Barcelona, 2001–2006. Sci. Total Environ. 389:52–57.
- Hara, I. 1985. Health status and PCBs in blood of workers exposed to PCBs and of their children. Environ. Health Perspect. 59:85–90.
- Heilmann, C., Grandjean, P., Weihe, P., Nielsen, F., and Budtz-Jørgensen, E. 2006. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 3:311.
- Holladay, S. D., and Blaylock, B. L. 2002. The mouse as a model for developmental immunotoxicology. Hum. Exp. Toxicol. 21:525–531.
- Holladay, S. D., Lindstrom, P., Blaylock, B. L., Comment, C. E., Germolec, D. R., Heindell, J. J., and Luster, M. I. 1991. Perinatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p-dioxin (TCDD). Teratology 44:385–393.
- Huisman, M., Eerenstein, S. E., Koopman-Esseboom, C., Brouwer, M., Fidler, V., Muskiet, F. A., Sauer, P. J., and Boersma, E. R. 1995. Perinatal exposure to polychlorinated biphenyls and dioxins through dietary intake. Chemosphere 31:4273–4287.
- Imanishi, J., Nomura, H., Matsubara, M., Kita, M., Won, S. J., Mizutani, T., and Kishida, T. 1980. Effect of polychlorinated biphenyl on viral infections in mice. Infect. Immun. 29:275–277.
- Ismail, A. A., Rohlman, D. S., Abdel Rasoul, G. M., Abou Salem, M. E., and Hendy, O. M. 2010. Clinical and biochemical parameters of children and adolescents applying pesticides. www.thejoem.com 1:132–143.
- Janeway, C. A., Travers, P., Walport, M., and Shlomchik, M. (Eds.) 2001. The recognition of antigen. In: Immunobiology, New York: Churchill Livingstone, pp. 93–122.
- Kamel, F., Engel, L. S., Gladen, B. C., Hoppin, J. A., Alavanja, M. C., and Sandler, D. P. 2007. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum. Exp. Toxicol. 26:243–250.
- Kidd, P. 2003. TH1/TH2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 8:223–246.
- Koppe, J. G., Olie, K., and van Wijnen, J. 1992. Placental transport of dioxins from mother to fetus. II. PCBs, dioxins and furans and Vitamin K metabolism. Dev. Pharmacol. Ther. 18:9–13.
- Lehmann, I., Thoelke, A., Rehwagen, M., Rolle-Kampczyk, U., Schlink, U., Schulz, R., Borte, M, Diez, U., and Herbarth, O., 2002. The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T-cells. Environ. Toxicol. 17:203–210.
- Lin, P. P., Hu, W., and Chang, T. H. 2003. Correlation between gene expression of aryl hydrocarbon receptor (AhR), hydrocarbon receptor nuclear translocator (Arnt), cytochromes P4501A1 (CYP1A1) and 1B1 (CYP1B1), and inducibility of CYP1A1 and CYP1B1 in human lymphocytes. Toxicol. Sci. 71:20–26.
- Luster, M. I., Johnson, V. J., Yucesoy, B., and Simeonova, P. P. 2005. Biomarkers to assess potential developmental immunotoxicity in children. Toxicol. Appl. Pharmacol. 206:229–236.
- Ma, X., Buffler, P. A., Gunier, R. B., Dahl, G., Smith, M.T., Reinier, K., and Reynolds, P. 2002. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ.Health Perspect. 110:955–960.
- Malia, R., Preston, F., and Mitchell, V. 1980. Evidence against Vitamin K deficiency in normal neonates. Thromb. Haemost. 44:159–160.
- Miller, T. L. 1978. Interaction of hydroxychlorobiphenyls-polychlorinated biphenyl metabolites with the human erythrocyte membrane. J. Environ. Pathol. Toxicol. 1:459–474.
- Mills, K. H. 2004. Regulatory T-cells: Friend or foe in immunity to infection. Nature Rev. Immunol. 4:841–855.
- Mokhles, M., Tawfik, M., and Abou-Arab, A.A. 2006. Pesticides detection in hepatocellular carcinoma (HCC) hepatitis C virus (HCV)-infected male patients in Egypt. Sci. J. Az. Med. Fac (Girls) 27:1115–1125.
- Motz, R. 1992. Late hemothorax after oral Vitamin K. New Z. Med. J. 105:459.
- Mukhopadhyay, I., Nazir, A., Saxena, D. K., and Chowdhuri, D. K. 2003. Heat shock response: hsp70 in environmental monitoring. J. Biochem. Mol. Toxicol. 17:249–254.
- Neal, R. 1985. Mechanisms of the biological effects of PCBs, polychiorinated dibenzodioxins and polychiorinated dibenzofurans in experimental animals. Environ. Health Perspect. 60:41–46.
- O’Neill, L. A. 2002. Toll-like receptor signal transduction and the tailoring of innate immunity: A role for Mal? Trends Immunol. 22:296–300.
- Oesterle, D., and Deml, E. 1983. Promoting effect of polychlorinated biphenyls on development of enzyme-altered islands in livers of weanling and adult rats. J. Cancer Res. Clin. Oncol. 105:141–147.
- Parkinson, A. 1996. Biotransformation of xenobiotics. In: Toxicology, The Basic Science of Poisons (Klaassen, C. D., Ed.), New York: McGraw-Hill, pp. 113–186.
- Parron, T., Hernandez, A. F., Pla, A., and Villanueva, E. 1996. Clinical and biochemical changes in greenhouse sprayers chronically exposed to pesticides. Human Exp. Toxicol. 15:957–63.
- Prapamonatal, T., and Stevenson, D. 1991. Rapid method for the determination of organochlorine pesticides in milk. J. Chromatogr. 552:249–257.
- Raulf, M., and Konig, W. 1991. In vitro effects of polychlorinated biphenyls on human platelets. Immunology 72:287–291.
- Reed, A., Dzon, L., Loganathan, B.G., Whalen, M.M. 2004. Immunomodulation of human natural killer cell cytotoxic function by organochlorine pesticides. Hum Exp. Toxicol. 23:463–471.
- Rogan, W. J., Gladen, B. C., Hung, K. L., Koong, S. L., Shih, L. Y., Taylor, J. S., Wu, Y. C., Yang, D., Ragan, N. B., and Hsu, C. C. 1988. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science 241: 334–336.
- Ross, G. 2004. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol. Environ. Safety 59:275–291.
- Safe, S. H. 1986. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Ann. Rev. Pharmacol. Toxicol. 26:371–399.
- Safe, S. H. 1990. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of Toxic Equivalency Factors (TEFs). Crit. Rev. Toxicol. 21:51–88.
- Safe, S. H. 1994. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses and implications for risk assessment. Crit. Rev. Toxicol. 24:87–149.
- Smit, L. A., van–Wendel-de-Joode, B. N., Heederick, D., Peiris-John, R. J., and van der Hoek, W. 2003. Neurological symptoms among SriLankan farmers occupationally exposed to acetylcholinesterase-inhibiting insecticides. Am. J. Ind. Med. 44:254–264.
- Sonne, C., Dietz, R., Larsen, H. J., Loft, K. E., Kirkegaard, M., Letcher, R. J., Shahmiri, S., and Moller, P. 2006. Impairment of cellular immunity in West Greenland sledge dogs (Canis familiaris) dietary exposed to polluted Minke whale (Balaenoptera acutorostrata) blubber. Environ. Sci. Technol. 40:2056–2062.
- ten Tusscher, G. W., Steerenberg, P. A., van Loveren, H., Vos, J. G., von dem Borne, A. E., Westra, M., van der Slikke, J. W., Olie K., Pluim, H. J., and Koppe, J. G. 2003. Persistent hematologic and immunologic disturbances in 8-year-old Dutch children associated with perinatal dioxin exposure. Environ. Health. Perspect. 111, 1519–1523.
- Trotter, W. J. 1975. Removing the interference of DDT and its analogs in the analysis for residues of polychlorinated biphenyls. Assoc. Off. Anal. Chem. 58:461–465.
- Weisglas-Kuperus, N., Patandin, S., Berbers, G. A., Sas, T. C., Mulder, P. G., Sauer, P.J., and Hooijkaas, H. 2000. Immunological effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ. Health Perspect. 108:1203–1207.
- Wortthing, C. R. (Ed.) 1987. The Pesticides Manual. A World Compendium. London: British Crop Protection Council.
- Xiao, C., Chen, S., Li, J., Hai, T., Lu, Q., Sun, E., Wang R., Tanguay, R., and Wu, T. 2002. Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones 7:396–402.
