Abstract
Natural products have been used as potentially important sources of anti-inflammatory drugs. This study examined the effects of pinocembrin against lipopolysaccharide (LPS)-induced endotoxemia to ascertain whether pinocembrin could protect mice from ensuing death. Cytokine responses were also assessed in serum isolated from blood collected at 0, 2, 4, 6, 8, and 24 h after LPS administration of the mice (with or without drug treatment). The results showed that there was a lower production of TNFα, IL-6, and IL-1β in the serum of LPS-challenged mice that had been pre-treated with pinocembrin. In addition, pre-treatment with pinocembrin improved host survival against the LPS-induced lethal endotoxemia. These results suggest that this new flavonoid could potentially be a novel candidate for preventing development/mitigation progression of septic shock.
Introduction
Flavonoids display a variety of pharmacological properties of interest in the therapy of several diseases; these including use as cytotoxic, anti-angiogenic, or anti-vascular agents in treating cancer (Touil et al., Citation2009). They have long been recognized to possess anti-inflammatory, anti-oxidant, anti-allergic, anti-thrombotic, anti-viral, and anti-carcinogenic activities (Middleton et al., 2000). Due to the wide range of flavonoids routinely consumed by humans and animals (Hanasaki et al., Citation1994), research is needed to clarify the effects of this class of agents on inflammation.
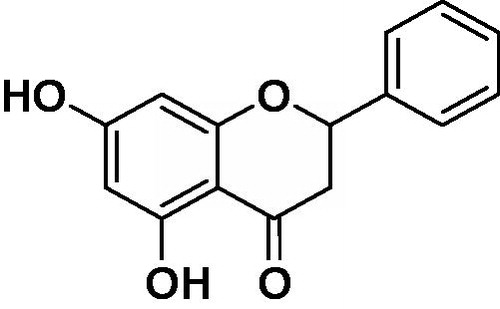
Pinocembrin (5,7-dihydroxyflavanone, C15H12O4, ) is a flavanone, a type of flavonoid isolated from the seeds of Alpinia katsumadai Hayata (Huang et al., Citation2007). Like many flavonoids, pinocembrin has been shown to relax the contraction evoked by KCl in rat thoracic aortic rings (Calderone et al., Citation2004). Pinocembrin inhibited the up-regulation of the receptor for advanced glycation end product (RAGE) transcripts and protein expression both in vivo and in vitro, and regulated mitochondrion-mediated apoptosis by restoration of B-cell lymphoma 2 and cytochrome c and inactivation of caspase 3 and caspase 9 (Liu et al., Citation2012). Other studies showed that pinocembrin reduced glutamate-induced SH-SY5Y cell injury and (primary) cultured cortical neuron damage in oxygen–glucose deprivation/re-oxygenation (OGD/R) scenarios (Gao et al., Citation2008a), alleviated cerebral ischemic injury in middle cerebral artery occlusion in rats (Gao et al., Citation2008b), and improved cognition by protecting cerebral mitochondria structure and function against chronic cerebral hypoperfusion in rats (Guang & Du, Citation2006). Although those previous studies have clearly shown the beneficial effects of pinocembrin in animals, little is known about its anti-inflammatory effects in general.
Inflammation is a localized protective response elicited by injury or destruction of tissues that serves to destroy, dilute, or sequester the injurious agent and injured tissue. Sepsis is considered a systemic inflammatory disorder and a serious clinical problem with a high mortality. The acute-phase response is a major pathophysiological phenomenon that accompanies inflammation and is associated with increased activity of pro-inflammatory cytokines (Kalantar-Zadeh et al., Citation2003). Injection of lipopolysaccharide (LPS) endotoxin into a mouse (or any other host) induces an acute inflammation that is associated with significantly increased levels of pro-inflammatory mediators. Major cytokines that participate in the inflammatory response after LPS challenge include tumor necrosis factor (TNF)-α, as well as interleukin (IL)-1, -2, -6, and -8 (Namas et al., Citation2009). It would seem logical that any means to suppress an excessive systemic inflammatory response would be a promising potent strategy for treating endotoxic sepsis. Accordingly, agents that could regulate inflammatory cytokine release and lead to increased survival in hosts with established endotoxemia would have potentially widespread therapeutic use. Therefore, in this study, the ability of pinocembrin to mitigate endotoxemia, and an initial defining of some mechanisms by which this might occur, were evaluated.
Materials and methods
Chemical and reagents
Pinocembrin (purity >99.7%) was purchased from the Sichuan Research Center of Traditional Chinese Medicine (Chengdu, China). Dimethyl sulfoxide (DMSO) and lipopolysaccharide (LPS, Escherichia coli 055:B5) were purchased from Sigma (St. Louis, MO). TNFα, IL-1β, and IL-6 ELISA kits were purchased from Biolegend (San Diego, CA).
Animals
Male BALB/c mice (18–20 g, 6–8-weeks-old) were purchased from the Center of Experimental Animals of the Baiqiuen Medical College of Jilin University (Jilin, China). The mice were housed in a temperature-controlled room (24 ± 1 °C) with a relative humidity of 40–80% and a 12-h light–dark cycle. Throughout the experiments, all mice had ad libitum access to standard laboratory chow and filtered water. Mice were acclimated for 3 days prior to initiation of any experimental protocols. All studies were approved by the Animal Use Committee of Jilin University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Bethesda, MD).
Induction of shock and treatment regimen
To determine a suitable concentration of LPS for inducing endotoxemia, 48 mice were divided into four groups (n = 12/group) and challenged with LPS (5–40 mg/kg) via a single intraperitoneal (IP) injection. The mice were then observed for mortality twice a day for 7 days, and survival rates were recorded.
To test the effect of the pinocembrin against LPS-induced mortality (using an established LPS dose that was 80–90% lethal), the drug was administered IP at 10, 20, or 50 mg/kg at 1 h prior to the LPS challenge. For this study, five groups of mice (n = 12/group) were created prior to any injections, i.e. control (vehicle), LPS (20 mg/kg) only, and pinocembrin (10, 20, or 50 mg/kg) + LPS. Survival in each group was assessed every 12 h for a period of 7 days.
Cytokine assays
The cytokine concentrations in the serum samples were determined by enzyme-linked immunosorbent assays (ELISA) using commercial kits specific for the mouse and following the manufacturer’s instructions. Pinocembrin (50 mg/kg) was administered IP; control mice received an equal volume of vehicle instead of pinocembrin. One hour later, all mice received a single dose of LPS (20 mg/kg) by IP injection. At 0, 2, 4, 6, 8, and 24 h after administration of the LPS, blood was recovered from the tail vein of each mouse and serum isolated; the serum was stored at −70 °C until used in ELISAs for TNFα, IL-1β, and IL-6. The levels of sensitivity for the TNFα, IL-1β, and IL-6 kits were, respectively, 1.5, 16, and 2 pg/ml.
Statistics
Data are presented as mean ± SD. Differences in survival of the groups were assessed with a Fisher’s exact test. A one-way analysis of variance (ANOVA) was used for comparisons between different groups. Cytokine concentrations between treatment groups were evaluated by a t-test. A p value < 0.05 was considered statistically significant.
Results
Effect of pinocembrin on LPS-induced mortality and cytokine responses in vivo
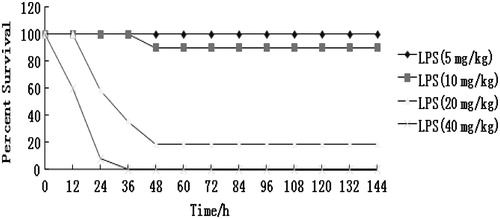
The protective effect of pinocembrin was evaluated in a standard model of murine endotoxemia. After LPS challenge, mice showed signs of severe sepsis symptoms such as decreased activity, conjunctivitis, diarrhea, lethargy, piloerection, huddling, etc. The deaths were observed as early as 12 h after the start of LPS treatment (40 mg/kg); in groups of mice given 5, 10, or 20 mg LPS/kg, the mortality rates were, respectively, 0, 10, and 81% (). Therefore, in the subsequent experiments here, mice were injected with the 20 mg LPS/kg as a clearly lethal dose that induced endotoxemia.
Figure 2. Survival rate (%) of mice challenged with LPS of different doses. Mice were injected IP with 5, 10, 20, or 40 mg LPS/kg (n = 12/group) without any treatment with pinocembrin. Survival was then monitored every 12 h for 7 days (p < 0.05).
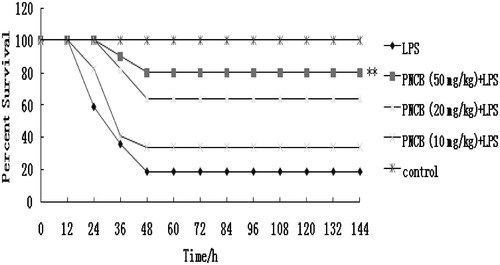
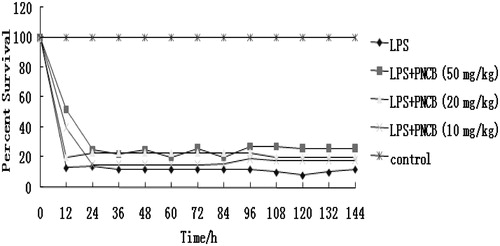
To assess the potential effect of pinocembrin against endotoxin-mediated mortality, its effect on mortality in mice with lethal endotoxemia was monitored. As shown in , pre-treatment with pinocembrin significantly reduced LPS-induced mortality in mice. However, the lower dose of pinocembrin (10 mg/kg) did not provide meaningful protection. Doses at 20 or 50 mg/kg yielded protection of 64 and 80%, respectively. To ascertain if pinocembrin was therapeutically effective, other sets of mice were injected with pinocembrin 1 h post-LPS injection. The results showed that the drug did not exhibit any therapeutic effects if administered after LPS ().
Effect of pinocembrin on LPS-induced cytokine responses in vivo
Growing evidence suggests that LPS does not directly cause septic shock and tissue injury; rather, LPS stimulates the release of pro-inflammatory cytokines (e.g. TNFα and IL-1β) that mediate the damage (Beutler et al., Citation1985; Hotchkiss & Karl, Citation2003; Tracey et al., Citation1987). To investigate the anti-inflammatory effects of pinocembrin, levels of pro-inflammatory cytokines mechanistically linked to endotoxemia were measured in blood samples collected from mice 0, 2, 4, 6, and 8 h after an LPS challenge. Serum concentrations of TNFα, IL-1β, and IL-6 in mice receiving 20 mg LPS/kg were compared with those in mice receiving the same dose of LPS and a single high dose of pinocembrin (50 mg/kg). As shown in , serum cytokine levels in pinocembrin-pre-treated, LPS-challenged mice significantly differed from those of hosts that received LPS only. Overall, levels of TNFα, IL-1β, and IL-6 production in mice treated with pinocembrin (50 mg/kg) were consistently decreased as compared to that in mice that received the LPS alone. In contrast, IL-10 levels were slightly up-regulated in sera of LPS-challenged mice after pre-treatment with pinocembrin ().
Figure 5. Influence of a single dose of pinocembrin on cytokine responses induced by a lethal dose of LPS. Mice were injected IP with pinocembrin 1 h before challenge with the LPS (20 mg/kg). Blood was isolated from the tail vein of each mouse at 0, 2, 4, 6, and 8 h post-LPS challenge and serum levels of TNFα, IL-1β, IL-6, and IL-10 then measured using ELISA. Data are presented as means (±SE) from n = 12 mice/group. **p < 0.01 versus LPS-only group.

Discussion
To our knowledge, the current study is the first to demonstrate that administration of pinocembrin can protect a host from LPS-induced death. Furthermore, levels of pro-inflammatory cytokines important as mediators of endotoxin shock, e.g. TNFα, IL-1β, IL-6, and IL-10, were also regulated in pinocembrin-treated mice. These outcomes indicated that cytokine regulation by this drug could be useful as part of a novel strategy prevention/mitigation of septic shock.
In the presence of stimuli such as LPS, activated macrophages produce several inflammmatory cytokines, including TNFα, IL-10, IL-6, and IL-1β, as well as nitric oxide (NO), and various prostaglandins (including PGE2) (Guha & Mackman, Citation2001). Several of these agents play key roles in mediating acute inflammatory responses and sepsis (Lantz et al., Citation2005). Thus, agents that can act to inhibit formation/release of these inflammatory mediators/cytokines have to be considered potential candidates as anti-inflammatory drugs (Choi & Park, Citation2012). Previous studies in our laboratory showed that in vitro pre-treatment of RAW 264.7 cells with pinocembrin (100–300 μg/ml) remarkably regulated the production of TNFα, IL-6, and IL-1β by inhibiting the phosphorylation of IκBα/NF-κB, ERK1/2, JNK, and p38MAPK (Soromou al., Citation2012), each key factors involved in cell signaling pathways. It should be noted that this inhibitory effect was not due to any cytotoxic activity of pinocembrin per se, as cell viability was unaffected by treatment.
Injection of LPS in mice induces acute inflammation accompanied by increased levels of pro-inflammatory mediators such as TNFα, IL-1β, and IL-6 in the blood. Normal production of these cytokines helps the innate immune response, but over-production results in acute phase endotoxemia and causes tissue injury, septic shock, and even death (Chao et al., Citation2011). The study here demonstrated the in vivo effects of pinocembrin on production of these pro-inflammatory mediators in LPS-challenged mice. Recently, new approaches for the use of Chinese herbal plants to prevent and treat inflammatory responses by inhibiting inflammatory cytokines, such as TNFα, IL-1β, and IL-6, have become an important area of investigation (Kim et al., Citation2005). TNFα is a target cytokine in the development of new medications development for disease involving inflammation, such as rheumatoid arthritis. Inhibition of elevated TNFα induced by LPS has been a common practice for evaluation of anti-inflammatory effect of drug candidates (Wang et al., Citation2007; Wu & Gu, Citation2009). TNFα is also regarded as the predominant cytokine that mediates LPS-induced lethality (Beutler et al., Citation1985). The results here showed that there was a lower level of TNFα in the serum of LPS-challenged mice treated with pinocembrin, confirming TNFα as a target in the anti-inflammatory strategy.
IL-6 is produced by a number of normal and transformed cells (Bartold & Haynes, Citation1991; Van et al., Citation1990). IL-1 is a multi-functional cytokine that is responsible for various processes including host defense, inflammation, and response to injury. Many types of cells (predominantly macrophages) produce IL-1 after stimulation with viruses or bacterial components (such as lipopolysaccharide [LPS]) or phorbol esters (Kielian et al., Citation2004; Morrissey & Charrier, Citation1994). Therefore, inhibiting secretion of these cytokines is a key issue during sepsis treatment. As shown here, pinocembrin treatment (50 mg/kg, IP) inhibited in situ release of TNFα, IL-1β, and IL-6 in a time-dependent manner, indicating that the protective role in endotoxemia may be attributable, at least in part, to inhibition of pro-inflammatory cytokine release. This effect may be particularly important when considering that these inflammatory mediators induce sickness behavior characterized by fever, lethargy, hypophagia, anxiety, and depression, etc. (Guyon et al., Citation2008). Studies have also reported that IL-10 plays an essential role in the suppression of the production of pro-inflammatory cytokines like TNFα (Dhingra et al., Citation2009) by inhibiting NF-κB activity in purified T-lymphocytes (Zemse et al., Citation2010).
In this study, we found that PNCB administrated 1 h ahead of LPS administration slightly increased the levels of anti-inflammatory cytokine IL-10 while causing a significant decrease in pro-inflammatory cytokines, illustrating that PNCB had a protective role against the inflammation related to sepsis potentially through a shifting of the cytokine balance towards anti-inflammatory. This would parallel the findings of another previous study wherein pinocembrin treatment led to a significant lowering of the number of total cells, neutrophils, macrophages, and lymphocytes during an LPS-induced septic event. Those authors also demonstrated histologically that pinocembrin could reduce pulmonary injury and attenuate edema accumulation in the lung (Soromou et al., Citation2012). The fact that the in vivo results appear to correlate well with in vitro outcomes suggests that the deactivation of resident and infiltrating macrophages could be the major mechanism involved in the protective effect of pinocembrin. Thus, it would seem important that the use of pinocembrin could be a key to maintaining/affecting the careful balance between pro- and anti-inflammatory cytokines during pathologic states, including sepsis, such that the host is not ushered into an unhealthy ‘over-inflammatory’ status nor cast into a situation where the host will not be able to properly mitigate any ongoing bacterial/pathogen-based challenge.
The pathogenesis of lethal sepsis remains obscure, but is associated with dysregulated production of inflammatory mediators (Cohen, Citation2002; Parrillo, Citation1993). The early events of severe sepsis set in motion a cascade of events that significantly contributes to the morbidity and mortality observed during the first few days of this syndrome (Benjamim et al., Citation2004). The data presented here suggest the possibility that, in addition to protection via suppression of pro-inflammatory cytokine expression, pinocembrin treatment can also protect from endotoxin shock via preventing the development of clinical manifestations of LPS morbidity (including decreased activity, lethargy, diarrhea, piloerection, huddling). Survival (to 60 h) among mice that also received 10 mg pinocembrin/kg was indistinguishable from mice that did not receive pinocembrin (group of mice given LPS alone). However, pre-treatment of pinocembrin (20 or 50 mg/kg) significantly lowered LPS-induced mortality. It would be beneficial in future studies to explore the therapeutic potential of pinocembrin against other inflammatory pathologies or inflammation-based diseases.
Conclusions
This study clearly indicated that pinocembrin could inhibit the release of the pro-inflammatory cytokines and improve survival against lethal endotoxemia. These results suggest that this new flavonoid could potentially be a novel candidate for prevention of the onset of septic shock.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors thank the National Nature Science Foundation of China (No.31172172) and Chinese postdoctoral station of Jilin University (No.20090461034) for their financial support in this research.
References
- Bartold, P. M., and Haynes, D. R. 1991. IL-6 production by human gingival fibroblasts. J. Periodontal. Res. 26:339–345
- Benjamim, C. F., Hogaboam, C. M., and Kunkel, S. L. 2004. The chronic consequences of severe sepsis. J. Leukocyte Biol. 75:408–412
- Beutler, B., Milsark, I. W., and Cerami, A. 1985. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229:869–871
- Calderone, V., Chericoni, S., Martinelli, C., et al. 2004. Vasorelaxing effects of flavonoids: Investigation on possible involvement of potassium channels. Naunyn-Schmied. Arch. Pharmacol. 370:290–298
- Chao, W. W., Kuo, Y. H., Hsieh, S. L., and Lin, B. F. 2011. Inhibitory effects of ethyl acetate extract of Andrographis paniculata on NF-κB trans-activation activity and LPS-induced acute inflammation in mice. Evid. Based Complement Alt. Med. doi:10.1093/ecam/nep120
- Choi, Y. H., and Park, H. Y. 2012. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 19:31
- Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885–891
- Dhingra, S., Sharma, A. K., Arora, R. C., et al. 2009. IL-10 attenuates TNFα-induced NF-κB pathway activation and cardiomyocyte apoptosis. Cardiovasc. Res. 82:59–66
- Gao, M., Liu, R., Zhu, S. Y., and Du, G. H. 2008b. Acute neurovascular unit protective action of pinocembrin against permanent cerebral ischemia in rats. J. Asian Nat. Prod. Res. 10:551–558
- Gao, M., Zhang, W. C., Liu, Q. S., et al. 2008a. Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of bax/bcl-2 ratio. Eur. J. Pharmacol. 591:73–79
- Guang, H. M., and Du, G. H. 2006. Protections of pinocembrin on brain mitochondria contribute to cognitive improvement in chronic cerebral hypo-perfused rats. Eur. J. Pharmacol. 542:77–83
- Guha, M., and Mackman, N. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 13:85–94
- Guyon, A., Massa, F., Rovere, C., and Nahon, J. L. 2008. “How cytokines can influence the brain: A role for chemokines?” J. Neuroimmunol. 198:46–55
- Hanasaki, Y., Ogawa, S., and Fukui, S. 1994. Correlation between active oxygen scavenging and anti-oxidative effects of flavonoids. Free Rad. Biol. Med. 16:845–850
- Hotchkiss, R. S., and Karl, I. E. 2003. The pathophysiology and treatment of sepsis. New Engl. J. Med. 348:138–150
- Huang, W. Z., Zhang, C. F., Zhang, M., and Wang, Z. T. 2007. A new biphenyl-propanoid from Alpinia katsumadai. J. Chin. Chem. Soc. 54:1553–1556
- Kalantar-Zadeh, K., Ikizler, T. A., Block, G., et al. 2003. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am. J. Kidney Dis. 42:864–881
- Kielian, T., Bearden, E. D., Baldwin, A. C., and Esen, N. 2004. IL-1 and TNFα play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J. Neuropathol. Exp. Neurol. 63:381–396
- Kim, S. J., Jeong, H. J., Moon, P. D., et al. 2005. Anti-inflammatory activity of gumigangh-waltang through the inhibition of NF-κB activation in peritoneal macrophages. Biol. Pharm. Bull. 28:233–237
- Lantz, R. C., Chen, G. J., Solyom, A. M., et al. 2005. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 12:445–452
- Liu, R., Wu, C. X., Zhou, D., et al. 2012. Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptors for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med. 10:105
- Middleton, E. J., Kandaswami, C., and Theoharides, T. C. 2000. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52:673–751
- Morrissey, P. J., and Charrier, K. 1994. Treatment of mice with IL-1 before infection increases resistance to a lethal challenge with Salmonella typhimurium. The effect correlates with the resistance allele at the Ity locus. J. Immunol. 153:212–219
- Namas, R., Ghuma, A., Hermus, L., et al. 2009. The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: Current state and emerging prospects. Libyan J. Med. 4:97–103
- Parrillo, J. E. 1993. Pathogenetic mechanisms of septic shock. New Engl. J. Med. 328:1471–1477
- Soromou, L. W., Chu, X., Jiang, L., et al. 2012. In vitro and in vivo protection provided by pinocembrin against lipopolysaccharide-induced inflammatory responses. Int. Immunopharmacol. 14:66–74
- Touil, Y. S., Fellous, A., Scherman, D., and Chabot, G. G. 2009. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer 61:310–321
- Tracey, K. J., Fong, Y., Hesse, D. G., et al. 1987. Anti-cachectin/-TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature 330:662–664
- Van, M. E., Sawamura, Y., Diserens, A. C., et al. 1990. Human glioblastoma cells release IL-6 in vivo and in vitro. Cancer Res. 50:6683–6688
- Wang, Q., Zhang, Y., Hall, J. P., et al. 2007. A rat pharmacokinetic and pharmacodynamic model for assessment of lipopolysaccharide-induced TNFα production. J. Pharmacol. Toxicol. Meth. 56:67–71
- Wu, M., and Gu, Z. 2009. Screening of bioactive compounds from Moutan Cortex and their anti-inflammatory activities in rat synoviocytes. Evidence-Based Complement Alt. Med. 6:57–63
- Zemse, S. M., Chiao, C. W., Hilgers Rob, H. P., and Webb, R. C. 2010. IL-10 inhibits the in vivo and in vitro adverse effects of TNFα on the endothelium of murine aorta. Am. J. Physiol. Heart Circ. Physiol. 299:1160–1167