Abstract
The concept of immunological surveillance implies that immunogenic variants of tumor cells arising in the organism can be recognized by the immune system. Tumor progression is provided by somatic evolution of tumor cells under the pressure of the immune system. The loss of MHC Class I molecules on the surface of tumor cells is one of the most known outcomes of immune selection. This study developed a model of immune selection based on the immune response of TCR 1d1 single β-chain transgenic B10.D2(R101) (KdIdDb) mice to allogeneic EL4 (H-2b) thymoma cells. In wild-type B10.D2(R101) mice, immunization with EL4 cells induced a vigorous CTL response targeted to the H-2Kb molecule and results in full rejection of the tumor cells. In contrast, transgenic mice developed a compromised proliferative response in mixed-lymphocyte response assays and were unable to reject transplanted allogeneic EL4 cells. During the immune response to EL4 cells, CD8+ T-lymphocytes with endogenous β-chains accumulated predominantly in the spleen of transgenic mice and only a small part of the T-lymphocytes expressing transgenic β-chains became CD8+CD44+CD62L− effectors. Then, instead of a full elimination of tumor cells as in wild-type mice, a reproducible prolonged equilibrium phase and subsequent escape was observed in transgenic mice that resulted in death of 90% of the mice in 40–60 days after grafting. Prolonged exposure of tumor cells to the pressure of the immune system in transgenic mice in vivo resulted in a stable loss of H-2Kb molecules on the EL4 cell surface. Genetic manipulation of the T-lymphocyte repertoire was sufficient to reproduce the classic pattern of interactions between tumor cells and the immune system, usually observed in reliable syngeneic models of anti-tumor immunity. This newly-developed model could be used in further studies of immunoregulatory circuits common for transplantational and anti-tumor immune responses.
Introduction
Accumulating evidence shows that tumor formation is accompanied by both genetic and epigenetic alterations of the genome (Felsher, Citation2003). Tumors in mice and man express multiple tumor-specific as well as tumor-associated antigens encoded by mutant and normal cellular genes, correspondingly. The idea that the immune system that effectively protects a host from microbial pathogens might also recognize and destroy tumor cells was first discussed more than 100 years ago by Paul Ehrlich, then modified by Frank M. Burnet, has been reviewed in detail (Dunn et al., Citation2002). Despite tumor immune surveillance, tumors do develop in the presence of a functioning immune system and, therefore, the updated concept of tumor immunoediting provides a more complete explanation for the role of the immune system in tumor development.
Tumor immunoediting can be divided into three phases: elimination, equilibrium, and escape (Dunn et al., Citation2004). The elimination phase of cancer immunoediting is exactly the same process described in the initial theory of tumor immune surveillance, by which the immune system detects and eliminates tumor cells that have developed as a result of failed intrinsic tumor suppressor mechanisms. The elimination phase can be complete when all tumor cells are cleared, or incomplete when only a portion of the cells is eliminated. In the case of partial tumor elimination, the concept of immunoediting implies a temporary state of equilibrium between the immune system and the developing tumor. During this period it is suggested that tumor cells either remain dormant or continue to evolve and accumulate further changes (such as DNA mutations or changes in gene expression) that can modulate tumor-specific antigens and stress-induced antigens that tumor cells express. As this process continues, the immune system eliminates susceptible tumor clones where possible. Pressure exerted by the immune system during this phase is sufficient to control tumor progression but, eventually, if the immune response still fails to completely eliminate the tumor, the process results in selection of tumor cell variants that are able to resist, avoid, or suppress the anti-tumor immune response, leading to the escape phase. During that phase, the immune system is no longer able to prevent tumor growth, resulting in a progressively growing tumor (Swann & Smyth, Citation2007).
Historically, the first animal models of anti-tumor immunity were based on transplantation of tumor cells to allogeneic recipients. As a rule, these models reproduced well only the initial elimination phase of cancer immunoediting, because differences in histocompatibility antigens caused strong immune response and complete rejection of transplanted tumors (Little, Citation1924). The problem became apparent when it was realized that immunization with tumor would also immunize the host against normal tissue of the donor and that normal tissues of the donor could also immunize the host against the tumor (Woglom, Citation1929). Although these studies forced the development of transplantational immunology and immunogenetics, they resulted in a long-lasting and popular belief that allogeneic models are inapplicable to studies of the anti-tumor immunity. This opinion was strengthened by our observation that difference even in one product of MHC Class I was sufficient for rejection of allogeneic tumors.
In our studies, B10.D2(R101) (KdIdDb) mice successfully rejected thymoma EL4 (KbDb) and sarcoma MC-11(KbDb) cells (Brondz et al., Citation1995; Kazansky et al., Citation1998). Immunization of R101 mice with these tumor cells induced vigorous CTL response targeted to the H-2Kb molecule, which resulted in complete rejection of tumor cells, indefinite survival of mice, and formation of CD8+ memory T-lymphocytes. Using the capability of CD8+ memory cells to proliferate in vitro in response to heat-shocked allogeneic stimulator cells, we were able to obtain T-lymphocyte hybridomas of memory cells that, in turn, provided an opportunity for molecular identification and cloning of their T-cell receptors (TCR) (Kazansky et al., Citation1999; Zvezdova et al., Citation2008). The next step was to generate hosts with transgenic expression of individual TCR chains (Silaeva et al., Citation2013; Zvezdova et al., Citation2010). Due to full or partial (functional) allelic exclusion of TCR β- and α-chains, the repertoire of T-lymphocytes in these animals is altered in varying degrees, that allows us to investigate the impact of individual chains of TCR on a great number of immunological phenomena, such as intrathymic selection of T-lymphocytes, their survival in the periphery, allogeneic recognition, autoimmunity, and the formation of memory T-lymphocytes.
One newly-identified TCR was 1D1 TCR, originated from a memory T-hybridoma specific to the H-2Kb molecule; this TCR is composed of Vα11 and Vβ6 chains. In the work here, we investigated the impact of single transgenic 1D1 β-chain expression on the capability of B10.D2(R101) mice to reject thymoma EL4 cells. We have shown that, instead of complete elimination of thymoma cells as in wild-type mice, a reproducible prolonged equilibrium phase and subsequent escape of tumor cells took place in the transgenics. Prolonged exposure of tumor cells to the pressure of immune system in transgenic mice in vivo resulted in the loss of H-2Kb MHC Class I molecule on the surface of EL-4 cells. The developed model reproduces the classical pattern of interactions between tumor cells and the immune system and can be applied to other studies in immunopharmacology and immunotoxicology.
Materials and methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the N. N. Blokhin Cancer Research Center (Moscow, Russia).
Animals
Mice of the C57BL/6 (H-2b), C57BL/10 (H-2b), B10.D2(R101) (H-2g1, KdI-AdI-EdDb), and FVB (H-2q) strains were obtained from the breeding facility of the N. N. Blokhin Cancer Research Center. Transgenic mice that express TCR β-chains of the memory hybridoma 1D1 were generated in the Laboratory of Transgenesis, Institute of Gene Biology, Russian Academy of Sciences, and bred in the Laboratory of Regulatory Mechanisms in Immunity, N. N. Blokhin Cancer Research Center at the Russian Academy of Medical Sciences (Silaeva et al., Citation2013).
Cell lines
EL4 thymoma cells were transplanted into syngeneic C57BL/6 mice at 3–5 × 106) per mouse and grown as ascites tumors. In 10–14 days, the ascites were collected aseptically by aspiration and washed 3-times by centrifugation in cold phosphate-buffered saline (PBS, pH 7.4) at 4 °C. Viable cells were then counted after trypan blue-eosin staining.
Immunization
Recombinant B10.D2(R101)(KdIdDb) mice and transgenic 1D1bFM mice on the genetic background of B10.D2(R101) mouse strain were immunized intraperitoneally with 1 ml of cell suspension (2 × 107 EL4 cells in PBS) at room temperature. Control non-immunized mice were injected with PBS only. At 12 days post-immunization, the mice were euthanized by cervical dislocation and their spleens isolated to prepare cell suspensions.
Cell suspension
Splenocytes were gently squeezed from each spleen in a Potter homogenizer with a conic pestle in cold PBS at 4 °C and centrifuged (200 g, 5 min). Red blood cells were removed by hypotonic shock and the ‘purified’ mononuclear cells were washed 3-times by centrifugation in cold PBS at 4 °C. Mononuclear cells were re-suspended in PBS for subsequent staining with monoclonal antibodies or in complete medium for a mixed-lymphocyte reaction (MLR). Viable cells were counted after trypan blue-eosin staining.
Mixed lymphocyte reactions
T-Lymphocyte proliferation was measured by [3H]-thymidine (Saint-Petersburg ‘Izotop’, Saint-Petersburg, Russia) incorporation after a 3-day co-incubation of 4 × 105 splenocytes with 4 × 105 irradiated (25 Gy) or mitomycin C-treated (50 μg/ml, 30 min, 37 °C) stimulatory splenocytes in 96-well plates (Corning Costar, Corning, NY). All reactions took place in a 150 μl total volume of RPMI-1640 (GIBCO BRL, Grand Island, NY) supplemented with 5% fetal bovine serum (FBS), 20 mM L-glutamine (GIBCO), and 50 µM 2-ME (Merck, Darmstadt, Germany).
Staining and analysis of T-lymphocytes on flow cytometer
To analyze T-lymphocyte subpopulations, the following antibodies were used: FITC-, eFluor®450-, and APC-conjugated anti-CD8α (Clone 53-6.7, eBioscience, San Diego, CA), FITC-conjugated anti-CD4 (Clone GK1.5, eBioscience), PE-conjugated anti-mouse TCR Vβ6 (Clone RR4-7, BD Pharmingen, Franklin Lakes, NJ), APC/Cy7-conjugated anti-CD62L (Clone MEL-14, BioLegend, San Diego, CA), APC-conjugated anti-CD44 (Clone IM7, eBioscience), Alexa Fluor®647- and eFluor 450-conjugated anti-CD3 (Clone 17A2, eBioscience), or FITC-conjugated anti-Kb (Clone AF6-88.5.5.3, eBioscience).
Cells (1 × 106) were stained with antibodies at 4 °C for 35 min. Analysis was then performed in a BD FACSCanto II flow cytometer using a FACSDiva 6.0 program (BD Pharmingen). Dead cells were excluded from analysis based on scatter signals and staining with PI (75 µM; Sigma, St. Louis, MO) or 7AAD Viability Staining Solution (BioLegend). No less than 105 events/sample were collected to characterize peripheral T-lymphocyte populations. Further processing of results was performed using Flow Jo 7.6 software (TreeStar Inc., Ashland, OR).
Statistical analysis
All data are presented as mean ± SEM. All statistical analyses were performed using a Student’s t test. A p value ≤ 0.05 was used to determine statistical significance.
Results
Primary allogeneic response of splenocytes of 1D1bFM mice in vitro
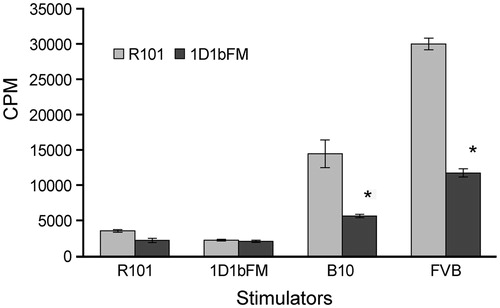
We have shown previously that expression of 1D1 TCR transgenic β-chain results in profound changes in the surface activation phenotype of peripheral T-lymphocytes. In transgenic mice, almost all T-lymphocytes that express the transgenic 1D1 β-chain (Vβ6+) have the phenotype of naïve T-lymphocytes CD44−CD62L+, whereas T-lymphocytes with expressed endogenous β-chains (Vβ6− T-cells) are CD44+CD62L− that represent the phenotype of activated effector T-lymphocytes (Silaeva et al., Citation2013). We sought to determine how expression of the transgenic β-chain affects functional characteristics of T-lymphocytes. Taking into account the specificity of original 1D1 TCR to H-2Kb molecule, we studied MLR responses of splenocytes isolated from the 1D1bFM mouse strain to mitomycin C-treated stimulator allogeneic splenocytes of C57BL/10 (H-2b) mice. Primary proliferative responses of transgenic mouse splenocytes were decreased as compared with the response of wild-type B10.D2(R101) (KdIdDb) mice (). Moreover, the response to third-party allogeneic stimulators from FVB (H-2q) mice was also compromised. Therefore, it was evident that (1) T-lymphocytes that express the transgenic 1D1 TCR β-chain do not reproduce specificity of original 1D1 TCRα/β, and (2) their capability to respond on alloantigens in vitro is impaired in comparison with normal T-lymphocytes of wild-type animals.
Figure 1. Proliferation in response to allogeneic stimulators. MLR responses of splenocytes from transgenic 1D1bFM and wild-type B10.D2(R101) mice to syngeneic B10.D2(R101), syngeneic 1D1bFM, allogeneic C57BL/10 (B10), and third-party FVB stimulators, treated with mitomycin C. Transgenic mice were at least 8-times back-crossed to B10.D2(R101) mice. Data shown are average of three replicates (± SEM). * p < 0.05 vs corresponding control. Similar data were obtained in three independent experiments.
Primary allogeneic response of 1D1bFM mice to thymoma EL4 cells in vivo
Our next step was to investigate the capability of transgenic mice to reject transplanted allogeneic tumor cells in vivo. In 12 days after intraperitoneal injection of EL4 thymoma cells both wild-type B10.D2(R101) and transgenic 1D1bFM mice have elevated percents of CD8+ T-lymphocytes in spleen (from 28.24 [± 0.74] to 41.93 [± 1.87], p < 0.001 in wild-type mice, and from 32.86 [± 0.63] to 39.51 [± 1.54], p < 0.01 in transgenics, ). Evidently, in wild-type mice, this increase did not show any selectivity in relation to T-lymphocytes that express Vβ6 (). In contrast, in transgenic mice, T-lymphocytes with rearranged endogenous β-chains (Vβ6−) took priority over transgenic CD8+ T-lymphocytes (Vβ6+) in response to the allogeneic thymoma (). At the same time, the percentage of effector CD44+CD62L− CD8+ T-lymphocytes in non-immunized transgenics (6.50 [± 0.61], , bottom panel, left) was decreased compared with that in wild-type mice (23.10 [± 2.70], p < 0.001, , left panel) and only slightly increased after immunization (21.55 [± 4.71], p < 0.01, , bottom panel, right). Further, immunization of transgenic mice by EL4 cells resulted in only slight increases in the percentage of effector CD8+ T-lymphocytes with rearranged endogenous β-chains (Vβ6−) (from 64.27 [± 2.70] in non-immunized to 76.56 [± 2.83] in immunized transgenics, p < 0.01, , upper panel). These results suggested that development of CD8+ CD44+CD62L− effector T-lymphocytes of transgenic mice in response to allogeneic EL4 cells was impaired.
Figure 2. Flow cytometric analysis of splenocytes from wild-type B10.D2(R101) and 1D1bFM transgenic mice on genetic background B10.D2(R101) 12 d after immunization with EL4 thymoma cells. Ex vivo isolated splenocytes of intact (left panel) and immunized mice (right panel) were stained by antibodies and analyzed: (A, C) CD4, CD8 expression on surface of CD3+ splenocytes. (B, D) Vβ6 expression on CD3+CD8+ splenocytes. Data from one representative staining are shown. Similar results were obtained in three independent experiments.
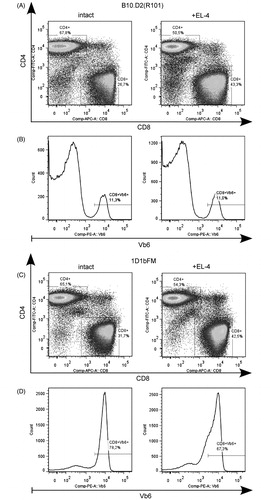
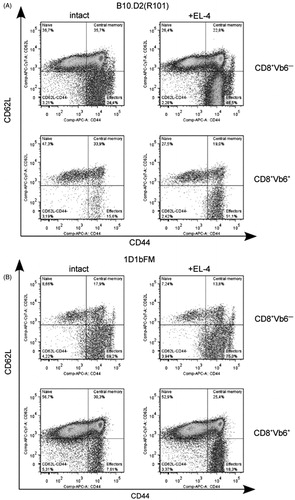
Figure 3. Flow cytometric analysis of co-expression of CD62L and CD44 molecules on the surface of different subpopulations of CD8+ T-lymphocytes in spleen 12 days after immunization with EL4 thymoma cells. Splenocytes of (A) wild-type B10.D2(R101) and (B) 1D1bFM transgenic mice on genetic background B10.D2(R101) were ex vivo isolated from intact (left panel) and immunized (right panel) mice, stained by antibodies, and analyzed. Upper panels show CD8+Vb6− T-lymphocytes and bottom panels shown CD8+Vβ6+ T-lymphocytes. Data from one representative staining are shown. Similar results were obtained in three independent experiments. Only singlets were gated for analysis. Relative number of CD8+ T-lymphocytes with the surface phenotype CD44+CD62L− in transgenic mice significantly differed from those in wild-type animals, both in control and immunized animals (p < 0.05).
Survival rates of transgenic 1D1bFM mice after immunization by thymoma EL4 cells
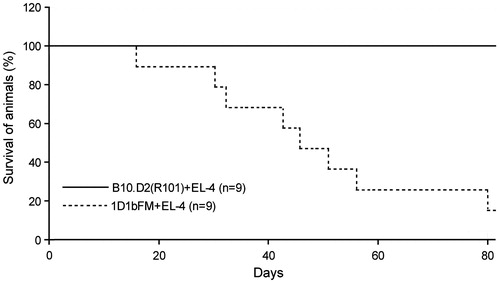
As the results above show, the pool of effector T-lymphocytes arising in the course of the immune response to allogeneic thymoma cells was decreased in transgenic mice as compared to in wild-type animals. To investigate the impact of this decrease on survival rates of transgenic mice, 2 × 107 EL4 thymoma cells were injected intraperitoneally into 1D1bFM and wild-type mice. Obviously, normal B10.D2(R101) mice successfully reject transplanted EL4 cells within 12–14 days with indefinite survival of these mice and formation of CD8+ memory T-lymphocytes. In contrast, 90% of 1D1bFM mice developed ascitic tumors and died within 80 days; peak-death was observed 40–60 days after injection of the allogeneic tumor ().
Figure 4. Survival rates of wild-type B10.D2(R101) and 1D1bFM transgenic mice on genetic background B10.D2(R101) after immunization by thymoma EL4 cells. Nine control and nine transgenic mice were immunized with EL4 thymoma cells (2 × 107 cells) and then examined every day up to 80 days thereafter. Similar data were obtained in three independent experiments.
Immune selection of tumor cells in transgenic 1D1bFM mice
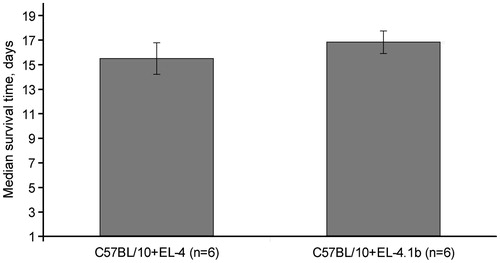
Allogeneic difference between B10.D2(R101) (KdIdDb) mice and thymoma EL4 (H-2b) cells is only in one product of MHC, the H-2Kb molecule, because this thymoma does not express MHC Class II molecules. This difference is enough for induction of efficient CTL response, tumor rejection, and formation of the pool of memory CD8+ cells. In contrast to wild-type mice, 1D1 TCR β-chain transgenic animals were not able to efficiently eliminate tumor cells. Nevertheless, tumor development in transgenic animals was delayed as compared with in syngeneic animals. This could be due to the pressure of an immune response yet incapable of rejecting the tumor completely, but leading to immune selection of tumor cells, further tumor escape, and progression. We surmised that the selection of thymoma EL4 cells would result in the loss of H-2Kb molecule expression. To verify this, we isolated cells of ascitic tumors and analyzed H-2Kb molecule expression using thymoma cells grown in C57BL/10 mice as a control. Indeed, thymoma cells in 1D1bFM mice lost the H-2Kb molecule from the surface (). The loss of expression was stable and retained during more than 21 days in culture in vitro (). In spite of the loss of the H-2Kb molecule, this sub-line (entitled EL-4.1b) did not acquire a capability to grow better in syngeneic recipients; the lifespans of C57BL/10 mice bearing EL-4.1b did not differ from that of mice injected with the original variant of EL4 ().
Figure 5. Stable loss of H-2Kb molecule expression on the surface of thymoma cells 30 days after immunization of 1D1bFM transgenic mice. (A) EL4.1b cells were ex vivo isolated from ascites of transgenic 1D1bFM mice, stained by antibodies, and analyzed by flow cytometry (solid line) in a comparison with an original variant of EL4 cells (dotted line). (B) An EL4.1b H-2Kb-negative sub-line was additionally propagated in vitro during 21 days, stained, and analyzed by flow cytometry (solid line) in a comparison with an original variant of EL4 cells (dotted line). This experiment was reproduced 4-times with near-identical results.
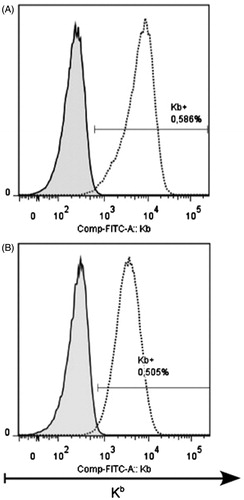
Figure 6. Mice of C57BL/10 strain succumb to injection of both original EL4 and H-2Kb-negative EL4.1b thymoma cells. Mice were intraperitoneally transplanted with original EL4 cells or EL4.1b cells that previously passed immune selection in 1D1bFM mice. Thereafter, mice were examined every day after the transplantation. Differences in lifespan of mice in control and experimental groups were not statistically significant. Each group contained six animals.
Discussion
Although many immunodeficient mouse models were developed up to now to investigate anti-tumor immunity, none of them was based on partial restriction of diversity of T-lymphocyte repertoire due to transgenic expression of single chains of TCR (Belizario, Citation2009). Existing models with transgenic TCRα/β have grave shortcomings that do not allow using them directly in anti-tumor immunity studies. Among these shortcomings are: abnormal intrathymic differentiation of T-lymphocytes and an unphysiologically high frequency of T-lymphocytes with the same specificity, compelling investigators to apply invasive methods and approaches, e.g. adoptive transfers of transgenic T-lymphocytes into normal or lymphopenic wild-type animals. In this respect, the model developed by us has benefits, because it allows formation of a diverse repertoire of TCRs due to rearrangements of endogenous α-chains. The most evident outcome of single transgenic β-chain expression is a biased repertoire of T-cells due to inhibitory effects of TCR transgenic chain expression on rearrangement of endogenous chains of TCR. As a result, in transgenic animals, many peripheral T-lymphocytes express TCR with an invariant β-chain that affects overall TCR diversity (Biro et al., Citation1999; Gui et al., Citation2001).
Functional consequences of such restriction of the T-lymphocyte repertoire remain largely unknown. Earlier, we generated a transgenic mouse strain expressing the transgenic β-chain of TCR 1D1 (belonging to the Vβ6 family) on the genetic background B10.D2(R101). We have shown that intrathymic development of T-lymphocytes in these transgenics was not impaired. The repertoire of peripheral T-lymphocytes in these mice contained 70–80% of T-lymphocytes expressing transgenic β-chains and 20–30% expressing endogenous β-chains. The ratio of peripheral CD4+CD8− and CD4−CD8+ T-lymphocytes remained unchanged in transgenic animals. The percentage of T-lymphocytes with the phenotype CD44−CD62L+ was significantly increased in a subpopulation expressing the transgenic β-chain of TCR 1D1. In contrast, T-lymphocytes expressing endogenous β-chains had a surface phenotype like that of activated CD44+ T-lymphocytes (Silaeva et al., Citation2013).
In the present work, we have shown that in 1D1bFM transgenic mice the capability of T-lymphocytes repertoire to respond to alloantigens in vitro is impaired as compared with wild-type B10.D2(R101) mice. This observation complies with results previously described by Listman et al. (Citation1996), who found that expression of TCR DO-11.10 (Vβ8.2) transgenic β-chain resulted in decreased responses of T-lymphocytes to different antigens, including allogeneic MHC molecules. The most interesting finding of the current study was that single transgenic TCR β-chain expression was sufficient for substantial transformation of the mode of interactions between allogeneic tumor and the immune system of recipient. Instead of full elimination of thymoma cells in wild-type mice, well reproducible prolonged equilibrium phase and subsequent escape of tumor cells were observed in transgenic mice. Obviously, this pattern of interactions is typical for syngeneic animal models. Evidently, this feature could be due to a deficiency in effector CD8+ T-lymphocytes observed in our experiments in transgenic animals after immunization with EL4 cells.
Despite of the increase in the percent of CD8+ cells, the immune response was not able to destroy tumor cells completely. Reasons for this could be decreased frequencies of CTL clones capable of high avidity interactions with allogeneic MHC Class I molecule on the surface of thymoma cells (Suchin et al., Citation2001). This made possible the survival of thymoma cells in the allogeneic recipient and the immune selection of tumor cells. Prolonged exposure of tumor cells to the pressure of the immune system in transgenic mice in vivo can be evidenced by the loss of H-2Kb MHC Class I molecules on the surface of the EL-4 cells. After this, transgenic mice could not further control tumor growth and 90% die within 40–60 days of immunization.
The loss of MHC molecules is a regular event observed in the course of tumor progression (Algarra et al., Citation2004; Fassati and Mitchison, Citation2010). It is believed that this represents one of the key mechanisms of tumor escape (Garrido et al., Citation2010). Therefore, although the H-2Kb molecule is not a tumor-specific antigen, the model developed reproduces key features of anti-tumor responses. The lifespan of C57BL/10 mice after intraperitoneal injection of EL4 cells lacking H-2Kb did not differ substantially from that of mice injected with the original variant of thymoma. Evidently, natural killer (NK) cells destroying MHC Class I-negative cells (Oberoi et al., Citation2013) were not effective in our experimental model. This observation is in accordance with results previously described by Smithson et al. (Citation1991), who determined resistance of different lymphoma cells to cytotoxic effects of NK cells. Therefore, we have developed a transgenic experimental model that allows one to observe a weak allogeneic response to tumor cells, resulting in the loss of MHC Class I molecules. The results obtained show that there is no considerable gap between recognition of tumor antigens and alloantigens. The developed model reproduced the classic pattern of interactions between tumor cells and the immune system and could likely be applied in future studies of immunoregulatory circuits common for transplantational and anti-tumor immune responses.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by grants from the Russian Foundation for Basic Research (Nos. 08-04-00563 and 11-04-00700), Introduction Center Protek, and State contract No. 14.518.11.7040 from the Ministry of Industry and Commerce.
References
- Algarra, I., García-Lora, A., Cabrera, T., et al. 2004. The selection of tumor variants with altered expression of classical and non-classical MHC Class I molecules: Implications for tumor immune escape. Cancer Immunol. Immunother. 53:904–910
- Belizario, J. E. 2009. Immunodeficient mouse models: An overview. Open J. Immunol. 2:79–85
- Biro, J., Würch, A., Potocnik, A. J., et al. 1999. Regulation of T-cell receptor (TCR)-β gene expression by CD3 complex signaling in immature thymocytes: Implications for TCRβ allelic exclusion. Proc. Natl. Acad. Sci. USA 96:3882–3887
- Brondz, B. D., Kazansky, D. B., Chernyshova, A. D., and Ivanov, V. S. 1995. Peptides of a major histocompatibility complex class I (Kb) molecule cause prolongation of skin graft survival and induce specific down-regulatory T-cells demonstrable in the mixed lymphocyte reaction. Immunology 86:219–223
- Dunn, G. P., Bruce, A. T., Ikeda, H., et al. 2002. Cancer immune-editing: From immunosurveillance to tumor escape. Nat. Immunol. 3:991–998
- Dunn, G. P., Old, L. J., and Schreiber, R. D. 2004. The three Es of cancer immunoediting. Ann. Rev. Immunol. 22:329–360
- Fassati, A., and Mitchison, N. A. 2010. Testing the theory of immune selection in cancers that break the rules of transplantation. Cancer Immunol. Immunother. 59:643–651
- Felsher, D. W. 2003. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 3:5–10
- Garrido, F., Cabrera, T., and Aptsiauri, N. 2010. “Hard” and “soft” lesions underlying the HLA Class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer 127:249–256
- Gui, M., Li, J., Wen, L. J., et al. 2001. TCRβ chain influences but does not solely control autoreactivity of Vα 14J281 T-cells. J. Immunol. 167:6239–6246
- Kazansky, D. B., Chernysheva, A. D., Sernova, N. V., et al. 1998. The nature of epitopes, recognized by T-lymphocytes in the allogenic immune response. Mol. Biol. (NY) 2:574–583
- Kazansky, D. B., Petrishchev, V. N., Shtil’, A. A., et al. 1999. Heat shock of antigen-presenting cells as a method for functional testing of the T-cells of allospecific memory. Russ. J. Bioorg. Chem. 25:99–109
- Listman, J. A., Rimm, I. J., Wang, Y., et al. 1996. Plasticity of the T-cell receptor repertoire in TCRβ-chain transgenic mice. Cell. Immunol. 167:44–55
- Little, C. C. 1924. The genetics of tissue transplantation in mammals. Cancer Res. 8:75–95
- Oberoi, P., Jabulowsky, R. A., Bähr-Mahmud, H., and Wels, W. S. 2013. EGFR-targeted granzyme B expressed in NK cells enhances natural cytotoxicity and mediates specific killing of tumor cells. PloS One 8:e61267
- Silaeva, Y. Y., Kalinina, A. A., Vagida, M. S., et al. 2013. Decrease in pool of T-lymphocytes with surface phenotypes of effector and central memory cells under Influence of TCR transgenic β-chain expression. Biochemistry (Moscow) 78:549–559
- Smithson, G., Bittick, T. H., Chervenak, R., et al. 1991. The role of NK cells in regulation of experimental metastasis in a murine lymphoma system. J. Leukocyte Biol. 49:621–629
- Suchin, E. J., Langmuir, P. B., Palmer, E., et al. 2001. Quantifying the frequency of alloreactive T-cells in vivo: New answers to an old question. J. Immunol. 166:973–981
- Swann, J. B., and Smyth, M. J. 2007. Immune surveillance of tumors. J. Clin. Invest. 117:1137–1146
- Woglom, W. H. 1929. Immunity to transplantable tumors. Cancer Rev. 4:129–214
- Zvezdova, E. S., Grinenko, T. S., Pobezinskaya, E. L., et al. 2008. Co-receptor function of CD4 in response to the MHC Class I molecule. Mol. Biol. (NY) 42:588–597
- Zvezdova, E. S., Silaeva, Y. Y., Vagida, M. S., et al. 2010. Generation of transgenic animals expressing the α and β chains of the autoreactive T-cell receptor. Mol. Biol. (NY) 44:277–286

