Abstract
People are constantly exposed to environmental chemicals through contact with the atmosphere or by ingestion of food. Therefore, when conducting safety assessments, the immunotoxic effects of combinations of chemicals in addition to toxicities produced by each chemical alone should be considered. The objective of the studies reported here were to demonstrate the combined effects of three well-known environmental immunotoxic chemicals -- methoxychlor (MXC), an organochlorine compound; parathion (PARA), an organophosphate compound; and piperonyl butoxide (PBO), an agricultural insecticide synergist -- by using a short-term oral exposure method. Seven-week-old Balb/cAnN mice received daily oral exposure to either one or two of the environmental immunotoxic chemicals for 5 consecutive days. On Day 2, all mice in each group were immunized with sheep red blood cells (SRBC), and their SRBC-specific IgM responses were analyzed by using an enzyme-linked immunosorbent assay and plaque-forming cell assay. T- and B-cell counts in the mouse spleens were also assessed via surface antigen expression. Mice that received MXC + PARA and PBO + MXC treatment showed marked decreases in SRBC-specific IgM production and T- and B-cell counts compared with those in mice that received vehicle control or the corresponding individual test substance. This suggests that simultaneous exposure to multiple environmental chemicals increases the immunotoxic effects of the chemicals compared to individual exposure.
Introduction
Humans are exposed daily to a vast range of products that contain environmental agents (e.g. cosmetics, pesticides, drugs, and biotechnology-derived products) and to multiple environmental chemicals in the atmosphere and in food (Gilbert et al., Citation2011; Groten et al., Citation1997; Kortenkamp et al., Citation2007; Teuschler et al., Citation2002). Because of this constant exposure, when conducting safety assessments one must take into consideration the effects of combined exposure. For example, combined exposure to pesticides and heavy metals is known to enhance overall toxicity compared with that from exposure to the individual agents (Institoris et al., Citation1999, Citation2002). Approaches to assess effects from combined exposures have been described (Feron et al., Citation1995; Groten et al., Citation2001; Hernandez et al., Citation2013; Simmons, Citation1995). However, most toxicity assessments are conducted based on exposure to individual substances and, as such, mechanisms of effects from combined exposure to environmental chemicals remain unclear. Therefore, the objective of the studies reported here were to investigate the combined toxicologic effects of multiple chemicals.
In the study reported here, the toxic effects from combined exposure to three common environmental chemicals were investigated by examining the impact on immune functions. It is well known that exposure to environmental agents can compromise immunologic function (Fukuyama et al., 2010, 2013; Nishino et al., Citation2013). For example, several animal studies have shown there are alterations of primary humoral responses induced by immunotoxicants like dioxins and pesticides (Flipo et al., Citation1992; Smialowicz et al., Citation1997). To avoid these risks, immunotoxicity tests have been developed for evaluating the safety of environmental chemicals and pharmaceuticals (Holsapple, Citation2003; Luster et al., Citation1988). Based on those analyses, guidelines have been introduced over the years to regulate exposure to many agents; these include those published by the US Environmental Protection Agency (EPA, Citation1998), the Food and Drug Administration (FDA, Citation2002), the European Medicines Agency (Committee for Proprietary Medicinal Products, Citation2000), and the International Conference on Harmonization (ICH, Citation2006).
Our laboratories previously developed a short-term oral exposure method for assessment of the immunosuppressive potential of environmental chemicals (Fukuyama et al., 2013). In the current study, using this method, we demonstrate the combined immunotoxic effects of three well-known environmental chemicals, i.e. methoxychlor (MXC) – an organochlorine compound, parathion (PARA) – an organophosphate compound, and piperonyl butoxide (PBO) – an agricultural insecticide synergist. These three chemicals were selected on the basis of previous studies: MXC exposure causes atrophy of CD4+CD8+ T-cells in the thymus (Takeuchi et al., Citation2002a,Citationb); PARA markedly inhibits antigen-specific IgM production (Casale et al., Citation1984); and PBO depletes T-cells in the spleen and thymus, induces bone marrow hypoplasia, and inhibits T-cell proliferation in lymphoid tissues (Diel et al., Citation1999; Battaglia et al., Citation2010; Mitsumori et al., Citation1996). We also previously showed that MXC, PARA, and PBO exposure results in increased thymocyte apoptosis, markedly inhibited sheep red blood cell (SRBC)-specific IgM production, and aggravation of immune disorders such as atopic dermatitis and allergic airway inflammation (Fukuyama et al., 2011; Nishino et al., Citation2013).
Materials and methods
Chemicals
Standard MXC (C16H15Cl3O2, >97% pure), standard PARA (C10H14NO5PS, 99.5% pure), standard PBO (C19H30O5, >98% pure), and dimethyl sulfoxide (DMSO) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Corn oil was purchased from Hayashi Chemicals (Tokyo, Japan). For the in vivo portion of this study, MXC, PARA, or PBO diluted in corn oil to a fixed final volume was orally administered to mice. Based on the EPA Immunotoxicity Guidelines (1998) that states doses should ‘not produce significant stress, malnutrition, or fatalities’, doses used in this study were < 1/5 the median lethal dose (LD50; dose at which ≥50% of animals would be expected to die) and administered concurrently to avoid induction of clear general or immune toxicity (i.e. changes in appearance, posture, behavior, respiration, consciousness, neurologic status, temperature, excretion, etc.) (Fukuyama et al., 2013). The single-chemical dosages used in this study were: MXC, 100 mg/kg day; PARA, 1.0 mg/kg day; and PBO, 100 mg/kg day. Combination dosages were prepared by mixing each chemical so that the final concentration of each chemical was half that of the single dosage. Actually, there were no abnormal signs during the examination period. With regard to body weight measurements, treated groups’ values were comparable with those of the vehicle control and intact groups (data not shown). Therefore, we selected relatively high doses compared with actual human exposures. Actual doses and preparation of the test substances are presented in .
Table 1. Chemicals and dose settings.
Animals
Balb/cAnN mice (female, 6-weeks-old) were purchased from Charles River Laboratories (Atsugi, Kanagawa, Japan) and housed individually in cages under controlled lighting (lights on, 07:00–19:00), temperature (22 ± 3 °C), humidity (55% ± 15%), and ventilation (at least 10 complete fresh-air changes/h). Standard rodent chow (Certified Pellet Diet MF; Oriental Yeast Co., Tokyo) and filtered water were available ad libitum.
Female mice were selected as the model for this study because the EPA Immunotoxicity Guidelines (EPA, Citation1998) consider the mouse a model species for use in immunotoxicity studies that examine effects of agricultural chemicals (see Casale et al., Citation1984; Diel et al., Citation1999; Battaglia et al., Citation2010). The guideline indicates that either rats or mice may be used. Additionally, if ADME data are similar between species, then either rats or mice may be used. According to our preliminary immunotoxicity study data for MXC, PARA, and PBO, mice were more sensitive than rats. Therefore, we selected mice for the current study. Furthermore, in immunotoxicity studies, only one gender need be evaluated; in general, females are considered to yield more consistent outcomes than male animals when evaluating humoral immune responses. Consequently, Balb/cAnN mice were selected because our laboratory has historical immunotoxicity study data for our selected chemicals on this strain (data not shown). All aspects of the current study were conducted in accordance with the Animal Care and Use Program of the Institute of Environmental Toxicology, Japan (IET IACUC Approval No. 12027).
Chemical exposure of mice
After a 1-week acclimatization period, mice (now 7-weeks-old) were allocated randomly to two groups (n = 8 mice/group): treatment/vehicle control and to a no treatment (intact group). On Days 1–5, mice were given an oral dose (by gavage, without anaesthesia) of a single or combination test solution (MXC, PARA, PBO, MXC + PARA, PARA + PBO, PBO + MXC) or vehicle only. On Day 2, a solution of SRBC (6 × 107 cells/animal; Nippon Bio-Supp. Center, Tokyo) was injected via the tail vein into all test and control mice for immunization. One day after the final oral administration (i.e. on Day 6 of study), all mice were anaesthetized with Isoflurane and blood samples taken from the inferior vena cava. Serum samples were assayed for SRBC-specific serum IgM. After exsanguination from the abdominal aorta, the thymus of each animal was carefully removed and weighed. The spleen was removed and placed in phosphate-buffered saline (PBS, pH 7.4; Life Technologies Co., Ltd., Tokyo). Single-cell splenocyte suspensions in 5 ml modified Eagle’s medium supplemented with 5% heat-inactivated fetal bovine serum (FBS, Life Technologies) were prepared by passage through a stainless-steel screen and sterile 70-µm nylon cell strainer (Falcon, Tokyo). Numbers of lymphocytes in each suspension were determined using a Z2 Coulter Counter (Beckman Coulter, Tokyo).
Determination of serum SRBC-specific IgM response
Levels of SRBC-specific IgM in the serum were determined using a modified version of the method of Temple et al. (Citation1993). In brief, SRBC-membrane antigen was extracted with Tris-HCl and 0.1% sodium dodecyl sulfate in PBS. The samples were then dialyzed for 2 days against PBS. The protein content of each conjugated sample was determined using the method of Lowry et al. (Citation1951). SRBC-specific IgM levels were then measured by means of enzyme-linked immunosorbent assay (ELISA) in flat-bottomed microplates (Nalge Nunc, Tokyo) whose wells had been coated with SRBC-membrane antigen (2 µg/ml coating buffer; BD Pharmingen, Tokyo) during an overnight incubation at 4 °C. Following washing of each well 5-times with wash buffer (BD Pharmingen) and blocking of potential non-specific binding by incubation for 2 h at room temperature (RT) with assay diluent (BD Pharmingen), a dilution of each mouse serum sample (in assay diluent, from 1:4 to 1:16384) was added to each well and the plates incubated a further 2 h at RT. After gentle rinsing with wash buffer to remove all unbound materials, peroxidase-conjugated anti- mouse IgM (secondary antibody, Rockland Inc., Gilbertsville, PA; dilution 1:15 000) was added to each well and the plate incubated for 2 h at RT. The wells were then rinsed again to remove non-adherent anti-mouse IgM. Finally, to quantify the amount of bound antibodies in each well, tetramethylbenzidine (100 µl/well) was added to each well and the plate incubated in the dark at RT for 30 min. Optical density was then measured at 450 nm by in a Spectra MAX 190 microplate reader (Molecular Devices, Osaka).
Assessment of the splenocyte IgM plaque-forming cell response to SRBC
The IgM plaque-forming cell (PFC) response to SRBC was determined using a modified version of the methods of Cunningham (1965) and Jerne & Nordin (Citation1963). Briefly, ≈1 × 106 cells were incubated with 1% SRBC and a 1:30 dilution of guinea pig complement (Denka Seiken Co., Tokyo) for 10 min at 4 °C. The cells were then applied to a Cunningham chamber (Takahashi Giken Glass Co., Tokyo) and incubated for 1.5 h at 37 °C in a 5% CO2 atmosphere. The number of plaques in each sample was then counted using a stereomicroscope.
Flow cytometric analysis
Isolated splenocytes were stained all at one time with fluorescein isothiocyanate (FITC)- conjugated anti-peanut agglutinin (Vector Laboratories, Inc., Burlingame, CA) and the monoclonal antibodies (MAb) phycoerythrin-cyanine-7-conjugated anti-mouse CD4 (PE-Cy7, clone RM- 4-5), allophycocyanin-cyanine-7-conjugated anti-mouse CD8 (APC-Cy7, clone 53-6.7), APC-conjugated anti-mouse CD3 (clone 145-2C11), and peridinin chlorophyll protein-Cy5.5-conjugated rat anti-mouse CD19 (PerCP-Cy5, clone 1D3 (all BD Pharmingen). To avoid non-specific binding, 106 cells were incubated with 1 µg Mouse Fc BlockTM (BD Pharmingen) for 5 min at RT, followed by incubation with MAb for 30 min at 4 °C in the dark. Cells were then washed twice with 5% FBS in PBS, re-suspended at 106 cells/tube in 500 µl PBS and then analyzed on a FACSVerse flow cytometer (BD Pharmingen) using FACSuite software. A minimum of 20 000 events/sample was collected and analyzed for antigen expression.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Analysis of variance (ANOVA) was used to evaluate the results. For significant results, differences between vehicle control and treatment groups were then assessed using a Dunnett’s multiple comparison test. Statistical significance of differences between single-chemical and combination-treatment groups was determined using a Student’s t-test. p values <0.05 were considered significant in each test.
Results
Overall toxicity of the various treatments to the mice
Throughout the studies, there were no abnormal clinical signs (e.g. decreased activity) or changes in body weight or body weight gain due to any of the treatment regimens.
Thymus weights
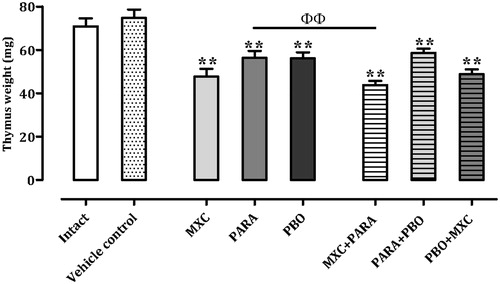
Thymus weights are shown in . All treatment groups had significantly (p < 0.01) decreased values compared with those seen with the vehicle control mice. The MXC + PARA mice did display a significant decrease (≈22.2%, p < 0.01) compared against the values for the PARA treatment mice, and also a decrease of 8.1% versus MXC mice; however, this decrease was not significant. The PBO + MXC mice had average decreases of thymus weight of ≈13.0% vs the PBO hosts, but this decrease too was not significant. Thymus weights in the PARA + PBO mice were comparable with those of the PARA or PBO hosts.
Figure 1. Absolute thymus weights. Mice were treated with nothing (intact naive), vehicle, methoxychlor (MXC), parathion (PARA), piperonyl butoxide (PBO), or combinations of the agents (two at a time). Absolute thymus weights are expressed as mean ± SD (mg; n = 8 per group). **p < 0.01 (Dunnett’s multiple comparison test) vs vehicle control group; ΦΦ p < 0.01 (Student’s t-test) vs single test substance groups.
Serum SRBC-specific IgM responses
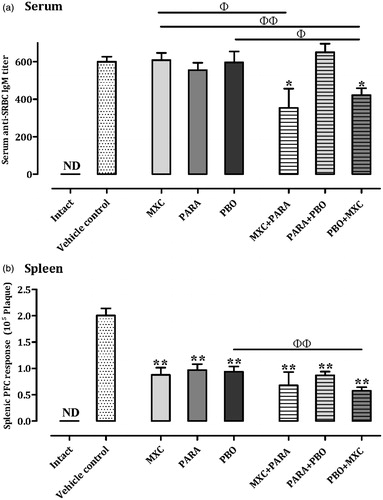
Serum SRBC-specific IgM responses are shown in . In the PARA, PBO, and PARA + PBO treatment groups, SRBC-specific IgM responses were comparable with that of the vehicle control group. However, the MXC + PARA and PBO + MXC treatment groups displayed significantly decreased (p < 0.05) SRBC-specific IgM responses compared with that by the vehicle controls – decreases of ≈40.9% and 29.5%, respectively. Furthermore, the MXC + PARA- treated mice had a significantly decreased (≈41.8%) response compared with that of the MXC-only mice. Further, while the MXC + PARA mice displayed a decreasing trend in response compared with that by the PARA-only mice, the net difference (a decrease of ≈36.3%) was not significant. Similarly, the PBO + MXC mice had a significantly decreased response compared with the PBO ≈29.2%, p < 0.05) and MXC (30.6%, p < 0.01) treatment mice.
Figure 2. Serum and splenic IgM responses. Mice were treated as described in the legend. (a) Serum and (b) spleen IgM responses are shown. IgM responses are expressed as mean ± SD (titre; n = 8 per group). IgM responses in the spleen are expressed as mean ± SD (n = 8 per group). *p < 0.05 and **p < 0.01 (Dunnett’s multiple comparison test) vs vehicle control group; Φp < 0.05 and ΦΦp < 0.01 (Student’s t-test) vs single test substance groups.
Splenocyte IgM PFC response to SRBC
Splenocyte IgM PFC responses to SRBC are shown in . All treatment groups had significantly (p < 0.01) lower IgM PFC responses to SRBC compared with the vehicle control mice. The MXC + PARA mice had decreases of ≈22.7% vs the MXC and 29.9% vs the PARA groups, but the decreases were not significant. However, PBO + MXC treatment did cause a significant decrease (≈38.3%, p < 0.01) from PBO mice values; there was a decrease of ≈34.1% vs MXC mice values, but this was not significant. Splenocyte PFC responses with PARA + PBO mice were comparable to that seen with their PARA- or PBO-only counterparts.
Splenocyte T-cell counts
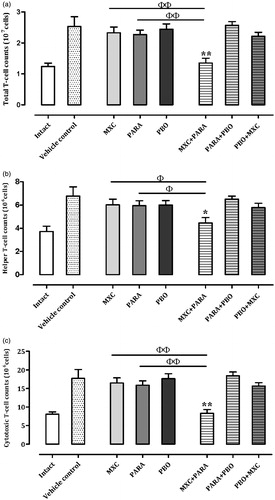
To evaluate the level of T-cell immunosuppression following the single or combination treatments, isolated lymphocytes were stained with anti-CD3, -CD4, and -CD8 antibodies. The numbers of total, helper, and cytotoxic T-cells are shown in . In the MXC, PARA, PBO, PARA + PBO, and PBO + MXC treatment groups, all T-cell counts were comparable with those of the vehicle control group. The PBO + MXC group showed a decrease in T-cell counts, but this was not statistically significant. The MXC + PARA group had significantly decreased total (p < 0.01), helper (p < 0.05), and cytotoxic (p < 0.01) T-cell counts compared with those of control, MXC, and PARA mice – decreases of, respectively, ≈52.5, 42.8, and 58.0% vs control; 42.1, 26.1, and 49.9% vs MXC alone; and 33.2, 20.0, and 39.6% vs PARA alone.
Figure 3. T-cell sub-type counts in spleens. Mice were treated as described in the legend. (a) Total, (b) helper, and (c) cytotoxic T-cell counts are shown. Results for intact, vehicle, and individual agent-treated mice are included in each chart. Cell counts are expressed as mean ± SD (n = 8 per group). *p < 0.05 and **p < 0.01 (Dunnett’s multiple comparison test) vs vehicle control group; Φp < 0.05 and ΦΦp < 0.01 (Student’s t-test) vs single test substance groups.
Splenocyte B-cell counts
To evaluate B-cell immunosuppression following the single or combination treatments, isolated lymphocytes were stained with anti-CD19 and anti-peanut agglutinin (PNA) antibodies (). In the MXC, PARA, PBO, PARA + PBO, and PBO + MXC treatment groups, total B-cell (CD19+) counts were comparable with that in the vehicle controls. The PBO + MXC group had a decrease in total B-cell counts, but this was not significant. The MXC + PARA group had significantly decreased total B-cell counts (p < 0.01) compared with the vehicle control, MXC, and PARA groups – decreases of, respectively, ≈50.9, 36.1, and 33.8%.
Figure 4. Total B-cell and germinal center B-cell counts in spleens. Mice were treated as described in the legend. (a) Total B-cell and (b) germinal center B-cell counts are shown. Results for intact, vehicle, and individual agent-treated mice are included in each chart. Cell counts are expressed as mean ± SD (n = 8 per group). *p < 0.05 and **p < 0.01 (Dunnett’s multiple comparison test) vs vehicle control group; Φp < 0.05 and ΦΦp < 0.01 (Student’s t-test) vs single test substance groups.
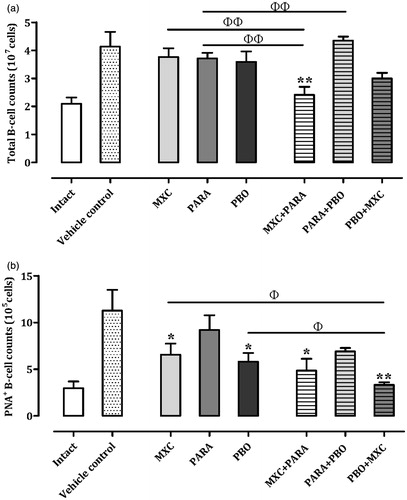
In all of the groups given a test substance, germinal center B-cell (CD19+PNA+) counts were lower than that in the vehicle control mice. In addition, the MXC (p < 0.05) PBO (p < 0.05), MXC + PARA (p < 0.05), and PBO + MXC (p < 0.01) groups had significantly decreased germinal center B-cell counts compared with that of the vehicle controls. The PBO + MXC treatment mice also had significantly decreased (p < 0.05) germinal center B-cell counts compared with the PBO and MXC treatment group – decreases of ≈42.7 and 49.2%, respectively.
Discussion
Our objective was to provide new insights into effects of combined exposures to three well-known environmental chemicals: methoxychlor, parathion, and piperonyl butoxide. This study examined immunotoxic effects of these chemicals in Balb/cAnN mice using a short-term oral exposure protocol. Changes in host immune status were assessed by measures of effects on thymus weight, anti-SRBC IgM responses, and T- and B-cell counts in serum and spleen.
Based on our previous report (Fukuyama et al., Citation2010), immunosuppressive environmental chemicals induce thymocyte apoptosis and reduced thymus weights. Thus, changes in thymus weights were analyzed as a general measure of in situ immunotoxicity from the test agents here as the thymus is a key lymphoid organ, and precursor T-cells migrate there to undergo maturation (Janeway et al., Citation2004). In our study, compared to what was seen with vehicle control mice, all treatments induced significant decreases in thymus weight. Among the combined exposure groups, the MXC + PARA mice had values significantly decreased compared with those of PARA-only mice (but not vs MXC mice). In contrast, while PBO + MXC mice had a trend toward decreasing values vs the same PBO hosts, the decrease was not significant. PARA + PBO mice had values not altered from their individual agent counterparts. These results suggested to us that, at the level of thymocyte damage (i.e. potential apoptosis), there was little to no interactive effect from the combined exposures to PARA + PBO and PBO + MXC exposures. In contrast, as MXC + PARA exposure appeared to have induced a more severe effect compared with exposure to each individual chemical, it is likely that some interactive effect (most likely synergistic) was occurring in situ to amplify the toxicities of each individual test agent. As will become clear below, this preferentially strong toxicity by MXC + PARA compared to the other combinational regimens becomes evident in several other aspects of these studies.
SRBC is a common antigen used to evaluate general immune status. After immunization with SRBC, SRBC-specific IgM responses in serum and spleen can be assessed using ELISA and PFC assays, respectively (Temple et al., Citation1993; White et al., Citation2010). Use of these two assays allows for evaluation of the mechanisms of action of xenobiotic-induced immunotoxicities (Herzyk & Holsapple, Citation2007). Compared with the vehicle control mice, MXC + PARA and PBO + MXC mice had significant decreases in serum SRBC-specific IgM responses, whereas MXC, PARA, PBO, and PARA + PBO mice did not. In addition, the IgM responses with the MXC + PARA mice were significantly decreased vs that of MXC mice, and PBO + MXC treatment led to significant decreases relative to those seen with PBO and MXC mice. In contrast, in all groups given a test substance, spleen SRBC-specific IgM PFC responses were significantly decreased relative to those seen with the vehicle control group. In addition, the SRBC-specific IgM responses with spleens from the PBO + MXC mice were significantly decreased compared to that of organs from PBO mice. Based on our historic data (Fukuyama et al., Citation2013), the peak response to SRBC for the SRBC-specific IgM ELISA occurs ∼2 days after the maxima that would be used to optimize results for a PFC. Thus, as we utilized a protocol that was focusing mainly on the PFC assay, it is not a complete surprise that the SRBC-specific IgM ELISA responses were weaker than the PFC ones. Under these conditions, MXC + PARA and PBO + MXC led to significant decreases relative to those seen with PBO and MXC in serum SRBC-specific IgM responses. These results suggested to us that MXC + PARA or PBO + MXC exposures induced a more severe reduction in humoral immune responses compared with exposure to any of the three individual chemicals.
To further clarify mechanisms of MXC + PARA- or PBO + MXC-induced immunosuppression, total, helper, and cytotoxic T-cell counts, as well as total and germinal center B-cell counts in the spleens were analyzed via flow cytometry based on cell-specific surface makers (Janeway et al., Citation2004). It was clear that the MXC + PARA combined treatment damaged T-cells. Total, helper, and cytotoxic T-cell counts in hosts that received this combined treatment were decreased compared with those in vehicle control, MXC, and PARA mice. In contrast, in the PBO + MXC hosts, the counts were comparable to those in PBO- and MXC-only mice. Similarly, in PARA + PBO mice, all sets of counts were comparable to those in PARA and PBO mice. These latter sets of results indicated that the toxicities of these two chemical combinations, at least in regard to impacts on T-cell populations in situ, were similar to those of any of their single chemical constituents. It is interesting to note that, with the MXC + PARA regimen, the changes in cell counts corresponded with the observed changes in SRBC-specific IgM responses. On the other hand, in the PBO + MXC group, although SRBC-specific IgM responses were decreased compared to the control, PBO, and MXC mice, there were no similar correspondence with the T-cell measures. This might suggest that the combined action of the PBO and MXC may have been directed more against the B-cell aspects of humoral responses than against T-cells; however, this still remains to be verified in more detailed studies.
In an immune response, local activated B-cells act as antigen-presenting cells for helper or cytotoxic T-cells (Goutet et al., Citation2005), proliferate, and differentiate into plasma cells to secrete antigen-specific antibodies. Some B-cells are activated at the T/B-cell border and migrate to form germinal centers (in primary follicles; Janeway et al., Citation2004); therefore, changes in the numbers of germinal centers and associated B-cells can reflect major responses to exposure to antigens or toxicants (Vieira & Rajewsky, Citation1990; Takahashi et al., Citation1998). A marked decrease in total B-cell counts was seen in the MXC + PARA-treated mice compared with that in MXC and PARA mice. Neither other combinational treatment had a similar significant effect. At the germinal center level, both MXC + PARA and PBO + MXC led to significant reductions in B-cell levels; PARA + PBO had no significant impact. Compared to their individual agents, MXC + PARA treatment caused even greater reductions in total B-cell levels, but had no effect at the germinal center level. This contrasts with PBO + MXC that had the opposite effect, i.e. no impact at total B-cell level but significantly-so at germinal centers. While these opposing outcomes are without explanation at this point, the upshot is that the combinational treatments with PBO + MXC or MXC + PARA are toxic to B-cells in situ. Toxicity from PARA + PBO is nominal at best.
The findings with the PBO + MXC mice supports our contention cited in the early paragraphs about potentially more selective effects on B-cells. That the MXC + PARA regimen also impacted on B-cells (beyond above-noted effects on thymic weights, T-cell counts, and IgM responses) suggested that this specific combination displayed a far more immunotoxic targeting than the other combined regimen. Whether such a divergent effect is due to differences in synergizing effects from each individual agent is an interesting possibility. Future studies with gradational combinations of each test chemical should allow us to ascertain which of the individual agents is driving any synergisms.
Conclusions
Our data show that combined exposure to certain environmental chemicals can induce immunotoxicity, as shown by effects on SRBC-specific IgM responses and T- or B-cell counts, compared to that by individual exposure to the chemicals in mixtures. However, this toxicity appears to differ, depending on which chemicals are combined. In particular, it was clear that, among the three combinations, MXC + PARA presented the most immunotoxic profile in the murine hosts. The combined toxicity may be affected by chemical structure, receptor binding, and immune pathways involved; further studies are currently in progress. It is expected that the results of this study will help others in their evaluation of immunotoxic combinational effects when conducting assessments of the safety of environmental/occupational chemicals.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This work was supported by a research Grant-in-Aid from the Ministry of Health, Labor, and Welfare of Japan. The authors wish to thank Mrs Y. Tajima and L. Miyashita of the Institute of Environmental Toxicology (Ibaraki, Japan) for their technical assistance.
References
- Battaglia, C. L., Gogal, R. M. Jr., Zimmerman, K., and Misra, H. P. 2010. Malathion, lindane, and piperonyl butoxide, individually or in combined mixtures, induce immune-toxicity via apoptosis in murine splenocytes in vitro. Int. J. Toxicol. 29:209–220
- Casale, G. P., Cohen, S. D., and DiCapua, R. A. 1984. Parathion-induced suppression of humoral immunity in inbred mice. Toxicol. Lett. 23:239–247
- Committee on Proprietary Medicinal Products. 2000. Note for Guidance on Repeat-Dose Toxicity, CPMP/SWP/1042/99. Available online at: http://www.emea.eu.int
- Cunningham, A. J. 1965. A method of increased sensitivity for detecting single antibody-forming cells. Nature 207:1106–1107
- Diel, F., Horr, B., Borck, H., et al. 1999. Pyrethroids and piperonyl-butoxide affect human T-lymphocytes in vitro. Toxicol. Lett. 107:65–74
- EPA (United States Environmental Protection Agency). 1998. Health Effects Test Guidelines: Immunotoxicity. 1998, OPPTS 870.7800. Available online at: http://www.epa.gov/opptsfrs/publications
- FDA (United States Food and Drug Administration). 2002. Guidance for Industry: Immuotoxicology Evaluation of Investigational New Drugs. Available online at: http://www.fda.gov/cder/guidance
- Feron, V. J., Groten, J. P., Jonker, D., et al. 1995. Toxicology of chemical mixtures: Challenges for today and the future. Toxicology 105:415–427
- Flipo, D., Bernier, J., Girard, D., et al. 1992. Combined effects of selected insecticides on humoral immune response in mice. Int. J. Immunopharmacol. 14:747–752
- Fukuyama, T., Kosaka, T., Hayashi, K., et al. 2013. Immunotoxicity in mice induced by short-term exposure to methoxychlor, parathion, or piperonyl butoxide. J. Immunotoxicol. 10:150–159
- Fukuyama, T., Tajima, Y., Ueda, H., and Kosaka, T. 2011. Prior exposure to immunosuppressive organophosphorus or organochlorine compounds aggravates the T(H)1- and T(H)2-type allergy caused by topical sensitization to 2,4-dinitrochlorobenzene and trimellitic anhydride. J. Immunotoxicol. 8:170–182
- Fukuyama, T., Tajima, Y., Ueda, H., et al. 2010. Apoptosis in immunocytes induced by several types of pesticides. J. Immunotoxicol. 7:39–56
- Gilbert, K. M., Rowley, B., Gomez-Acevedo, H., and Blossom, S. J. 2011. Co-exposure to mercury increases immunotoxicity of trichloroethylene. Toxicol. Sci. 119:281–292
- Goutet, M., Pepin, E., Langonne, I., et al. 2005. Identification of contact and respiratory sensitizers using flow cytometry. Toxicol. Appl. Pharmacol. 205:259–270
- Groten, J. P., Feron, V. J., and Suhnel, J. 2001. Toxicology of simple and complex mixtures. Trends. Pharmacol. Sci. 22:316–322
- Groten, J. P., Schoen, E. D., van Bladeren, P. J., et al. 1997. Subacute toxicity of a mixture of nine chemicals in rats: Detecting interactive effects with a fractionated two-level factorial design. Fundam. Appl. Toxicol. 36:15–29
- Hernandez, A. F., Parron, T., Tsatsakis, A. M., et al. 2013. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 307:136–145
- Herzyk, D. J., and Holsapple, M. 2007. Immunotoxicity evaluation by immune function tests: Focus on the T-dependent antibody response (TDAR). Overview of Workshop Session at 45th Annual Meeting of Society of Toxicology March 5-9, 2006 San Diego, CA. J. Immunotoxicol. 4:143–147
- Holsapple, M. P. 2003. Developmental immunotoxicity testing: A review. Toxicology 185:193–203
- ICH. 2006. International Conference on harmonization of technical requirements for registration of pharmaceuticals for human use. ICH Harmonized Tripartite Guideline Immunotoxicity Studies For Human Pharmaceuticals S8. Switzerland: ICH
- Institoris, L., Papp, A., Siroki, O., et al. 2002. Immuno- and neurotoxico- logical investigation of combined subacute exposure with the carbamate pesticide propoxur and cadmium in rats. Toxicology 178:161–173
- Institoris, L., Siroki, O., Undeger, U., et al. 1999. Immunotoxicological effects of repeated combined exposure by cypermethrin and the heavy metals lead and cadmium in rats. Int. J. Immunopharmacol. 21:735–743
- Janeway, C. A., Travers, P., Walport, M., and Shlomchik, M. J., (Eds.). 2004. Immunobiology, 6th Edition. New York: Grand Science
- Jerne, N. K., and Nordin, A. A. 1963. Plaque formation in agar by single antibody-producing cells. Science 140:405
- Kortenkamp, A., Faust, M., Scholze, M., and Backhaus, T. 2007. Low-level exposure to multiple chemicals: Reason for human health concerns? Environ. Health. Perspect. 115:106–114
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275
- Luster, M. I., Munson, A. E., Thomas, P. T., et al. 1988. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 10:2–19
- Mitsumori, K., Takegawa, K., Shimo, T., et al. 1996. Morphometric and immunohistochemical studies on atrophic changes in lymphohematopoietic organs of rats treated with piperonyl butoxide or subjected to dietary restriction. Arch. Toxicol. 70:809–814
- Nishino, R., Fukuyama, T., Tajima, Y., et al. 2013. Prior oral exposure to environmental immunosuppressive chemicals methoxychlor, parathion, or piperonyl butoxide aggravates allergic airway inflammation in NC/Nga mice. Toxicology 309C:1–8
- Simmons, J. E. 1995. Chemical mixtures: Challenge for toxicology and risk assessment. Toxicology 105:111–119
- Smialowicz, R. J., DeVito, M. J., Riddle, M. M., et al. 1997. Opposite effects of 2,2′,4,4′,5,5′-hexachlorobiphenyl and 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antibody response to sheep erythrocytes in mice. Fundam. Appl. Toxicol. 37:141–149
- Takahashi, Y., Dutta, P. R., Cerasoli, D. M., and Kelsoe, G. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)-acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895
- Takeuchi, Y., Kosaka, T., Hayashi, K., et al. 2002b. Alterations in the developing immune system of the rat after perinatal exposure to methoxychlor. J. Toxicol. Pathol. 17:165–170
- Takeuchi, Y., Kosaka, T., Hayashi, K., et al. 2002a. Thymic atrophy induced by methoxychlor in rat pups. Toxicol. Lett. 135:199–207
- Temple, L., Kawabata, T. T., Munson, A. E., and White, K. L. Jr. 1993. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 21:412–419
- Teuschler, L., Klaunig, J., Carney, E., et al. 2002. Support of science-based decisions concerning the evaluation of the toxicology of mixtures: A new beginning. Regul. Toxicol. Pharmacol. 36:34–39
- Vieira, P., and Rajewsky, K. 1990. Persistence of memory B-cells in mice deprived of T-cell help. Int. Immunol. 2:487–494
- White, K. L., Musgrove, D. L., and Brown, R. D. 2010. The sheep erythrocyte T-dependent antibody response (TDAR). Meth. Mol. Biol. 598:173–184

