Abstract
Recently, there has been a renewed interest in the use of the minipig as an alternative to dogs and non-human primates for conducting toxicological assessments in non-rodent species. Since the T-dependent antibody response (TDAR) is one of the most widely-accepted assays used in the assessment of immunocompetence, the present study was undertaken to characterize the primary and secondary TDAR to keyhole limpet hemocyanin (KLH) in the Göttingen Minipig®. Following primary immunization with either 2 or 10 mg KLH, anti-swine IgM and IgG ELISAs were optimized and individual animal responses were evaluated over time. Immunization with 10 mg KLH on Day 0 promoted primary IgM responses that peaked 6–9 days after antigen administration, while primary IgG levels peaked on Day 13 or 14. Secondary IgG antibody levels (following secondary injection with 2 mg KLH on Day 14) plateaued on Days 20–22. Anti-KLH antibody levels were decreased in minipigs treated with cyclophosphamide (CPS), a known immunosuppressant, at doses ranging from 12.5–50 mg/kg/day, while antibody levels in animals treated with 2.5 mg CPS/kg/day were similar to levels in saline-treated swine. These results demonstrate that the Göttingen Minipig® can be a useful alternative non-rodent species to the dog and the non-human primate for evaluating the TDAR to KLH in regulatory assessments of immunotoxicity.
Introduction
For centuries, swine have been used in biomedical research, with reports of swine surgical dissections (for research in anatomy) dating back to ancient Greece in 200 AD. In the 1940s, the use of swine in research began to increase; however, their large size presented a number of difficulties, leading to the development and subsequent use of a number of miniature pig breeds for a variety of medical research applications (Köhn, Citation2012). Recently, minipigs have increasingly been recognized by regulatory agencies such as the US Food and Drug Administration (USFDA) as a suitable alternative to non-human primates for conducting toxicological evaluations of pharmaceuticals in non-rodent species under Good Laboratory Practice (GLP) regulations (Bode et al., Citation2010; van der Laan et al., Citation2010).
Minipigs have been well established as non-rodent models for dermal and cardiotoxicity studies. Although they are not frequently used at present for other types of toxicological assessments, swine are generally regarded as excellent models for drug and chemical-induced toxicity (Svendsen, Citation2006), due in part to their anatomical and physiological similarities to humans (Swindle & Smith, Citation1998). The Göttingen Minipig® was developed at the University of Göttingen in the 1960s and is a crossbreed of the Minnesota minipig, the Vietnamese potbelly pig, and the German Landrace (Svendsen, Citation2006). This outbred minipig reaches sexual maturity ≈3–5 months after birth (Köhn, Citation2012), at which time, based upon growth modeling conducted in this species, its approximate body weight ranges from 5–12 kg (Köhn et al., Citation2007). One year after birth, the average Göttingen Minipig® weighs ≈25 kg, while 35 kg is the average adult weight at 2 years of age (Köhn et al., Citation2007; Köhn, Citation2012).
The Göttingen Minipig® is often a preferred choice for non-rodent toxicity testing due to its manageable size, a lower age of sexual maturity as compared to dogs and non-human primates, the significant background toxicological control database available for this breed (Svendsen, Citation2006), and its well-documented disease-free (viral, parasitic, fungal, bacterial) status (Gad et al., Citation2007). Furthermore, this breed has relatively few spontaneously occurring histopathological lesions (Svendsen et al., Citation1998), with rare spontaneous effects (interstitial focal mononuclear cell infiltration) on the primary lymphoid organs, i.e., the spleen and the thymus (Gad et al., Citation2007; Svendsen et al., Citation1998). With regard to the immune system, the pig is considered a suitable model for humans, in spite of a few minor differences, including an ‘inverted’ lymph node structure, in which cortical tissue extends into the medulla and the germinal centers are primarily found in the cortex (Gad et al., Citation2007; Rothkötter, Citation2009; Swindle & Smith, Citation1998). Other notable differences in the immune system of pigs include: the presence of Peyer’s patches in swine ileum, the absence of maternal antibody transfer to the fetus in pigs, and greater percentages of γδT-cells in the swine peripheral blood (Bode et al., Citation2010; Rothkötter, Citation2009; Yang & Parkhouse, Citation1996).
In regulatory immunotoxicity evaluations of pharmaceuticals, the T-dependent antibody response (TDAR) is one of the recommended functional evaluations of the immune system (USFDA, Citation2006), due in part to its sensitivity to detecting modulation of immune function (Luster et al., Citation1992). This functional assay was also recommended as part of the ICH S8 Immunotoxicology Harmonization Document (ICH, Citation2005). The purpose of the present study was to characterize the primary IgM, primary IgG, and secondary IgG antibody responses of Göttingen Minipigs® to keyhole limpet hemocyanin (KLH), a T-dependent antigen, using an enzyme-linked immunosorbent assay (ELISA). Time-course studies identified the optimum sampling windows for the anti-KLH antibody responses of this breed. The anti-KLH responses of Göttingen Minipigs® treated with cyclophosphamide (CPS), a known immunosuppressive drug, were evaluated to validate that the TDAR could be modulated in the Göttingen Minipig® and to establish a dose of CPS that could be used as a positive control in future studies.
Materials and methods
Animals and animal husbandry
The in-life phase of these studies was conducted at WIL Research in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Eighteen male and four female Göttingen Minipigs®, maintained in a colony by WIL Research, were randomly selected for use in these studies. This number provided a sufficient sample size to evaluate the TDAR to KLH in saline-treated (vehicle control) and CPS-treated pigs. Animals were ≈4–8 months-of-age at the start of the studies, with body weights ranging from ≈9.5–18 kg. Animals were observed twice daily for signs of moribundity, and detailed physical examinations were conducted weekly. Body weights for all animals were obtained weekly beginning on study Day 0.
Pigs were generally housed two per cage (by both sex and group) in clean canine/minipig caging units in an environmentally controlled room (22 ± 3 °C; 50–70% relative humidity; 12-h light/dark cycle). Animals were separated within each cage (i.e., one on each side). Cages were elevated above stainless steel flush pans, which were cleaned daily. Twice daily, all animals were offered appropriate quantities of food (PMI 5K99 Certified Lab Minipig Grower LabDiet®) according to facility standard operating procedures. Reverse-osmosis treated tap water was provided ad libitum.
Experimental design
Seven groups of animals were utilized in this study in order to optimize and assess the TDAR to KLH in vehicle (saline-treated) and CPS-treated minipigs. summarizes the treatment and immunization doses for each group. Four males and one female were randomly assigned to each of Groups 1 and 2. Two males were randomly assigned to each CPS treatment group (Groups 3–7), and one female was randomly assigned to each of Groups 6 and 7. Once daily on Days 0–4 for the primary IgM and IgG responses, and on Days 14–18 for the secondary IgG response, animals were treated by subcutaneous (SC) inguinal injection with 0.9% saline (Groups 1 and 2) or the assigned dose of CPS (Groups 3–7) in a volume of 1 ml/kg. One animal in Group 5 was not treated with CPS (25 mg/kg/day) on Days 14–18 due to concern for overt toxicity at this dose of CPS.
Table 1. Treatment groups.
Six days prior to KLH immunization, all animals were initially bled to evaluate background levels of anti-KLH antibodies. KLH was obtained from biosyn Corporation (Carlsbad, CA). Intravenous (IV) administration was selected as the route of KLH immunization, based on the experience of the authors, in order to provide a robust antibody response to the antigen. On the day of immunization (‘Day 0’), all pigs were immunized by IV injection via the ear vein with KLH (2 or 10 mg/animal; see ) in a volume of 1 ml/animal. These immunization levels were chosen based on the experience of the authors and on previous reports (Penninks & van Mierlo, Citation2012). Once daily, on Days 5–9 and on Days 12–14, blood was collected from all animals to assess primary IgM and primary IgG antibody levels, respectively. Following blood collection on Day 14, all animals received a secondary challenge of KLH (2 mg/animal; IV via ear vein), and blood was collected on Days 19–23 to assess the secondary IgG response. All blood samples were collected via the jugular vein (≈5 ml/interval). Serum was obtained from each sample, transferred to cryotubes identified by animal number and day of collection, and stored at ≈−70 °C until shipment to ImmunoTox®, Inc. for evaluation. All samples were shipped frozen on dry ice via overnight carrier and arrived frozen and in good condition at ImmunoTox®, Inc.
KLH ELISA development
Several parameters were initially evaluated for optimal performance in developing the KLH ELISA, including the choice of the solid phase (i.e., 96-well ELISA plate), coupling buffer, antigen concentration for coupling to the plate, and the assay buffer (i.e., ‘blocking buffer’). Following these initial studies, optimum secondary antibody concentrations were determined for the anti-swine IgM and IgG ELISAs. All secondary antibodies (goat anti-pig IgM and goat anti-pig IgG, conjugated with the enzyme horseradish peroxidase [HRP]) were obtained from AbD Serotec (Raleigh, NC). The optimum dilutions of secondary antibody for the IgM and IgG ELISAs were selected based on their ability to produce robust responses without significant non-specific binding. The substrate utilized in all experiments was 2,2′-azino-bis(3ethyl-benzothia-zoline-6-sulfonic acid) diammonium salt (ABTS; Sigma, St. Louis, MO), prepared at 0.1 mg/ml in a phosphate-citrate buffer (Sigma). This substrate was selected due to its slower rate of color development, allowing for multiple plates to be run in one assay without need for a stop solution or the limitation of reading the plates within a short time period.
Previous reports identified Day 7 post-immunization as the peak day for IgM antibody response in Göttingen Minipigs® (Hinton & Khan, Citation1989). Therefore, serum samples obtained on Day 7 were used for optimizing the various ELISA parameters. After optimization, all samples obtained in this study were analyzed using the optimized anti-swine ELISA standard operating procedures in order to determine the time-courses of the primary IgM and primary and/or secondary IgG responses to KLH.
ELISAs were conducted using the following standard operating procedure. Flat-bottomed 96-well ELISA plates were coated overnight at 2–8 °C with KLH (100 μl/well), diluted to the optimum concentration in the optimum coating buffer. On the day of assay, plates were washed three times (200 μl/well/wash) with assay buffer, after which 200 μl/well of assay buffer was added to all wells, and the plates were incubated for 1 h at room temperature. Next, the plates were washed three additional times (200 μl/well/wash) with assay buffer, and samples were added in a volume of 200 μl/well at an appropriate starting dilution in assay buffer and serially-diluted (1:2 in assay buffer) across the plate. At least 11 dilutions per sample were evaluated to ensure that during analysis the linear region could be identified and the prozone region could be avoided. After samples were added, plates were incubated for 1 h at room temperature, washed three times with assay buffer (200 μl/well/wash), and secondary antibody was added in a volume of 100 μl/well at the desired dilution. After an additional hour, plates were again washed three times as before, and ABTS substrate was subsequently added in a volume of 100 μl/well. After the addition of the substrate, all plates were read at 15-min intervals over the course of 1 h on a Thermomax microplate reader (Molecular Devices Corp., Sunnyvale, CA) at 405 nm. Results were obtained using SoftMax (Molecular Devices, v. 2.3.2) by identifying the linear portion of a log–log plot of optical density (OD) versus dilution and interpolating at 0.5 OD. Titer was defined as the reciprocal of the dilution corresponding to 0.5 OD, and the data were reported in terms of log2 (titer), the appropriate metric when using doubling serial dilutions.
Data analysis
Statistical analysis was not conducted due to the small sample size of each treatment group. However, results are discussed in terms of how the serum anti-KLH antibody levels after sensitization compare to background levels (pre-sensitization).
Results
General observations
All minipigs tolerated immunization with KLH very well. Animals treated with CPS at 2.5 and 12.5 mg/kg/day tolerated the drug well, and no clinical signs of distress were noted. Both male minipigs treated with 25 mg/kg/day CPS displayed decreased appetite. One animal was noted with emesis on Day -6 (day of pre-bleed). Other clinical findings for this animal included emesis, inappetance, and decreased activity on Days 6–8, and the animal was treated on each of these days with antibiotics (Naxcel®; 0.08 ml/kg) by intramuscular [IM] injection. This animal did not receive a second treatment regimen of CPS. The other male minipig treated with 25 mg CPS/kg/day displayed decreased appetite beginning on Day 6. On Days 6–8, the animal was administered flunixinamine (0.02 ml/kg, IM; a non-steroidal analgesic/anti-inflammatory veterinary drug), and the animal was monitored closely for signs of moribundity. Following a second administration of CPS on Days 14–18, decreased activity and increased drainage from the eyes were noted on Day 19, and the animal was humanely euthanized. No secondary IgG antibody titers were evaluated for this animal. No animals treated with 50 mg CPS/kg/day survived beyond Day 7.
Primary IgM response to KLH
and show the anti-KLH antibody responses over time for each of the vehicle control (saline-treated) Göttingen Minipigs® immunized with KLH. The responses of vehicle animals receiving a primary immunization of 2 mg KLH are shown in , while the responses of those immunized with 10 mg KLH are depicted in . In the figures, the individual animal responses are labeled A–E. The lefthand panel for each animal gives the primary IgM and primary IgG responses for that animal, while the righthand panels give the secondary IgG responses.
Figure 1. Primary IgM, primary IgG, and secondary IgG anti-KLH antibody responses of Göttingen Minipigs® immunized with 2 mg KLH. Saline-treated minipigs (males: animals A–D; female: animal E) were immunized with 2 mg KLH by IV injection via ear vein on Day 0. On Day 14, all animals received a secondary immunization of 2 mg KLH by IV injection via ear vein. Primary antibody levels (left panel for each animal) were evaluated in serum samples obtained on Days -6, 5, 6, 7, 8, 9, 12, 13, and 14. Secondary IgG antibody levels (right panel for each animal) were evaluated in serum samples obtained on Days 19, 20, 21, 22, and 23. Results are presented as mean ± SE in terms of log2 (titer). Dotted horizontal line: baseline (i.e. Day -6) primary IgM antibody levels. Solid horizontal line: baseline (i.e. Day -6) primary IgG antibody levels.
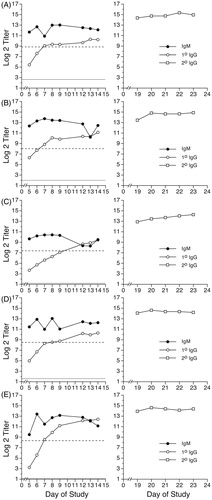
Figure 2. Primary IgM, primary IgG, and secondary IgG anti-KLH antibody responses of Göttingen Minipigs® immunized with 10 mg KLH. Saline-treated minipigs (males: animals A–D; female: animal E) were immunized with 10 mg KLH by IV injection via ear vein on Day 0. On Day 14, all animals received a secondary immunization of 2 mg KLH by IV injection via ear vein. Primary antibody levels (left panel for each animal) were evaluated in serum samples obtained on Days -6, 5, 6, 7, 8, 9, 12, 13, and 14. Secondary IgG antibody levels (right panel for each animal) were evaluated in serum samples obtained on Days 19, 20, 21, 22, and 23. Results are presented as mean ± SE in terms of log2 (titer). Dotted horizontal line: baseline (i.e. Day -6) primary IgM antibody levels. Solid horizontal line: baseline (i.e. Day -6) primary IgG antibody levels.
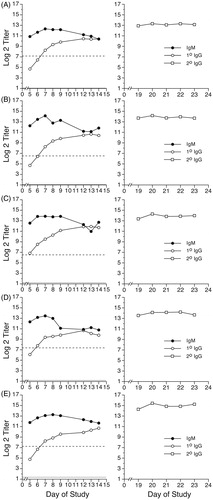
The peak primary IgM responses, compared to background, of saline-treated Göttingen Minipigs® immunized with KLH occurred on Days 7, 8, or 9 for the male minipigs in the 2 mg KLH group (, animals A–D), while the IgM levels of the lone saline-treated female minipig receiving the 2 mg KLH challenge peaked on Day 6 (, animal E). For the animals immunized with 10 mg KLH (, left panels), the peak IgM responses occurred on Day 7 (males) or on Day 8 (female).
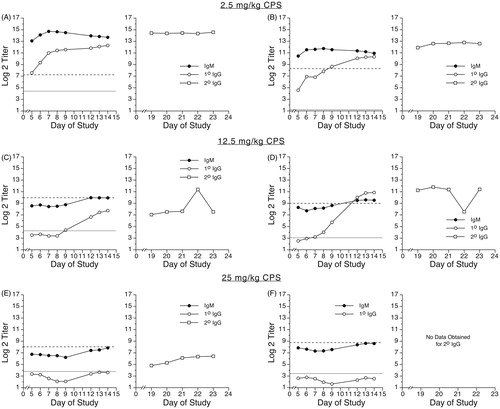
The anti-KLH IgM responses of CPS-treated minipigs are shown in . Treatment with 2.5 mg CPS/kg/day (, animals A and B) resulted in primary IgM antibody responses of similar magnitude to the responses of the vehicle group, as shown in (Group 2). Anti-KLH IgM antibody titers in animals treated with 12.5 mg CPS/kg/day (, animals C and D) were decreased to levels below background (Day -6) through at least to Day 10. At the high dose (25 mg CPS/kg/day; , animals E and F), the antigen-specific IgM titers were decreased below background levels throughout the sampling period (Days 5–14). Treatment with 50 mg CPS/kg/day also decreased IgM antibody titers in all samples obtained prior to the loss of the animals (data not shown).
Figure 3. Primary IgM, primary IgG, and secondary IgG anti-KLH antibody responses of Göttingen Minipigs® treated with cyclophosphamide. Male minipigs were treated with 2.5 (animals A and B), 12.5 (animals C and D), or 25 mg CPS/kg/day (animals E and F) daily on Days 0–4 and on Days 14–18. Animal E received no CPS treatment on Days 14–18. All animals were immunized with 10 mg KLH by IV injection via ear vein on Day 0. On Day 14, all animals received a secondary immunization of 2 mg KLH by IV injection via ear vein. Primary antibody levels (left panel for each animal) were evaluated in serum samples obtained on Days -6, 5, 6, 7, 8, 9, 12, 13, and 14. Secondary IgG antibody levels (right panel for each animal) were evaluated in serum samples obtained on Days 19, 20, 21, 22, and 23. Results are presented as mean ± SE in terms of log2 (titer). Dotted horizontal line: baseline (i.e., Day -6) primary IgM antibody levels. Solid horizontal line: baseline (i.e., Day -6) primary IgG antibody levels.
Primary IgG response to KLH
In addition to the primary IgM responses, the lefthand panels of and show the primary IgG responses of the saline-treated minipigs immunized with 2 mg or 10 mg KLH, respectively. At both immunization levels, the primary IgG responses of all animals increased over time. The maximum primary IgG response was observed on Day 14 for all vehicle-treated animals receiving the 2 mg KLH immunization (). For animals immunized with 10 mg KLH, the individual peak responses for primary IgG generally occurred on either Day 13 or Day 14 (, left panels).
The primary IgG responses of CPS-treated pigs are shown in . In animals treated with 2.5 mg CPS/kg/day (animals A and B), primary IgG anti-KLH antibody titers were similar to levels observed in saline-treated minipigs (see ). At the 12.5 mg CPS/kg/day dose (, animals C and D), CPS treatment decreased primary IgG antibody titers to levels below background through Day 7 or Day 9, after which titers increased to levels several log2 units above background by Day 14. However, titers for one of the two animals in this group remained below titers of control animals, even through Day 14. In animals treated with 25 mg CPS/kg/day (, animals E and F), primary anti-KLH IgG antibody titers were decreased below background levels through Day 14. Animals treated with 50 mg CPS/kg/day also displayed lower serum anti-KLH IgG primary antibody titers than titers of saline-treated control animals (data not shown).
Secondary IgG response to KLH
The right panels of and show the individual secondary IgG responses of the vehicle-treated minipigs following primary immunization with 2 mg or 10 mg KLH, respectively. All animals received a second immunization with 2 mg KLH on Day 14, after collection of the Day 14 blood sample. The secondary IgG antibody responses of all vehicle animals were fairly constant over the 5-day sampling period. Secondary anti-KLH IgG levels in CPS-treated animals are shown in . As observed with the primary anti-KLH IgG antibody response, the secondary anti-KLH IgG levels in animals treated with 2.5 mg CPS/kg/day were similar to levels in vehicle control animals. At the 12.5 mg CPS/kg/day dose, one animal had secondary IgG titers several log2 units below those of animals in the vehicle group, while one did not. At 25 mg CPS/kg/day, the animal receiving only one 5-day treatment regimen of CPS (Days 0–4) had lower secondary IgG titers, which persisted throughout the sampling period (, animal E). The second animal in the 25 mg CPS/kg/day group received a second 5-day treatment with CPS on Days 14–18 and began to display signs of moribundity on Day 19. This animal was promptly and humanely euthanized; secondary IgG titers for this animal were not obtained. Animals treated with 50 mg CPS/kg/day did not survive to the day of secondary immunization.
Discussion
In the present study, optimum primary IgM, primary IgG, and secondary IgG antibody responses to KLH in the Göttingen Minipig® were observed following a primary immunization of 10 mg KLH and subsequent challenge 14 days later with 2 mg KLH. The optimal immunization and sampling windows are summarized in . The Göttingen Minipig® should be bled on Days 6–9 post-primary immunization for primary IgM levels, on Days 12–14 for primary IgG evaluation, and on Days 20–22 (with secondary immunization on Day 14) for evaluating secondary IgG antibody levels.
Table 2. Optimum immunization and serum sampling parameters for assessing the TDAR in Göttingen Minipigs®.
Because Göttingen Minipigs® are outbred animals, blood should be obtained at multiple time points in order to identify the peak antibody response for each individual animal. A similar approach should be used with outbred animals from other species. Unfortunately, this is not frequently done in most laboratories, resulting in high variability in the responses of the outbred animals when all bleeding is conducted on the same day. Obtaining blood at multiple time points from the same animals does not increase the number of animals needed for a study and is consistent with the 3Rs for the use of animals in research (replace, refine, reduce). While evaluating multiple blood collections does increase the time needed to evaluate the ELISA data, the data obtained represent more accurate and reliable scientific results.
Early immunological studies in pigs demonstrated that Berkshire and Yorkshire pigs (Mackie, Citation1981) and Veredeltes Landschwein piglets (Thalhammer et al., Citation1978) were capable of mounting a TDAR to sheep red blood cells (SRBC). In addition, the TDAR to KLH, albeit with the use of an adjuvant, was demonstrated in four breeds of pigs (Joling et al., Citation1993). In Hormel-Hanford miniswine, primary and secondary antibody responses to SRBC (evaluated using a hemolysin assay) each peaked 7 days after the last (i.e., first for primary responses or second for secondary responses) immunization (Hinton & Khan, Citation1989). These results are within the optimum response windows for primary anti-KLH IgM (6–9 days post-primary immunization) and secondary anti-KLH IgG (20–22 post-primary immunization, which corresponds to 6–8 days post-secondary immunization) that are reported here.
Recently, Penninks & van Mierlo (Citation2012) described an ELISA to evaluate the TDAR to KLH in the Göttingen Minipig® following intramuscular (IM) injection with KLH. The time-course results obtained by those authors, with peaks for primary IgM and secondary IgG occurring ≈7 days and 21 days post-primary immunization, are consistent with the anti-KLH IgM and IgG responses demonstrated herein. However, these authors did not report individual animal responses, opting instead to present the data in terms of mean ± SD, which is not altogether appropriate due to the outbred nature of these animals. Indeed, these authors themselves acknowledged the variability of the anti-KLH antibody response in their studies, where only 50% of control animals of each sex developed detectable anti-KLH titers.
The studies conducted by Penninks and van Mierlo also failed to generate substantial primary IgG antibody responses up to 10 days post-primary immunization, which was the last timepoint they evaluated prior to secondary immunization. A failure to develop a substantial antibody response to KLH was not observed in any of the vehicle control minipigs in our studies, nor was there any difficulty in either generating or detecting primary IgG antibody levels within 10 days of primary immunization. In fact, in our studies, all saline-treated animals demonstrated significant primary anti-KLH IgG titers, which were several log2 units above background levels, by this timepoint. This disparity may be explained by differences in the ELISA parameters and techniques, which may impart an increased sensitivity to our anti-swine ELISA. In addition, the disparity between the results of the present studies and those conducted by Penninks and van Mierlo suggests that IV may be preferable over IM as the route of KLH immunization for TDAR assessments in the Göttingen Minipig®.
CPS, a well-documented immunosuppressive drug, was used to validate this swine model based on its ability to suppress the TDAR to KLH. CPS treatment at 12.5 and 25 mg/kg/day decreased the TDAR to KLH to below background levels, although the decreases in the primary IgG response did not persist at the 12.5 mg CPS/kg/day dose level beyond Day 9, resulting in primary IgG antibody titers similar to those observed in vehicle control pigs on Days 12–14, which is the optimum response window for primary IgG TDAR to KLH in this model. Furthermore, at this dose of CPS, anti-KLH secondary IgG levels of one animal (but not the other) were lower than levels of animals in the saline-treated group. These results suggest that the threshold for CPS-induced suppression of the TDAR to KLH may be ≈12.5 mg CPS/kg/day.
A single 5-day treatment regimen with 25 mg CPS/kg/day (on Days 0–4 but not on Days 14–18) was sufficient to decrease primary IgM, primary IgG, and secondary IgG antibody levels through Day 23, as compared to levels in saline-treated minipigs, suggesting that the second 5-day treatment regimen (on Days 14–18) at this dose level may not be necessary. Indeed, the one animal that received the second treatment regimen of 25 mg CPS/kg/day was euthanized due to signs of moribundity. Therefore, when selecting a positive control for assessments of the primary IgM, primary IgG, and secondary IgG TDAR in this breed of swine, the use of a single 5-day treatment (on Days 0–4) of 25 mg CPS/kg/day produces the desired suppression of the antibody response throughout the entire sampling period, while avoiding the possibility of overt toxicity in study animals, which may be associated with a second round of CPS treatment at this dose in the Göttingen Minipig®.
Conclusions
The TDAR to KLH in the Göttingen Minipig® has been characterized in the present study, and a sensitive ELISA system for evaluating antibody responses in this breed of swine has been developed and validated using the immunosuppressive compound CPS. Immunization with 10 mg KLH/animal on Day 0 produced optimum primary IgM antibody responses between Days 6–9, while optimum primary IgG antibody responses were observed between Days 12–14 post-immunization. Secondary IgG responses, elicited by a challenge injection of 2 mg KLH administered 14 days following the 10 mg KLH primary immunization, reached constant levels between Days 20 and 22 post-primary KLH immunization. Furthermore, a single 5-day treatment regimen with 25 mg CPS/kg/day on Days 0–4 decreased both primary and secondary antigen-specific antibody responses throughout the sampling period in this animal model, demonstrating the utility of this non-rodent species for detecting suppression of the TDAR.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
| Abbreviations | ||
| AAALAC, | = | Association for the Assessment and Accreditation of Laboratory Care International; |
| ABTS, | = | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; |
| CPS, | = | cyclophosphamide; |
| ELISA, | = | enzyme-linked immunosorbent assay; |
| GLP, | = | Good Laboratory Practices; |
| HRP, | = | horseradish peroxidase; |
| ICH, | = | International Conference on Harmonization; |
| IM, | = | intramuscular; |
| IV, | = | intravenous; |
| KLH, | = | keyhole limpet hemocyanin; |
| OD, | = | optical density; |
| SC, | = | subcutaneous; |
| SE, | = | standard error; |
| SRBC, | = | sheep red blood cells; |
| TDAR, | = | T-dependent antibody response; |
| USFDA, | = | United States Food and Drug Administration. |
Acknowledgements
The authors wish to extend special thanks to Taneishia Edwards Taylor, Joseph Hoehn, Justin Metheny, and Julie Varsho for their expert assistance with this project.
References
- Bode, G., Clausing, P., Gervais, F., et al. (2010). The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Meth. 62:196–220
- Gad, S. C., Dincer, Z., Svendsen, O., and Skaanild, M. T. (2007). The minipig. In: Animal Models in Toxicology, 2nd ed. (Gad, S. C., Ed.). Boca Raton, FL: CRC Press, pp. 731–771
- Hinton, D. M., and Khan, M. A. (1989). Evaluation of the miniature swine for use in immunotoxicity assessment: I. Quantitation of humoral responses to sheep red blood cells and effects of cyclophosphamide. J. Am. Coll. Toxicol. 8:1039–1052
- ICH. (2005). S8 Immunotoxicity Studies for Human Pharmaceuticals. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available online at: http://www.ich.org/products/guidelines/safety/safety-single/article/immunotoxicity-studies-for-human-pharmaceuticals.html
- Joling, P., Mok, K. S., de Vries Reilingh, V., et al. (1993). An evaluation of immune competence in different swine breeds. Vet. Quart. 15:9–15
- Köhn, F. (2012). History and development of miniature, micro- and minipigs. In: The Minipig in Biomedical Research. (McAnulty, P. A., Dayan, A. D., Ganderup, N., and Hastings, K. L.). Boca Raton, FL: CRC Press, pp. 3–15
- Köhn, F., Sharifi, A. R., and Simianer, H. (2007). Modeling the growth of the Gottingen minipig. J. Anim. Sci. 85:84–92
- Luster, M. I., Portier, C., Pait, D. G., et al. (1992). Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 18:200–210
- Mackie, E. J. (1981). Immunosuppressive effects of cyclophosphamide in pigs. Am. J. Vet. Res. 42:189–194
- Penninks, A. H., and van Mierlo, G. J. (2012). Immunotoxicology studies in the minipig. In: The Minipig in Biomedical Research. (McAnulty, P. A., Dayan, A. D., Ganderup, N., and Hastings, K. L.). Boca Raton, FL: CRC Press, pp. 397–411
- Rothkötter, H. J. (2009). Anatomical and particularities of the porcine immune system - a physician's view. Dev. Comp. Immunol. 33:267–272
- Svendsen, O. (2006). The minipig in toxicology. Exp. Toxicol. Pathol. 57:335–339
- Svendsen, O., Skydsgaard, M., Aarup, V., and Klastrup, S. (1998). Spontaneously occurring microscopic lesions in selected organs of the Göttingen minipig. Scand. J. Lab. Anim. Sci. 25:231–234
- Swindle, M. M., and Smith, A. C. (1998). Comparative anatomy and physiology of the pig. Scand. J. Lab. Anim. Sci. 25:11–21
- Thalhammer, J. G., Stockl, W., and Reyero, C. (1978). Effects of the B-cell activators lipid A and dextran sulphate on the antibody response to sheep red blood cells in piglets. Immunology 35:793–799
- USFDA (US Food and Drug Administration). (2006). S8 Immunotoxicity Studies for Human Pharmaceuticals. Washington, DC: U.S. Department of Health and Human Services
- van der Laan, J. W., Brightwell, J., McAnulty, P. A., et al. (2010). Regulatory acceptability of the minipig in the development of pharmaceuticals, chemicals and other products. J. Pharmacol. Toxicol. Meth. 62:184–195
- Yang, H., and Parkhouse, R. M. (1996). Phenotypic classification of porcine lymphocyte sub-populations in blood and lymphoid tissues. Immunology 89:76–83

