Abstract
Evidence has previously been demonstrated for the role of NK cells in specific elimination of healthy stem cells (e.g. hMSC, hDPSC, hESC, hiPSC) as well as cancer stem cells, but not their differentiated counterparts. There is also a stage-wise susceptibility to NK cell-mediated cyto-toxicity in tumors, in which case the poorly-differentiated tumors are lysed much more than moderately-differentiated tumors. Well-differentiated tumors were lysed the least compared to either moderately- or poorly-differentiated tumors. It has also been reported that inhibition of differentiation or reversion of cells to a less-differentiated stage by blocking NF-κB or by gene deletion of COX2 significantly augmented NK cell cytotoxicity against both transformed and healthy cells. Additionally, the cytotoxic function of NK cells was severely inhibited against stem cells when they were cultured in the presence of monocytes. Therefore, it is proposed that CD16+CD56dimCD69− NK cells were important for the selection of stem cells, whereas the CD16dim/−CD56dim/+CD69+ anergized NK cells were important for differentiation and eventual regeneration of the tissues and the resolution of inflammation, thus potentially serving as regulatory NK (NKreg) cells. The concept of ‘split anergy’ in NK cells and the generation of NKreg cells with regard to contributions to cell differentiation, tissue repair and regeneration and in tumor resistance are discussed in this review.
Introduction
Effectors of the immune system are thought to shape the survival and maturation of tumor cells and to select for cancers with reduced immunogenicity. However, recent data from our laboratory indicted that the same effector mechanisms are likely responsible for shaping the survival and maturation of healthy stem cells for the ultimate goal of the regeneration of damaged tissues and the resolution of inflammation. Although immunosuppression and tumor escape from immune recognition are thought to be major factors responsible for the establishment and progression of cancer, neither their underlying physiological significance nor the exact mechanisms by which immunosuppression occurs are completely understood.
NK cells arise from the bone marrow and constitute 5–15% of total lymphocytes in the peripheral blood. They are known to mediate direct natural cytotoxicity as well as antibody-dependent cellular cytotoxicity (ADCC). The key involvement of CD16 receptors in ADCC has clearly been shown previously by many laboratories; however, its significance in NK cell direct killing is far less known, although tumor cell ligands have previously been identified that bind NK cell CD16 receptors (Mandelboim et al., Citation1999). By producing key cytokines and chemokines NK cells are known to regulate the functions of other immune cells (Farag & Caligiuri, Citation2006; Fildes et al., Citation2008). Conventional human NK cells are identified by the expression of CD16 and CD56, and by the lack of surface CD3 expression. NK cells mediate their function through a number of important activating and inhibitory cell receptors listed in (Pegram et al., Citation2011). It is thought that the balance between activating and inhibitory signals which NK cells receive from their surface receptors determines their functional fate. Many of the receptors listed in including CD16, killer immunoglobulin like receptors (KIR), NKG2 family of receptors that form a heterodimer with CD94, NKG2D, and natural cytotoxicity receptors (NCR) have all been subjects of many studies. Likewise, several key cytokines, chemokines, and adhesion molecules were found to have significant roles in maturation, differentiation, and effector function of NK cells. However, little is known regarding the function of Toll-Like Receptors (TLR), NOD-Like Receptors (NLR), and RIG like Receptors (RLR) in NK cell effector functions.
Table 1. List of NK cell activating and inhibitory surface receptors and their ligands.
Even much less is known regarding the interaction of NK cells with other immune effectors or with effectors in connective tissue in a tumor microenvironment. Considering that NK cells may reside primarily in the immune rich compartments, it is likely that other immune effectors may shape the phenotype and function of NK cells. In this regard, very little is known regarding the interaction of NK cells with Myeloid-Derived Suppressor Cells (MDSC) that is known to induce suppression in T-cells (Marigo et al., Citation2008; Yu et al., Citation2013).
The association of distinct effector functions with certain NK cell subsets is believed to be developmentally-regulated (Strowig et al., Citation2010). In this regard, previous studies have identified two distinct NK cell sub-populations, i.e. CD56brightCD16dim and CD56dimCD16bright, based on phenotypic and functional analyses (Farag & Caligiuri, Citation2006). The CD56dimCD16bright NK cells are the major subset in peripheral blood that mediates cytotoxicity, whereas CD56brightCD16dim constitutes a minor NK sub-population in the peripheral blood and plays a role in secretion of cytokines. CD56brightCD16dim cells do not mediate cytotoxicity, and they are thought to be precursors of the CD56dimCD16bright NK subset (Romagnani et al., Citation2007).
Although a lot is known about the inhibitory and activating receptors that modulate NK cell functions, and many previous studies have indicated that NK cells may recognize and become activated by irradiated or stressed cells (Farag & Caligiuri, Citation2006; Pegram et al., Citation2011), no previous studies have shown the role of NK cells in recognition, selection, and differentiation of stem cells and their potential role in resolution of inflammation. This review provides a summary of factors and mechanisms involved in shaping the function of NK cells in cancer and after interaction with healthy stem cells, and, further, discusses the emerging view from our laboratory that indicates that NK cells may behave as the effectors of selection and differentiation of stem cells and their subsequent resistance to NK cell-mediated cytotoxicity.
Split anergy in NK cells is induced after their binding to sensitive but not resistant tumors and after the triggering of CD16 receptors
We have previously shown that K562 (an NK sensitive tumor) causes loss of NK cell cytotoxicity while triggering significant induction of tumor necrosis factor (TNF)-α and interferon (IFN)-γ from the NK cells (Jewett & Bonavida, Citation1995, Citation1996). In contrast, NK resistant tumors such as RAJI cells induce very little loss of NK cell cytotoxicity or secretion of cytokines (Jewett & Bonavida, Citation1995, Citation1996). Indeed, there is an inverse relationship between target cell susceptibility to NK cell lysis and its ability to inactivate the cytotoxic function of NK cells (). Moreover, following NK cell cultures with sensitive tumor-target cells but not resistant tumors, the target-binding NK cells undergo phenotypic and functional changes. Target cell-inactivated NK cells express a CD16−CD56dim/−CD69+ phenotype (Jewett & Bonavida, Citation1995, Citation1996).
Table 2. Susceptibility of a number of different stem cells – but not their differentiated counterparts – to NK cell-mediated cytotoxicity.
This phenotype has also been observed in several disease manifestations including HIV infection (Hu et al., Citation1995). Significant down-modulation of CD16 receptor expression and decreased NK cell cytotoxic function were also seen in several cancer patients, including those of the oral and ovarian cancer patients (Lai et al., Citation1996; Kuss et al., Citation1999). In addition, down-regulation of CD16 surface receptors on NK cells was also observed when NK cells were treated with CA125 isolated from ovarian tumor cells (Patankar et al., Citation2005). The decreases in CD16 surface receptors were accompanied by a major decrease in NK cell killing activity against K562 tumor cells. These observations suggested that CD16 receptors could likely play an important role in target cell induced loss of NK cell cytotoxicity. Indeed, CD16:Ig fusion proteins were shown to bind to a variety of tumor-target cells, indicating the existence of specific ligands for CD16 receptors on tumor cells (Mandelboim et al., Citation1999). Further, we previously showed that triggering of CD16 on untreated or interleukin (IL)-2 treated NK cells resulted in down-modulation of CD16 receptors and in a great loss of cytotoxicity in NK cells. In addition, a small subset of NK cells was programmed to undergo apoptosis (Jewett & Bonavida, Citation1995, Citation1996; Jewett et al., 1997). Cell death of NK cells was seen as regulated, in part, by endogenously secreted TNFα from the NK cells (Jewett et al., Citation1997).
Previous studies by other groups have also shown that a subset of IL-2 activated NK cells undergo cell death following cross-linking of the CD16 receptor (Azzoni et al., Citation1995; Ortaldo et al., Citation1995). Addition of antibodies to CD56 or LFA-1 did not cause any decrease in NK cell cytotoxicity, demonstrating the specificity of anti-CD16 monoclonal antibody (mAb) signaling in mediating inhibition of NK cell cytotoxicity (Jewett & Bonavida, Citation2000). Thus, we coined the term ‘split anergy’ for responses mediated by NK cells after their interaction with sensitive target cells or after the triggering of CD16 receptors by the antibody in combination with IL-2 treatment (Jewett & Bonavida, Citation1995, Citation1996; Jewett et al., 1997; Jewett et al., Citation2006a, Citation2008). Indeed, three sub-populations of NK cells, namely Free, Binder, and Killer NK cells – with varying degrees of loss of cytotoxicity – were identified after formation of conjugates with K562 targets. Free cells that did not bind or form conjugates with target cells were inactivated less, or exhibited the most cytotoxicity; both Binder – those that bound but did not kill their bound tumors – and Killer subsets that bound and killed their bound tumors, exhibited significant loss of cytotoxicity. In contrast, Binder and Killer subsets, but not Free NK subsets, secreted significant levels of cytokines and exhibited a CD16−CD56dim/−CD69+ phenotype (Bonavida et al., Citation1993; Jewett & Bonavida, Citation1994, Citation1995; Jewett et al., Citation1996).
Treatment of NK cells with IL-2 and anti-CD16mAb also induced split anergy by significantly decreasing NK cell cytotoxicity while increasing cytokine secretion capabilities of the NK cells. Furthermore, NK cells exhibited a CD16−CD56dim/−CD69+ phenotype after treatment with the combination of IL-2 and anti-CD16 mAb (Jewett & Bonavida, Citation2000; Jewett et al., Citation1997, Citation2006a, Citation2008). Loss of cytotoxicity in NK cells was significantly exacerbated when NK cells were either treated with F(ab)’2 fragment of anti-CD16 mAb with IL-2 or treated with a combination of MHC Class I and anti-CD16 mAb in combination with IL-2, while the same treatments resulted in an increased secretion of cytokines (Jewett & Bonavida, Citation2000; Jewett et al., Citation2008). Based on our recent results, NKp46 mAb were also able to induce significant NK cell anergy in the presence and absence of IL-2 correlating with their increased expression on untreated and IL-2 treated NK cells (manuscript submitted). Moreover, addition of bacteria or their extracts in the presence of CD16 receptor signaling and IL-2 was able to induce a synergistic decrease in NK cell cytotoxicity while increasing the induction of cytokine release substantially (manuscript in preparation).
Because anergy in NK cells is an active process and is induced via signaling receptors such as CD16 and NKp46 and not through LFA1 or LFA3 or CD56, it is likely that binding of agonistic antibodies or their ligands signal NK cells to become tolerant in a manner similar to that obtained when anti-CD3 antibody is administered to T-cells (Chatenoud, Citation2003). In addition, the magnitude of signaling through the receptors on NK cells may determine the extent and levels of anergy induced in NK cells. Therefore, these results suggested that receptor signaling in NK cells via key surface receptors in the presence of IL-2 is likely to result in a rapid loss of NK cell cytotoxicity while continuing to increase secretion of cytokines by the NK cells.
Localization of NK cells within immune-rich compartments away from epithelial tumor nests may provide clues to significance of split anergy in NK cells induced by other immune effectors
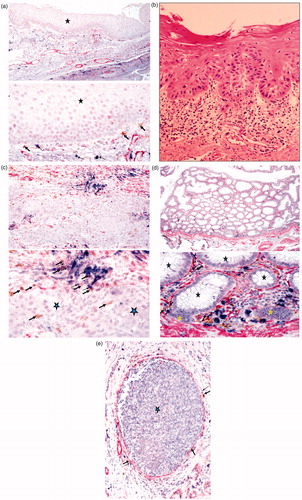
Most NK cells are found within immune rich compartments away from the tumor nests in several tumor types, including oral (), colon (), and breast tumors () (Jiang et al., Citation2013a, Citationb; Man et al., Citation2013). Although some NK cells may be found in the tumor nests, it appears that most cells are seen in the immune compartment associated with other immune effectors such as mast cells (). This indicates that perhaps NK cells might first encounter and bind to other immune effectors such as mast cells or monocytes known as Myeloid-Derived Suppressor Cells (MDSC). The interaction of NK cells with MDSC may, in turn, induce split anergy in NK cells and condition these cells to support differentiation of epithelial tumors as well as of surrounding immune effectors. Indeed, in normal colon tissue the NK cells are found under the myoepithelial layer bound to mast cells () or in oral mucosa they are found under the basal epithelium where undifferentiated proliferating cells reside (). Likewise, stem cells reside in the basal layer of oral mucosa (Squier & Kremer, Citation2001). Thus, it is likely that NK cells may be conditioned or anergized by other immune effectors or the effectors of connective tissue to support differentiation of the epithelial layer. In cancer, NK cells may obtain entry to the tumor site after the myoepithelial layer forming the tumor capsule is disrupted (Jiang et al., Citation2013a, Citationb; Man et al., Citation2013). As such, any NK cells that have escaped from the binding and anergizing effects of MDSC may be able to lyse some of the tumor stem cells, in addition to inducing differentiation in a fraction of the tumor cells ().
Figure 1. Immunohistochemical analysis of oral, colon, and breast tumor tissues. (a) Healthy inflammatory oral tissue was stained with antibodies to CD16 (Brown), mast cells (Blue), and Smooth Muscle Actin (SMA) (red). Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) are located below the normal oral epithelium (star), immediately below the basal layer where stem cells and undifferentiated epithelial cells reside. Please note that the epithelial layer is devoid of infiltrating immune cells. (b) Slides from OSCC were prepared and stained with H&E. Infiltration of immune effectors right beneath the epithelial layer can be seen in a connective tissue area where immune inflammatory cells are likely to condition NK cells to lose cytotoxicity and to support differentiation of epithelial cells. (c) Oral tumor tissue stained with the antibodies against CD16, mast cells, and SMA, as indicated above. Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) are located along oral tumor capsules, with only a few infiltrating immune cells seen within the epithelium (star). (d) Colorectal tumor tissue stained as above. Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) were located along colorectal epithelial capsules, with only a few infiltrating immune cells seen within the normal (black stars) and tumor (yellow star) epithelium. (e) Breast tumor tissue stained as described above. Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) were located along breast tumor capsules, with only a few infiltrating immune cells seen within the epithelium (star).
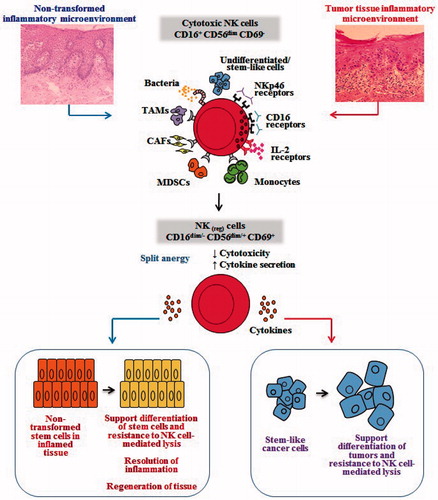
Potential roles of monocytes and other MSDC in induction of split anergy in NK cells
When either tumor cells or healthy non-transformed stem cells were cultured with viable or irradiated monocytes before being exposed to IL2-treated NK cells, a significant decrease in NK cell-mediated cytotoxicity could be observed against tumors and healthy stem cells (Jewett et al., Citation2010). Interestingly, significant lysis of the cells by untreated NK cells was also reproducibly blocked by addition of monocytes. Studies were then undertaken to determine whether decreased lysis of tumors or stem cells by NK cells was due to a competitive lysis of monocytes by the NK cells. These studies confirmed that the NK cells lysed the monocytes. Further, when tumors or healthy stem cells were co-cultured with monocytes and sorted to remove monocytes from the tumors or stem cells before assessing the NK cell killing function, one could still see some inhibition of NK-mediated lysis, indicating an ability of monocytes to induce protection of tumor cells or healthy stem cells from the NK lysis (Jewett et al., Citation2010). Thus, even though lysis of monocytes by NK cells may, in part, contribute to preventing NK cell lysis of stem cells, the interaction of monocytes with stem cells may result in their resistance against cytotoxicity. Cytokines such as IL-6 and TNFα produced by monocytes during interaction with the stem cells may increase NF-κB and result in an increased resistance of stem cells against NK cell-mediated cytotoxicity as reported previously (Jewett et al., Citation2010). In addition, IL-6 and TNFα may contribute to the increase in expression of surface receptors such as CD54 and MHC Class I on stem cells, resulting in their resistance to NK cell-mediated cytotoxicity (manuscript in preparation).
Decreases in NK cell lysis of tumors or healthy stem cells were paralleled by a significant induction of IFNγ. Indeed, when tumors or healthy stem cells were cultured with IL-2-treated NK cells alone, one could observe significant induction of IFNγ secretion. However, a synergistic increase was seen when IL2-treated NK cells were cultured with tumors or healthy stem cells in the presence of monocytes. Therefore, although decreased killing of tumors or healthy stem cells by the NK cells could be observed in the presence of monocytes, synergistic secretion of IFNγ by the NK cells in the presence of monocytes and stem cells could be seen, indicating an inverse relationship between cytotoxicity and IFNγ secretion (split anergy). This was similar to the profiles seen when NK cells were treated with IL-2 and anti-CD16 mAb in which significant decreases in cytotoxicity of NK cells could be observed in parallel with increased secretion of IFNγ (Jewett et al., Citation1997). Whether mast cells or other types of MDSC are able to induce split anergy in NK cells requires further investigation.
Degree of cellular differentiation determines sensitivity to NK cell lysis
Increased NK cell cytotoxicity and augmented secretion of IFNγ were observed when NK cells were co-incubated with stem-like OSCSC that released significantly lower levels of GM-CSF, IL-6, and IL-8, and also demonstrated decreased expression of phospho-Stat3, B7H1, and EGFR and much lower constitutive NF-κB activity when compared to that in differentiated OSCC. More importantly, OSCSC expressed CD133 and CD44bright oral stem cell markers (Jewett et al., Citation2010; Tseng et al., Citation2010); in contrast, differentiated OSCC expressed lower levels of CD44 surface receptors.
Table 3. Inverse association of target cell susceptibility to NK cell lysis and its ability to inactivate the cytotoxic function of NK cells.
To assess whether the stage of differentiation of other tumor types also correlated with their sensitivity to NK cell-mediated lysis, five pancreatic lines at different stages of differentiation were selected based on a number of criteria including sphere formation and immunohistochemical analysis (Sipos, Citation2003; Deer, 2010). In these studies (Jewett & Tseng, Citation2013), Panc-1 and MP-2 (two poorly-differentiated), BXPC3 and HPAF (two moderately-differentiated), and CAPAN-1 (a well-differentiated) pancreatic tumor cell lines were co-cultured with the NK cells and NK-mediated cytotoxicity was determined in a 4-h [51Cr] release assay. The results indicated there was a significant correlation between stage of differentiation of the tumors and level of NK cell-mediated lysis. The highest cytotoxicity was obtained against poorly-differentiated Panc-1 and MP-2 and intermediate lysis was seen against moderately differentiated BXPC3 and HPAF; the lowest lysis was obtained against well-differentiated CAPAN-1 cells. In addition, two glioblastoma multiforme (GBM) stem-like tumor cell lines (XO1-NS and XO2-NS) that were previously isolated and characterized (Inagaki et al., Citation2007; Oka et al., Citation2007; Soeda et al., Citation2008) were found to be significantly more susceptible to NK cell-mediated cytotoxicity as compared to differentiated U87 GBM tumors (Jewett & Tseng, Citation2013).
Because most stem-like tumors or poorly-differentiated cells were significantly more susceptible to NK cell cytotoxicity, it was reasoned that healthy, non-transformed primary stem cells may also be susceptible to NK-mediated cytotoxicity. Our laboratory had demonstrated previously that NK cells significantly lysed hMSC, hDPSC, and hESC (). All different types of stem cells became resistant to NK cell-mediated cytotoxicity once they were differentiated (). In addition, higher sensitivity of hiPSC to NK cell mediated lysis was also observed when compared to the parental line from which they were derived (Jewett et al., Citation2010; Tseng et al., Citation2010) (). Differentiation of XO1-NS and XO2-NS also rendered them more resistant to NK cell mediated cytotoxicity (; manuscript submitted). An increased lysis of cancer stem cells or non-transformed healthy stem cells may be attributed to the use of allogeneic NK cells; however, our previous work using autologous NK cells exhibited similar levels of cytotoxicity against hDPSC compared to the degree of lysis by allogeneic NK cells (Jewett et al., Citation2010). Taken together, these results indicated that undifferentiated cells were targets of both allogeneic and autologous NK cells, and that the degree of cellular differentiation correlated inversely with the level and extent of their susceptibility to NK cell-mediated cytotoxicity ().
De-differentiation of epithelial cells renders them susceptible to NK-mediated cytotoxicity
Since the degree of differentiation in the cells is predictive of their sensitivity to NK cell-mediated cytotoxicity, it was reasoned that blocking NF-κB in the cells may de-differentiate and subsequently increase their susceptibility to the cytotoxicity. Indeed, blocking NF-κB in oral tumors was found to increase their levels of CD44 surface receptor expression, one hallmark of stem cells (unpublished results). In addition, blocking of NF-κB nuclear function in a primary oral tumor OSCC and in non-tumorigenic oral cells (HOK-16B), as well as in an established tumor line (HEp-2 cells, known to be Hela contaminant) (Abdulkarim et al., 2002; Jewett et al., Citation2003; Murakami et al., Citation2004, Citation2005) augmented cytotoxicity and the release of key cytokines such as IFNγ from the NK cells (Jewett et al., Citation2003, Citation2006a). Similarly, inhibition of NF-κB by Sulindac increased the functional activation of NK cells and enhanced anti-tumor cytotoxic activity (Jewett et al., Citation2003, Citation2006b).
In agreement with those studies, a targeted deletion of Iκκβ in the epidermis of mice was shown to lead to inflammatory skin manifestations (Pasparakis et al., Citation2002). Elevated levels of cytokines and chemokines were also demonstrated in the epidermis of patients and animals with Iκκγ and Iκκβ deletions (Berlin et al., Citation2002; Pasparakis et al., Citation2002). Mice with a keratinocyte-specific deletion of Iκκβ demonstrated decreased proliferation of epidermal cells, and developed TNFα-dependent inflammatory skin disease (Pasparakis et al., Citation2002). In contrast, in other studies where NF-κB function was blocked in dermal keratinocytes by a mutant IκBα, an increased proliferation and hyperplasia (Seitz et al., Citation1998) and eventual development of cutaneous squamous cell carcinomas of skin was seen if mice were allowed to survive and reach adulthood (van Hogerlinden et al., Citation1999, Citation2004).
Since tumorigenic and non-tumorigenic human oral keratinocytes acquire sensitivity to NK cell-mediated lysis when NF-κB is inhibited, it is likely this phenomenon was not specific to cancer or oral keratinocytes, and may occur in other healthy non-transformed cell types. Indeed, when human primary monocytes were differentiated to dendritic cells (DC), they too became more resistant to NK cell-mediated cytotoxicity (Tseng et al., Citation2010). Moreover, knockdown of COX2 in primary mouse monocytes or mouse embryonic fibroblasts (unpublished observations) led to reversion or de-differentiation of the monocytes and fibroblasts, respectively, and activation of NK cell cytotoxicity. Indeed, it is likely that any disturbance in cellular differentiation might pre-dispose the cells to NK-mediated cytotoxicity. Since Stat3 is an important factor increased during differentiation, blocking Stat3 is also critical in the activation of immune effectors (Wang et al., Citation2004). In support of a critical role of Stat3 in immune evasion of tumor cells in humans, we and others have shown that GBM tumors display constitutive activation of Stat3 (Cacalano and Jewett, unpublished observation; Rahaman et al., Citation2002), and poorly induce activating cytokines and tumor-specific cytotoxicity in human peripheral blood mononuclear cells (PBMC) and NK cells. Ectopic expression of dominant-negative Stat3 in the GBM tumors increased lysis of the tumor cells by immune effectors and induced IFNγ production by the interacting immune effectors (unpublished observations).
Since NF-κB can regulate IL-6 secretion in OSCC, HOK-16B, and HEp2 cells, and secreted IL-6 in tumors can activate Stat3 expression and function, an increase in NF-κB nuclear function could, in turn, induce Stat3 activation and result in significant resistance of tumors to NK cell-mediated cytotoxicity. Indeed, inhibition of NF-κB in oral tumors resulted in significant decreases in IL-6 secretion by tumor cells and induction of NK cell IFNγ secretion (Jewett et al., Citation2006b, Citation2010). Therefore, targeted knockdown of Stat3 or signaling pathways upstream of Stat3 (such as NF-κB) may de-differentiate the cells and pre-dispose them to cytotoxicity.
Differentiation of the cells as a potential mechanism for the immunoselection
The three stages of selection, equilibrium, and escape have been hypothesized to govern the interaction of immune cells with their respective tumors (Dunn et al., Citation2002, Citation2004). Specific interaction of healthy stem cells with the immune cells has potential basis on selection, differentiation, and resolution of inflammation. Our own findings demonstrated there were two distinct mechanisms for the selection of stem cells: (1) mediated by perforin/granzme lysis and (2) mediated by synergistic action of cytokines induced by NK cells that eliminate poorly- and highly-differentiating target cells, respectively, leaving those that perhaps have intermediate phenotypes (manuscript in preparation). In this regard, at the initial stages of differentiation, some of the stem cells may be selected by the granule exocytosis. Those that survive the process of initial elimination by the NK cells will differentiate by the aid of cytokines produced by anergized NK cells. The second mechanism of selection will take place through synergistic action of cytokines in which case either those that have decreased capacity to differentiate are eliminated or those that are highly differentiated are subject to activation-induced cell death. At present, we are unable to differentiate between the two modes, although we believe activation-induced cell death may likely be the mechanism by which highly-differentiated cells are eliminated. It thus appears that selection by immune cells favors those that may not only have defects in differentiation but also those that perhaps have performed their tasks and are no longer needed.
Not all types of differentiated cells undergo selection through the function of cytokines. Our data demonstrated that oral tumors were clearly more sensitive to increased induction of death by the synergistic action of cytokines since the addition of the combination of cytokines induced cell death in a fraction of tumor cells. In contrast, lung tumors were induced to differentiate but did not undergo cell death by the addition of cytokines (manuscript in preparation). Indeed, both oral and lung tumor stem cells were susceptible to NK cell mediated lysis and both differentiated when either supernatants or fixed NK cells were added; however, only oral tumors were induced to undergo cell death by the action of cytokines. Differentiation of tumor cells by paraformaldehyde-fixed NK cells was found to be due to the synergistic actions of membrane-bound TNFα and IFNγ on NK cells (manuscript submitted). Similar to membrane-bound cytokines, secreted TNFα and IFNγ were also able to increase the differentiation of stem cells synergistically, albeit the levels of stem cell differentiation by membrane-bound cytokines were slightly lower than those seen induced by supernatants from the NK cells.
Split anergy in NK cells is a potential mechanism for induction of target cell differentiation whereby cells become resistant to NK cell functions; switch of NK cell function from effector to regulatory cells
Induction of split anergy in NK cells could be an important conditioning step responsible for the repair of tissues during pathological processes irrespective of the type of pathology. In tumors since the generation and maintenance of cancer stem cells is higher, the majority, if not all of the NK cells, may be conditioned to support differentiation and as such the phenotype of NK cells in tumor microenvironment as well as in the peripheral blood may resemble that of the anergic NK cells, i.e. decreased NK cell cytotoxicity, acquisition of a CD16−/dimCD56dim/+CD69+ phenotype, and augmented ability to secrete inflammatory cytokines. Of course, the degree of the loss of NK cell cytotoxicity may be directly proportional to the load of cancer stem cells, stage of the disease, and their metastatic potential. Therefore, the results suggest two very important functions for the NK cells: one is to limit the numbers of proliferating stem cells and immune inflammatory cells by selecting those with a greater potential to differentiate and the other is to support differentiation of the stem cells and subsequent regeneration of the tissues and the resolution of inflammation.
To achieve these tasks NK cells have to acquire two different phenotypes, and be conditioned to carry out both functions successfully. CD16+/CD56dimCD69− subsets of NK cells are cytotoxic and will mediate cytotoxicity depending on which cells they encounter first. As noted above, with respect to oral squamous cell carcinomas, since the majority of immune effectors are found at the connective tissue area, the chances are they may first encounter and interact with either other immune effectors or effectors of connective tissue, such as fibroblasts. However, there is also the possibility that NK cells may first encounter the stem cells at the epithelial layer base, in which case, by eliminating their bound stem cells, they too can become anergized. Surprisingly, allogeneic cytotoxic T-lymphocytes (CTL) were also found to target glioblastoma stem-like cells, but not their differentiated counterparts (unpublished observation).
By eliminating a subset of stem cells or after their interaction with other immune inflammatory cells or effectors of connective tissue, NK cells could then be in a position to support differentiation of a selected population of stem cells since they will be conditioned to lose cytotoxicity, induce cytokine and growth factor secretion, and gain a CD16−/dimCD56dim/+CD69+ phenotype. It is interesting to note that all immune effectors isolated from oral gingival tissues of healthy as well as diseased gingiva have a CD69+ phenotype, with the exception that numbers of immune effectors are much lower in healthy tissues compared to in the diseased ones (Jewett et al., Citation2013). Thus, these results suggest two very important functions for the NK cells. One is to kill and the other is to support differentiation for the repair and regeneration of tissues.
In vivo physiological relevance of the above-mentioned observations could be seen in a sub-population of NK cells in peripheral blood, uterine, and liver NK cells which express low or no CD16 receptors, have decreased capacity to mediate cytotoxicity, and are capable of secreting significant amounts of cytokines (Cooper et al., Citation2001; Nemeth et al., Citation2009). In addition, 70% of NK cells become CD16dim or CD16− immediately after allogeneic or autologous bone marrow transplantation (Cooper et al., Citation2001). Since NK cells lose their cytotoxic function and gain in cytokine secretion phenotype and down-modulate CD16 receptors after their interaction with tumor cells or the stem cells (Jewett & Bonavida, Citation1996; Jewett et al., Citation1997), it is tempting to speculate that in vivo-identified CD16− NK cells and in vitro tumor-induced CD16− NK cells have similar developmental pathways as they have similar if not identical functional properties.
This hypothesis was supported by the observation that anergized NK cells were directly responsible for the increased differentiation and resistance to NK cell-mediated cytotoxicity of a number of different stem cells, including cancer stem cells and dental pulp stem cells (manuscript submitted). As illustrated in , when OSCSC were cultured with supernatants or paraformaldehyde-fixed NK cells (manuscript submitted) treated with IL-2 and anti-CD16 mAb, they became resistant to cytotoxicity mediated by freshly-isolated untreated or IL-2-treated NK cells. IL-2-treated NK cell supernatants or paraformaldehyde-fixed IL-2 treated NK cells were also able to impart some resistance to OSCSC, but the highest levels of resistance were achieved when NK cells were treated with IL-2 and anti-CD16 mAb. No significant differences in resistance of OSCSC to NK cell-mediated lysis could be achieved by culture of the OSCSC with supernatants of either untreated or anti-CD16 mAb-treated NK cells. The resistance by OSCSC induced by anergized NK cells correlated with a decrease in CD44 receptor expression and in an increase in B7H1 expression (manuscript submitted), two surface receptors which were inversely expressed in differentiated OSCC and in OSCSC (Tseng et al., Citation2010). In addition, we now have evidence which supports the notion that the induction of anergy in NK cells is an active process which is induced by the triggering of CD16 receptor on the NK cells, and is regulated by the functions of cystatins and cathepsins, and is not due to degranulation and exhaustion of cytotoxic granules (unpublished results).
Figure 3. Supernatants from anergized NK cells induced the highest resistance of OSCSC against NK cell-mediated cytotoxicity. Highly-purified NK cells at 106 cells/ml were either left untreated or treated with IL-2 (1000 U/ml), anti-CD16 mAb (3 μg/ml), or a combination of IL-2 and anti-CD16 mAb for 24 h before they were harvested and used to induce differentiation of OSCSC. A total of at 106 OSCSC was added to each plate in 10 ml of media, and the cells were allowed to adhere before NK cell supernatants were added. A total of 180 µl supernatants were added at Days 1, 3, and 5; levels of NK cell cytotoxicity were then determined using freshly-isolated untreated NK cells in a 4-h [51Cr] release assay on Day 6.
![Figure 3. Supernatants from anergized NK cells induced the highest resistance of OSCSC against NK cell-mediated cytotoxicity. Highly-purified NK cells at 106 cells/ml were either left untreated or treated with IL-2 (1000 U/ml), anti-CD16 mAb (3 μg/ml), or a combination of IL-2 and anti-CD16 mAb for 24 h before they were harvested and used to induce differentiation of OSCSC. A total of at 106 OSCSC was added to each plate in 10 ml of media, and the cells were allowed to adhere before NK cell supernatants were added. A total of 180 µl supernatants were added at Days 1, 3, and 5; levels of NK cell cytotoxicity were then determined using freshly-isolated untreated NK cells in a 4-h [51Cr] release assay on Day 6.](/cms/asset/a7caff7b-fab4-408a-a740-d3695671f7a4/iimt_a_877104_f0003_b.jpg)
Our work collectively suggests that anergized NK cells are as important as the non-anergized NK cells in their effector functions. NK cells are not only important for the removal and shaping of the size of the stem cells which is mediated by the effector NK cells but also their differentiation, and the eventual regeneration of the new tissues which is mediated by their switch to anergized/regulatory NK cells. The task of NK cells in this regard goes above and beyond their most appreciated function of being the effectors of first line defense against viral infection and malignancies. They too can be effectors of differentiation and tissue regeneration.
Similarities in immune cell effector function in inflammatory tumor microenvironment and in non-transformed inflammatory microenvironment
The concept of tumor immunosurveillance has previously been expanded to include immunoediting as an important mechanism for the development of cancer (Dunn et al., Citation2002, Citation2004). It was suggested that cancer immunoediting was comprised of three phases: elimination, equilibrium, and escape. Elimination represents the classical concept of immunosurveillance. However, during equilibrium and escape the interaction and cross-signaling between the immune effectors including NK cells, the tumor cells, and perhaps the effectors of the connective tissue in the tumor microenvironment may result in the generation of tumors which are capable of gradual suppression of the NK cell cytotoxic function. The final stages of cancer development may result in the induction of resistant tumors in the presence of fewer immune effectors capable of lysing the tumors. Thus, pressures exerted by the tumor cells and immune effectors may eventually shape the microenvironment for the growth, expansion, and invasion of tumors.
Similarly, a variation of such interactions may also be observed during the interaction of NK cells with healthy non-transformed human stem cells in a non-transformed inflammatory microenvironment in which case the three phases of interaction may include elimination which marks a decrease in the numbers of proliferating stem cells or other immune effectors in the inflammatory microenvironment, potentially resulting in the selection of stem cells by the NK cells, induction of tolerance or anergy which denotes the conditioning of NK cells by the stem cells, and/or by the other effectors of the microenvironment to become regulatory cells and support maturation and differentiation of the remaining stem cells, and finally the resolution phase which denotes the elimination of anergized NK cells and generation of less immunogenic differentiated cells.
Immunosuppressive effectors in tumor and non-transformed inflammatory micro-environs
Both the tumor microenvironment as well as non-transformed inflammatory microenvironment consist of a number of heterogeneous cell populations with the ability to suppress and limit the function of cytotoxic immune effectors. Patients with cancer often have higher numbers of immature monocytes serving as Myeloid-Derived Suppressor Cells (MDSC) expressing a CD14+HLADR− phenotype (Vuk-Pavlovic, Citation2008; Greten et al. Citation2011). Tumor-associated macrophages (TAM) were previously shown to significantly influence and limit immune activation in the tumor microenvironment (Coffelt et al., 2009; Mantovani & Sica, Citation2010). In addition, MDSC that are comprised of a number of distinct cell populations of myeloid origin and whose roles in immunosuppression have received significant attention in recent years are major cells capable of suppressing the cytotoxic function of T and NK cells (Greten et al., Citation2011).
As noted above, NK cells are found in close proximity and often bound to mast cells, a subset of MDSC in both healthy and transformed inflammatory micro-environs (). T-cell dysfunction was induced by MDSC as a result of an increased secretion of IL-10, transforming growth factor (TGF)-β, induction of reactive oxygen species (ROS), and increased expression of arginase-1 and inducible nitric oxide synthase (iNOS). T-Regulatory (Treg) cells and regulatory DC (DCreg) were also recently shown to have significant immunosuppressive roles in the tumor microenvironment (Greten et al., Citation2011).
Perhaps one of the most intriguing observations regarding immunosuppressive effectors is identification of Cancer Associated Fibroblasts (CAF) and Mesenchymal Stem Cells (MSC) as potential tumor promoters. Fibroblasts from tumor tissues demonstrate an activated phenotype and the ability to secrete many immunosuppressive factors such as TGFβ, and VEGF (Yaguchi et al., Citation2011). Our own studies have found that undifferentiated fibroblasts, as well as MSC and CD14+HLA-DR− monocytes, were significantly more susceptible to NK cell-mediated cytotoxicity (Jewett et al., Citation2010); therefore, these cells may condition NK cells to undergo split anergy and become regulatory NK cells. Indeed, as shown above in oral epithelial tumors, the majority of recruited immune effectors were usually found in connective tissue areas where – through cell–cell interaction with immunosuppressive cells such as fibroblasts, monocytes–macrophages, and to a lesser extent T- and B-cells (Jewett et al., Citation2010) – regulatory NK cells can be generated, resulting in differentiation and increased resistance of the oral epithelial tumors.
Tumor microenvironment may shape NK cell function and phenotype
Based on the work presented in this review, it is possible that the resident and recruited immune effectors in a tumor microenvironment such as monocytes may serve as shields against NK cell lysis of cancer stem cells. Monocytes can shield cancer stem cells from killing by the NK cells by increasing the total IFNγ release from the NK cells while decreasing the cytotoxic function of NK cells, resulting in an increased protection and differentiation of cancer stem cells. Indeed, monocytes also increase TNFα IL-6, and VEGF secretion in the co-cultures of cancer stem cells with NK cells which could augment NF-κB and increase differentiation of cancer stem cells. The shielding effect of monocytes could be a more generalized function of other effectors since NK cells can also target fibroblasts (Jewett et al., Citation2010). Whether or not other MDSC (like neutrophils [PMN]) can also be targeted by NK cells awaits future investigation. This might have significant implications for the role of NK cells in not only limiting inflammation, but also the significance of other immune effectors in shielding and limiting the cytotoxic function of NK cells against cancer or healthy stem cells in order to raise maximally the secretion of key cytokines for speedy and optimal differentiation of cancer stem cells during inflammation (). This is precisely what is observed in cancer patients in whom a global decrease in NK, cytotoxic T-cells, and monocytes have all been reported (Jewett et al., Citation2006c).
Figure 4. Hypothetical model of induction of conditioned/regulatory NK cells by immune inflammatory cells and by the effectors of connective tissue to support differentiation of the non-transformed stem cells and cancer stem cells. Hypothetical model of NK cell conditioning in tumor microenvironment, as well as in non-transformed immune inflammatory microenvironment, is shown. Significant infiltration of immune effectors right beneath the epithelial layer can be seen in a connective tissue area where immune inflammatory cells are likely to condition NK cells to lose cytotoxicity and gain the ability to secrete cytokines, a term we previously coined ‘split anergy’ in NK cells, and to support differentiation of the basal epithelial layer containing stem cells. NK cells are likely to encounter and interact with other immune effectors such as monocytes/macrophages or other myeloid-derived suppressor cells (MDSC), or with connective tissue-associated fibroblasts (CAF), in order to be conditioned to form regulatory NK (NKreg) cells. NK cells may also directly interact with stem cells at the base of the epithelial layer, in which case by eliminating their bound stem cells, they can become conditioned to support differentiation of other stem cells. In addition, bacteria, through binding to Toll-like receptors can further aid in the generation of NKreg cells. All above-mentioned mechanisms may be operational during inflammatory processes in a tumor microenvironment or in a healthy non-transformed inflammatory microenvironment. NK cell-differentiated epithelial cells will no longer be killed or induce cytokine secretion by the NK cells, resulting in the resolution of inflammation.
Potential functional similarities between induced regulatory NK and T-regulatory cells
Because of their ability to drive differentiation, anergized NK cells may have the ability to halt inflammation since differentiated cells are no longer targeted by the NK cells and T-cells and they do not induce cytokine secretion by the NK and T-cells () (Jewett et al., Citation2010; Tseng et al., Citation2010). This function of NK cells is similar to T-regulatory cells since they are inhibitory and are capable of decreasing the magnitude of inflammation in a number of previous studies. Therefore, although immunosuppression in the tumor microenvironment is not advantageous for the patient, it is indeed an important function that may not only stimulate differentiation, but it may also halt inflammation.
The majority if not all of the effectors in the mucosal immune system including in the oral cavity are of activated phenotype, i.e. they express CD69 early activation antigen. These cells, including NK cells, are likely conditioned in the mucosa to support differentiation and resistance of the epithelial cells. Such an environment is anti-inflammatory since the majority of immune cells is tolerant of ingested food particles and self-tissues and is known to contain many regulatory cells including T-cells, DC, and likely regulatory NK cells. However, once the threshold that keeps the inflammation at bay is decreased in the mucosa, immune effectors are activated and may cause tissue damage and establishment of chronic inflammation. Indeed, in this regard, in our preliminary in vivo observations in humans, consuming a combination of proprietary probiotic bacterial strains with potent ability to condition NK cells to support OSCSC and hDPSC differentiation (manuscript in preparation) was able to relieve chronic inflammation and pain, and resulted in resolution of inflammatory mouth ulcers and oral edemas. Further, the number of PMN in the blood of a donor who had chronically decreased levels of PMN rose to normal levels and both the numbers and function of NK cells in the blood improved substantially after consumption of the probiotic bacterial strain. These results regarding conditioning of NK cells to become regulatory NK cells with receptor signaling in the presence of lipopolysaccharide (LPS) and IL-2 were in line with the numerous anti-inflammatory benefits that are achieved by consumption of probiotic bacteria.
Conclusions
Much work has been done to identify strategies for how tumor cells evade immune system functions. Altered expression of MHC molecules that block recognition/activation of T and NK cells are examples of means by which tumor cells evade the immune system. In addition, tumor cells – by releasing immunosuppressive factors like Fas, VEGF, TNFα, GM-CSF, and IL-1β, -6, and -10, induce T and NK cell apoptosis, block lymphocyte homing and activation, and dampen macrophage and DC function. Yet, these same effector functions are key in tissue repair.
Based on the accumulated work presented in this review, we suggest that NK cells may have two significant functions; one that relates to removal of excess proliferating stem cells and their subsequent selection. In this regard, NK cells could also lyse other effectors in the connective tissue in order to not only decrease inflammation but also be conditioned to promote differentiation/resistance of selected stem cells and eventual regeneration of the tissues (). The second important task for NK cells is thus to support differentiation and promote tissue regeneration after altering their phenotype to cytokine-secreting cells. This process will not only remove cells that are perhaps damaged and have flaws in the differentiation process or in general are more than needed, but also ensures proper regeneration of tissues and resolution of inflammation. Thus, any disturbance in NK cell function or in selection and differentiation of stem cells may result in chronic inflammation, causing continual tissue damage and recruitment of immune effectors to aid in tissue regeneration.
The inability of patient NK cells to contain cancer stem cells due to flooding of NK cells by proliferating cancer stem cells and conversion of NK cells to regulatory NK cells may likely be one mechanism by which cancer may progress and metastasize. There should thus be two distinct strategies by NK cells to eliminate tumors—one that targets stem cells and the other differentiated cells. Since cancer stem cells were found to be more resistant to certain chemo-therapeutic drugs but sensitive to NK cell-mediated killing while differentiated oral tumors were more resistant to NK-mediated killing but relatively more sensitive to chemotherapeutic drugs, combinational therapies should be used for eliminating both undifferentiated and differentiated tumors. In addition, since a great majority of patient NK cells may have switched to regulatory functions to support differentiation of the proliferating cancer stem cells, they may not be effective in eliminating cancer stem cells. Thus, these patients may benefit from repeated allogeneic NK cell transplantation to eliminate those stem cells. In this regard, depletion of immunosuppressive effectors in the tumor microenvironment that condition NK cells to become regulatory cells – either via radiation or chemotherapeutic drugs – should in theory provide a better strategy for successful targeting of tumors by the NK cells.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This work was supported in part by NIH, the UCLA Academic Senate, and School of Dentistry grants.
References
- Abdulkarim, B., Sabri, S., Deutsch, E., et al. 2002. Anti-viral agent Cidofovir restores p53 function and enhances radiosensitivity in HPV-associated cancers. Oncogene 21:2334–2346
- Azzoni, L., Anegon, I., Calabretta, B., and Perussia, B. 1995. Ligand binding to FcγR induces c-myc-dependent apoptosis in IL-2-stimulated NK cells. J. Immunol. 154:491–499
- Berlin, A. L., Paller, A. S., and Chan, L. S. 2002. Incontinentia pigmenti: A review and update on molecular basis of pathophysiology. J. Am. Acad. Dermatol. 47:169–187
- Bonavida, B., Lebow, L. T., and Jewett, A. 1993. Natural killer cell subsets: Maturation, differentiation and regulation. Nat. Immunol. 12:194–208
- Chatenoud, L. 2003. CD3-specific antibody-induced active tolerance: From bench to bedside. Nat. Rev. Immunol. 3:123–132
- Coffelt, S. B., Hughes, R., and Lewis, C. E. 2009. Tumor-associated macrophages: Effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta. 1796:11–18
- Cooper, M. A., Fehniger, T. A., and Caligiuri, M. A. 2001. The biology of human NK cell subsets. Trends Immunol. 22:633–640
- Deer, E. L., Gonzalez-Hernandez, J., Coursen, J. D., et al. 2010. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435
- Dunn, G. P., Bruce, A. T., Ikeda, H., et al. 2002. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 3:991–998
- Dunn, G. P., Old, L. J., and Schreiber, R. D. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21:137–148
- Farag, S. S., and Caligiuri, M. A. 2006. Human natural killer cell development and biology. Blood Rev. 20:123–137
- Fildes, J. E., Yonan, N., and Leonard, C. T. 2008. Natural killer cells and lung transplantation, roles in rejection, infection, and tolerance. Transplant. Immunol. 19:1–11
- Greten, T. F., Manns, M. P., and Korangy, F. 2011. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11:802–807
- Hu, P. F., Hultin, L. E., Hultin, P., et al. 1995. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J. AIDS Human Retrovirol. 10:331–340
- Inagaki, A., Soeda, A., Oka, N., et al. 2007. Long-term maintenance of brain tumor stem cell properties under at non-adherent and adherent culture conditions. Biochem. Biophys. Res. Commun. 361:586–592
- Jewett, A., and Bonavida, B. 1994. Activation of the human immature natural killer cell subset by IL-12 and its regulation by endogenous TNFα and IFNγ secretion. Cell. Immunol. 154:273–286
- Jewett, A., and Bonavida, B. 1995. Target-induced anergy of natural killer cytotoxic function is restricted to the NK-target conjugate subset. Cell. Immunol. 160:91–97
- Jewett, A., and Bonavida, B. 1996. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J. Immunol. 156:907–915
- Jewett, A., and Bonavida, B. 2000. MHC-Class I antigens regulate both the function and the survival of human peripheral blood NK cells: Role of endogenously-secreted TNFα. Clin. Immunol. 96:19–28
- Jewett, A., and Tseng H. C. 2013. Tumor microenvironment may shape the function and phenotype of NK cells through theinduction of split anergy and generation of regulatory NK cells. In: The Tumor Immunoenvironment. (Shurin, M., Umansky, V., and Malyguine, A., Eds.). Basel: Springer, pp. 361–384
- Jewett, A., Arasteh, A., Tseng, H. C., et al. 2010. Strategies to rescue mesenchymal stem cells (MSC) and dental pulp stem cells (DPSC) from NK cell-mediated cytotoxicity. PLoS One 5:e9874
- Jewett, A., Cacalano, N. A., Head, C., and Teruel, A. 2006a. Co-engagement of CD16 and CD94 receptors mediates secretion of chemokines and induces apoptotic death of naive natural killer cells. Clin. Cancer Res. 12:1994–2003
- Jewett, A., Cacalano, N. A., Teruel, A., et al. 2006b. Inhibition of NF-κB activity in oral tumor cells prevents depletion of NK cells and increases their functional activation. Cancer Immunol. Immunother. 55:1052–1063
- Jewett, A., Cavalcanti, M., and Bonavida, B. 1997. Pivotal role of endogenous TNFα in the induction of functional inactivation and apoptosis in NK cells. J. Immunol. 159:4815–4822
- Jewett, A., Gan, X. H., Lebow, L. T., and Bonavida, B. 1996. Differential secretion of TNFα and IFNγ by human peripheral blood-derived NK subsets and association with functional maturation. J. Clin. Immunol. 16:46–54
- Jewett, A., Head, C., and Cacalano, N. A. 2006c. Emerging mechanisms of immunosuppression in oral cancers. J. Dent. Res. 85:1061–1073
- Jewett, A., Man, Y. G., and Tseng, H. C. 2013. Dual functions of NK cells in selection and differentiation of stem cells: Role in regulation of inflammation and regeneration of tissues. J. Cancer 4:12–24
- Jewett, A., Teruel, A., Romero, M., et al. 2008. Rapid and potent induction of cell death and loss of NK cell cytotoxicity against oral tumors by F(ab')2 fragment of anti-CD16 antibody. Cancer Immunol. Immunother. 57:1053–1066
- Jewett, A., Wang, M. Y., Teruel, A., et al. 2003. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex Class I antigens by NF-κB in HEp2 tumor cell line: Effect on the function of NK cells. Human Immunol. 64:505–520
- Jiang, B., Mason, J., Jewett, A., et al. 2013a. Tumor-infiltrating immune cells: Triggers for tumor capsule disruption and tumor progression? Int. J. Med. Sci. 10:475–497
- Jiang, B., Mason, J., Jewett, A., et al. 2013b. Cell budding from normal appearing epithelia: Predictor of colorectal cancer metastasis? Int. J. Biol. Sci. 9:119–133
- Kuss, I., Saito, T., Johnson, J. T., and Whiteside, T. L. 1999. Clinical significance of decreased ζ chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin. Cancer Res. 5:329–334
- Lai, P., Rabinowich, H., Crowley-Nowick, P. A., et al. 1996. Alterations in expression and function of signal-transducing proteins in tumor-associated T and NK cells in patients with ovarian carcinoma. Clin. Cancer Res. 2:161–173
- Man, Y. G., Stojadinovic, A., Mason, J., et al. 2013. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: Existing theories. J. Cancer 4:84–95
- Mandelboim, O., Malik, P., Davis, D. M., et al. 1999. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:5640–5644
- Mantovani, A., and Sica, A. 2010. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 22:231–237
- Marigo, I., Dolcetti, L., Serafini, P., et al. 2008. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222:162–179
- Murakami, J., Asaumi, J., Kawai, N., et al. 2005. Effects of histone deacetylase inhibitor FR901228 on the expression level of telomerase reverse transcriptase in oral cancer. Cancer Chemother. Pharmacol. 56:22–28
- Murakami, J., Asaumi, J., Maki, Y., et al. 2004. Influence of CpG island methylation status in O6-methylguanine-DNA methyltransferase expression of oral cancer cell lines. Oncol. Rep. 12:339–345
- Nemeth, E., Baird, A. W., and O'Farrelly, C. 2009. Microanatomy of the liver immune system. Semin. Immunopathol. 31:333–343
- Oka, N., Soeda, A., Inagaki, A., et al. 2007. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochem. Biophys. Res. Commun. 360:553–559
- Ortaldo, J. R., Mason, A. T., and O'Shea, J. J. 1995. Receptor-induced death in human natural killer cells: Involvement of CD16. J. Exp. Med. 181:339–344
- Pasparakis, M., Courtois, G., Hafner, M., et al. 2002. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of Iκκ2. Nature 417:861–866
- Patankar, M. S., Yu, J., Morrison, J. C., et al. 2005. Potent suppression of NK cell response mediated by the ovarian tumor marker CA125. Gynecol. Oncol. 99:704–713
- Pegram, H. J., Andrews, D. M., Smyth, M. J., et al. 2011. Activating and inhibitory receptors of NK cells. Immunol. Cell. Biol. 89:216–224
- Rahaman, S. O., Harbor, P. C., Chernova, O., et al. 2002. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene 21:8404–8413
- Romagnani, C., Juelke, K., Falco, M., et al. 2007. CD56brightCD16– killer Ig-like receptor NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 178:4947–4955
- Seitz, C. S., Lin, Q., Deng, H., and Khavari, P. A. 1998. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc. Natl. Acad. Sci. USA 95:2307–2312
- Sipos, B., Moser, S., Kalthoff, H., et al. 2003. A comprehensive characterization of pancreatic ductal carcinoma cell lines: Towards the establishment of an in vitro research platform. Virchows Arch. 442:444–452
- Soeda, A., Inagaki, A., Oka, N., et al. 2008. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J. Biol. Chem. 283:10958–10966
- Squier, C. A., and Kremer, M. J. 2001. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 29:7–15
- Strowig, T., Chijioke, O., Carrega, P., et al. 2010. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood 116:4158–4167
- Tseng, H. C., Arasteh, A., Paranjpe, A., et al. 2010. Increased lysis of stem cells but not their differentiated cells by NK cells: De-differentiation or reprogramming activates NK cells. PLoS One 5:e11590
- van Hogerlinden, M., Rozell, B. L., Ahrlund-Richter, L., and Toftgard, R. 1999. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/NF-κB signaling. Cancer Res. 59:3299–3303
- van Hogerlinden, M., Rozell, B. L., Toftgard, R., and Sundberg, J. P. 2004. Characterization of the progressive skin disease and inflammatory cell infiltrate in mice with inhibited NF-κB signaling. J. Invest. Dermatol. 123:101–108
- Vuk-Pavlovic, S. 2008. Rebuilding immunity in cancer patients. Blood Cells Mol. Dis. 40:94–100
- Wang, T., G. Niu, G., Kortylewski, M., et al. 2004. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 10:48–54
- Yaguchi, T., Sumimoto, H., Kudo-Saito, C., et al. 2011. Mechanisms of cancer immunoescape and development of overcoming strategies. Int. J. Hematol. 93:294–300
- Yu, J., W. Du, W., Yan, F., et al. 2013. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 190:3783–3797