Abstract
Exposure to agriculture organic dusts, comprised of a diversity of pathogen-associated molecular patterns, results in chronic airway diseases. The multi-functional class A macrophage scavenger receptor (SRA)/CD204 has emerged as an important class of pattern recognition receptors with broad ligand binding ability. The objective was to determine the role of SRA in mediating repetitive and post-inflammatory organic dust extract (ODE)-induced airway inflammation. Wild-type (WT) and SRA knockout (KO) mice were intra-nasally treated with ODE or saline daily for 3 weeks and immediately euthanized or allowed to recover for 1 week. Results show that lung histopathologic changes were increased in SRA KO mice as compared to WT following repetitive ODE exposures marked predominately by increased size and distribution of lymphoid aggregates. After a 1-week recovery from daily ODE treatments, there was significant resolution of lung injury in WT mice, but not SRA KO animals. The increased lung histopathology induced by ODE treatment was associated with decreased accumulation of neutrophils, but greater accumulation of CD4+ T-cells. The lung cytokine milieu induced by ODE was consistent with a TH1/TH17 polarization in both WT and SRA KO mice. Overall, the data demonstrate that SRA/CD204 plays an important role in the normative inflammatory lung response to ODE, as evidenced by the enhanced dust-mediated injury viewed in the absence of this receptor.
Introduction
The major macrophage scavenger receptor class A (SRA/CD204), expressed preferentially on myeloid lineage cells but also on airway epithelial cells, is a multi-functional receptor with broad ligand binding potential (Canton et al., Citation2013; de Winther et al., Citation2000; Dieudonne et al., Citation2012; Seimon et al., Citation2006; Wyatt et al., Citation2005). SRA is well recognized for its role in atherosclerosis as it binds endogenous ligands including oxidized and acetylated low-density lipoproteins as well as apoptotic cells (de Winther et al., Citation2000). SRA has emerged as an important pattern recognition receptor because it recognizes several pathogen-associated molecular patterns (PAMPs) including endotoxin, lipoteichoic acid, fungal glucans, bacterial CpG, and double-stranded RNA. SRA has also been found to contribute to Toll-like receptor (TLR)-signaling pathways and, in particular, SRA may limit the inflammatory response to systemic endotoxin through its interaction with TLR4 (Areschoug & Gordon, Citation2009; Seimon et al., Citation2006; Yu et al., Citation2011, Citation2012).
Consistent with this protective role for SRA, absence of SRA has been associated with worsening outcomes following infections with Listeria (Ishiguro et al., Citation2001), Neisseria (Pluddemann et al., Citation2009), and pneumococcal (Arredouani et al., Citation2007) infections in mice. In contrast, others have reported the opposite: SRA knockout (KO) mice were protected from surgically-induced polymicrobial sepsis (Ozment et al., Citation2012) and Cryptococcus neoformans infections (Qiu et al., Citation2013). In addition to its role in modulating infectious insults, SRA has been implicated in regulating lung consequences following exposure to environmental particles such as crystalline silica and titanium dioxide (Beamer & Holian, Citation2005; Thakur et al., Citation2008). Namely, SRA KO animals were protected from the development of silica-induced lung fibrosis, but, yet, lung inflammatory cell accumulation was enhanced (Beamer & Holian, Citation2005). These data suggest that SRA could play an important role in regulating lung inflammatory outcomes following exposure to occupational organic dusts, which are complex mixtures of particulate matter and a wide-diversity of microbial components.
Chronic exposure to occupational agricultural organic dusts or bioaerosols results in significant airway inflammatory diseases. The chronic airway inflammatory response, which is marked by influx of inflammatory cells (neutrophils, macrophages, and lymphocytes) and pro-inflammatory cytokine/chemokine production, leads to progressive decline in lung function over time (May et al., Citation2012). Although wearing personnel protective equipment is recommended, respirator compliance is poor and treatment options are limited for symptomatic persons, even after removal from the exposure (May et al., Citation2012). Recent advances in understanding the host response have focused on acute and sub-chronic exposures; however, there is little information about the recovery phase of repetitive organic dust-induced airway inflammation, which may be a more relevant target area for improving disease outcomes once injury has occurred. Important etiologic factors identified within the complex organic dusts include Gram-negative endotoxins, Gram-positive peptidoglycans, fungal glucans, and bacterial CpG DNA (Poole & Romberger, Citation2012). Moreover, important roles for Toll-like receptor (TLR)-2, TLR4, TLR9, and MyD88 have been reported to mediate organic dust-induced airway inflammatory consequences in mice and humans (Bauer et al., Citation2013; Charavaryamath et al., Citation2008; Gao et al., Citation2013; Poole et al., Citation2011; Senthilselvan et al., Citation2009).
Because SRA/CD204 is a multi-functional receptor that recognizes a diversity of ligands that are predominate within complex agricultural environmental organic dust samples, we hypothesized that SRA would play an important functional role in modulating organic dust extract (ODE)-induced airway inflammation. We also aimed to investigate the lung response following a 1-week post-exposure recovery phase, focusing on a potential regulatory role for SRA. Our studies demonstrated that lung histopathologic changes were enhanced with increased numbers of CD4+ T-cells following repetitive ODE exposure in SRA KO mice. Increased inflammatory consequences remained pronounced at 1 week post-exposure in SRA KO mice compared to WT mice. These studies suggest that SRA plays an important role in regulating airway inflammation and the post-inflammatory lung tissue homeostasis with ODE exposures.
Materials and methods
Animals
C57BL/6 wild-type (WT) mice and SRA (CD204) knockout (KO, Msr1tm1Cskl−/−) mice on C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were house in specific pathogen-free facilities maintained at a set-point of ≈22 °C (range of 21–24 °C) and a range of 30–70% relative humidity and a 12-h light:dark cycle. All mice had ad libitum access to standard rodent chow and filtered water throughout the course of the studies. All experimental animal procedures were conducted according to the NIH guidelines for the use of rodents, and the University of Nebraska Medical Center Institutional Animal Care and Use Committee approved all procedures.
Organic dust extract (ODE)
Aqueous organic dust extract (ODE) was prepared from settled dust collected from horizontal surfaces (∼3 feet above floor) of local swine confinement feeding operations (housed ≈400–600 animals), and dust extracts were batch prepared utilizing previously described methods (Poole et al., Citation2009). Briefly, dust (10 g) was placed into sterile Hank’s Balanced Salt Solution (10 ml; Sigma, St. Louis, MO), incubated at room temperature for 1 h, and then centrifuged twice for 20 min at 2000 g. The final supernatant was filter-sterilized (0.22 µm), a process that removes coarse particles and also yields inhalable particle sizes (equal to and less than 0.22 µm size particles). Stock ODE was aliquoted and frozen until use in experimental assays.
For use in the experiments, stock ODE was diluted to a final concentration of 12.5% (vol/vol) in sterile phosphate-buffered saline (PBS; pH 7.4 [diluent]). The 12.5% ODE has been previously shown to elicit optimal and reproducible lung inflammation in mice and is well-tolerated by these hosts (Poole et al., Citation2009). Prior work to understand the components within these extracts have been previously published (Poole et al., Citation2010), and these previous studies demonstrated by gas chromatography-tandem mass spectrometry the presence of a high concentration of muramic acid (component of peptidoglycan) and 3-hydroxy fatty acid (component of endotoxin) and a low concentration of ergosterol (component of fungi). In these studies, independent batches of ODE were prepared and tested, but, due to the biologic complexity of the samples, inherent variability in the samples was inevitable. The range of these diluted 12.5% ODE samples contained 2.91–3.88 mg/ml of total protein, as measured by Nanodrop spectrophotometry (NanoDrop Technologies, Wilmington, DE) and confirmed using a Bradford protein assay. Endotoxin levels in the 12.5% ODE samples ranged from 22.1–91.1 EU/ml when evaluated using a limulus amebocyte lysate assay (according to manufacturer instructions; Sigma). Peptidoglycan levels were not quantitated, since commercially available measurement assays do not exist to the best of our knowledge.
Murine model
Per established protocol (Poole et al., Citation2009), mice received intra-nasal inhalation of 50 µl of sterile saline (PBS) or 12.5% ODE. For experimental studies, mice were treated daily for 3 weeks (with week-ends excluded) and immediately euthanized (repetitive exposure), or allowed to recover for 1 week without treatments (recovery studies). No mice exhibited respiratory distress or weight loss throughout the treatment periods.
Bronchoalveolar lavage fluid cell analysis
Bronchoalveolar lavage (BAL) was achieved using 3 × 1-ml instillates/recoveries of PBS. After pooling the three lavages, the total number of cells/mouse was enumerated and differential cell counts performed using cytospin-prepared slides (Cytopro Cytocentrifuge, Wescor Inc, Logan, UT) stained with DiffQuick (Siemens, Newark, DE).
Histopathology
Following lung lavage, the whole lungs were excised and slowly inflated (and hung under 20 cm H2O pressure) with 10% formalin (Sigma) for 24 h. Next, by routine histology processing, the fixed lung tissue was embedded in paraffin and entire lung sections cut (4–5 µm) and stained with hematoxylin and eosin (H&E). Each slide was entirely reviewed at scanning magnifications (2×, 4×, and 10× objectives; Nikon Eclipse Model E600 microscope, Nikon, Tokyo, Japan) and semi-quantitatively assessed for the degree and distribution of lung inflammation by a pathologist (W.W.W.) blinded to the treatment conditions, utilizing a previously published scoring system (Poole et al., Citation2009).
Flow cytometry phenotyping of whole lung cells
Cells were isolated from whole lungs as previously described (Poole et al., Citation2012a, Citationb). Briefly, 24 h after the final treatment condition, animals were euthanized and lung lavage performed. Next, after exposing the chest cavity, the right ventricle was infused with 10 ml sterile PBS to remove blood from the pulmonary vasculature and the lungs were harvested and subjected to an automated dissociation procedure using a gentleMAC Dissociator instrument (according to manufacturer instructions [Miltenyi Biotech, Auburn, CA]) in a solution containing collagenase type I (324 U/mL; Fisher, Pittsburgh, PA), bovine DNase (75 U/ml), porcine heparin (25 U/ml) and PBS with Ca2+ and Mg2+ (pH 7.4). The resulting solution was passed through a nylon mesh (40 µM; Thermo Fisher Scientific, Waltham, MA) to remove any large fragments, red blood cells were lysed using a 0.84% (w/v) ammonium chloride treatment (5 min at 4 °C), and after centrifugation at 422 x g, the remaining cells were re-suspended in PBS and final cell counts obtained using a hemocytometer. Viability of the final cell preparations was assessed by trypan blue exclusion. A LIVE/DEAD Fixable Violet Dead Cell Stain kit (Life Technologies, Carlsbad CA) was also used to assess cell viabilities; ultimately, < 1% of (gated) macrophages and lymphocytes were seen to be dead, with no differences noted between the saline and ODE-treated groups (data not shown). Whole lung cells from each animal were then incubated with anti-CD16/32 (Fc Block, BD Biosciences, San Jose, CA) to minimize non-specific antibody staining, and then stained with monoclonal antibodies (mAb) directed against CD11c, CD11b, IA-b, CD80, CD86, Ly-6G, CD3, CD4, and CD8; parallel cell preparations were treated with appropriate isotype controls (BD Biosciences). Compensation was performed with antibody capture beads (BD Biosciences) stained separately with each individual mAb used in test samples.
The gating strategy for CD11c+ macrophages, lymphocytes, and Ly6G+ neutrophils are depicted in Supplemental Figure 1 and follow that outlined in previously published reports (Osterholzer et al., Citation2011; Poole et al., Citation2012b). Briefly, initial gating on CD45+ lung leukocytes excludes debris, and the percentage of respective population (i.e. macrophage, lymphocyte, neutrophil) was determined from CD45+ leukocytes. This percentage (determined by flow cytometry) is multiplied by the original hemocytometer count of total cells recovered for each animal. All populations were gated by characteristic forward and side-scatter properties and antibody-specific staining fluorescence intensity using a FACSAria cell sorter system and associated software (BD Biosciences). In each case, a minimum of 25,000 CD45+ events/mouse sample was acquired for analysis.
Lung homogenate cytokine assays
Cytokine profiles were also characterized in total lung homogenates. Briefly, total lung homogenates were prepared by homogenizing lung samples in 500 µl sterile PBS, and cell-free homogenates were quantitated for cytokine contents using a cytometric bead array protein multiplex immunoassay array (interleukin [IL]-17A, IL-4, IL-10; BD Biosciences) or by ELISA kits (IL-1β and IL-12p40; R&D Systems, Minneapolis, MN).
Statistical methods
Data are presented as mean and standard error of mean (SEM). Statistical significance was assessed by one-way analysis of variance (ANOVA) and a two-tailed Mann-Whitney test, where appropriate. GraphPad (Version 5.02, La Jolla, CA) software was used. Significance was accepted at p values < 0.05.
Results
SRA KO mice display increased lung inflammatory histopathology following repetitive ODE treatment
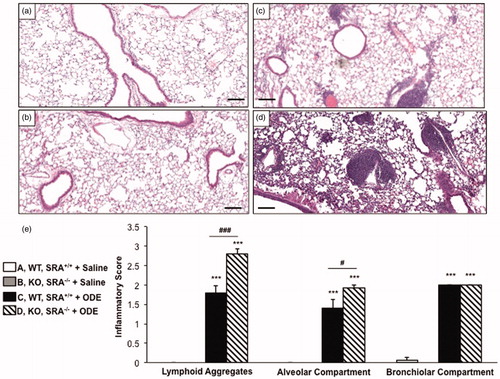
We have previously reported that repetitive ODE treatment induces lung inflammation in C57BL/6 WT animals (Poole et al., Citation2009); in this study, we sought to determine the role of SRA in regulating ODE-induced lung inflammation. WT and SRA KO mice were intra-nasally treated in side-by-side experiments with saline or ODE for 3 weeks, whereupon whole lung sections were processed and reviewed as described in the Methods section. By microscopic review there was a notable increase in inflammatory cells and the size and distribution of lymphoid aggregates in the SRA KO mice as compared to WT mice following repetitive ODE exposures (). The range of dust-induced histopathologic changes were semi-quantitatively assessed by reviewer (pathologist) blinded to treatment conditions; there were significant increases in the graded distribution of lung alveolar inflammation and lymphoid aggregates, but not bronchiolar inflammation, induced by repetitive ODE treatment in SRA KO mice as compared to WT mice (). Thus, these studies demonstrated enhanced pathologic injury in SRA KO mice following repetitive ODE treatment, suggesting a role for SRA in mediating lung inflammation consequences following ODE exposures.
Figure 1. Lung inflammation in SRA knockout mice after repetitive ODE exposures. Mice were intra-nasally treated with saline or organic dust extract (ODE) daily for 3 weeks. A representative 4–5-µm thick section (H&E stained) of one mouse per treatment group is shown (10 × magnification). (a) Wild-type (WT) SRA+/+ + saline. (b) Knockout (KO) SRA−/− + saline. (c) WT + ODE. (d) KO + ODE. (e) Mean semi-quantitative distribution of inflammatory scores of lung lymphoid aggregates, alveolar inflammation, and bronchiolar inflammation in mice (n = 4–5 mice/group). Error bars represent SE. Statistically significant (***p < 0.001) versus saline. #p < 0.05 and ###p < 0.001: significant differences between groups as indicated (1-way ANOVA with Tukey’s post-hoc comparison). Line scale represents 100 µm.
Lung inflammation in SRA KO mice remained increased following a 1-week recovery after repetitive ODE exposures
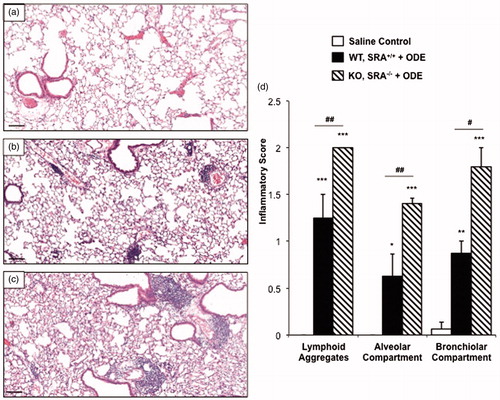
In the next set of experiments, we investigated lung inflammatory histopathology following a 1-week recovery phase (no treatment for 1 week) following the 3-week repetitive ODE treatment in WT and SRA KO mice. Although decreased compared to the effects from the 3-week repetitive ODE treatment, there remained notable evidence of inflammatory cells within the bronchiolar and alveolar compartments with a decreased number of lymphoid aggregates in WT mice at the end of the 1-week recovery period (). Interestingly, as compared to WT mice, SRA KO animals demonstrated increased lung inflammation at 1 week post-repetitive ODE treatments (). Significant differences were demonstrated in the range of inflammatory scores of lung lymphoid aggregates, bronchiolar inflammation, and alveolar inflammation between the exposed KO and WT treatment groups (). These studies show that the resolution of lung inflammation was slowed in SRA KO mice following ODE exposures, and suggest that SRA may play a significant role in post-inflammatory homeostasis.
Figure 2. Lung inflammation in SRA KO following 1 week recovery after repetitive ODE exposures. Mice intra-nasally treated with saline or ODE daily for 3 weeks and subsequently rested (no intra-nasal treatments) for a 1 week recovery phase. A representative 4–5-µm thick section (H&E stained) of one mouse per treatment group is shown (10 × magnification). (a) Saline. (b) Wild-type (WT) SRA+/+ treatment group. (c) KO SRA−/− treatment group. (d) Mean semi-quantitative distribution of inflammatory scores of lung lymphoid aggregates, bronchiolar inflammation and alveolar inflammation in mice (n = 4–5 mice/group). Error bars represent SE. Statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001) versus saline. #p < 0.05 and ##p < 0.01: significant differences between groups as indicated (1-way ANOVA with Tukey’s post-hoc comparison). Line scale represents 100 µm.
Differences in cell recruitment in lavage fluid demonstrated after daily repetitive ODE and following a 1-week recovery in SRA KO mice
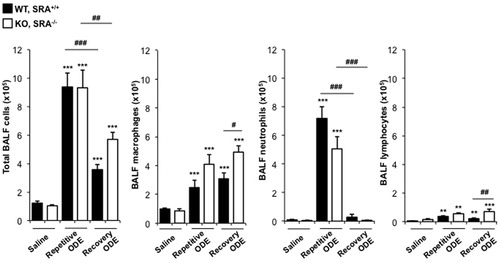
In these experiments, cellular influx in lavage fluid was determined in WT and SRA KO mice after daily repetitive ODE (or saline) and following a 1-week recovery. There were significant increases in total cells, neutrophils, macrophages, and lymphocytes after repetitive ODE treatments as compared to saline in the WT and KO mice (). Following the 1-week recovery, total cell influx marked by macrophages and lymphocytes, but not neutrophils, remained elevated as compared to in the saline control groups (). In comparing ODE-treated WT animals with SRA KO mice (), there was no significant difference in total cell influx at either time-point, but there were slight, but significant, differences observed with the numbers of macrophages, neutrophils, and lymphocytes. Namely, SRA KO mice demonstrated less neutrophil influx as compared to WT mice after the repetitive exposures, yet increased macrophages and lymphocytes after the 1-week recovery. These data suggest the role of SRA may be more important to specific cells (macrophages and lymphocytes) as opposed to all inflammatory cells in the airways.
Figure 3. Lavage fluid cell (type) levels indicative of recruitment after repetitive ODE treatments and following the 1 week recovery. Mice (WT and SRA KO) were intra-nasally treated with saline or ODE daily for 3 weeks (repetitive ODE) or ODE daily for 3 weeks followed by no treatments for 1 week (recovery ODE). Results shown are means (±SEM) bronchoalveolar lavage fluid counts of total cells, macrophages, neutrophils, and lymphocytes as determined by hemocytometer (n = 4–8 mice/group). Statistically significant (**p < 0.01, ***p < 0.001) versus saline. #p < 0.05, ##p < 0.01, ###p < 0.001: significant differences between groups as indicated by line (one-way ANOVA with Tukey’s post-hoc comparison).
Total lung cells and lung neutrophils following repetitive ODE and 1-week recovery in SRA KO mice
To further delineate the inflammatory cellular effect observed with SRA KO mice treated with ODE as compared to with WT hosts, whole lung cells following treatment conditions were dissociated and analyzed as described in the Methods; overall gating strategy is shown in Supplemental Figure 1. Repetitive ODE treatment resulted in increased total whole lung cell influx and increased neutrophil influx as compared to saline treatment (). Although trends for decreased total lung cells were observed in WT mice following the 1-week recovery as compared to after the repetitive ODE treatment, this did not reach statistical significance. There was also no difference in total lung cells between WT and SRA KO ODE treatment groups. Neutrophil numbers were significantly decreased (p < 0.001) after the 1 week recovery. The data suggest there were no pronounced differences in total lung cells between the WT and SRA KO mice following ODE treatments, but that neutrophil counts were diminishing after the 1-week post-exposure respite.
Figure 4. Total lung cells and lung neutrophils following repetitive ODE treatments and 1 week recovery in SRA KO mice. Mice were intra-nasally treated with saline or ODE daily for 3 weeks (repetitive ODE) or treated daily for 3 weeks followed by no treatment for 1 week (recovery ODE) whereupon mice were euthanized, lavage fluid removed, pulmonary vasculature perfused, and lung cells dissociated. (a) Mean total lung cells as determined by hemocytometer. (b) Mean total neutrophils (% LY6G+ neutrophils (see Supplemental Figure 1) of CD45+ cells [as determined by flow cytometry] multiplied by total lung cells) in WT and SRA KO mice. Error bars represent SE. Statistical significance (*p < 0.01, **p < 0.01, ***p < 0.001) versus saline. ###p < 0.001: significant difference between WT repetitive ODE and WT recovery ODE (1-way ANOVA with Tukey’s post-hoc test). n = 5–8 mice/group.
![Figure 4. Total lung cells and lung neutrophils following repetitive ODE treatments and 1 week recovery in SRA KO mice. Mice were intra-nasally treated with saline or ODE daily for 3 weeks (repetitive ODE) or treated daily for 3 weeks followed by no treatment for 1 week (recovery ODE) whereupon mice were euthanized, lavage fluid removed, pulmonary vasculature perfused, and lung cells dissociated. (a) Mean total lung cells as determined by hemocytometer. (b) Mean total neutrophils (% LY6G+ neutrophils (see Supplemental Figure 1) of CD45+ cells [as determined by flow cytometry] multiplied by total lung cells) in WT and SRA KO mice. Error bars represent SE. Statistical significance (*p < 0.01, **p < 0.01, ***p < 0.001) versus saline. ###p < 0.001: significant difference between WT repetitive ODE and WT recovery ODE (1-way ANOVA with Tukey’s post-hoc test). n = 5–8 mice/group.](/cms/asset/b43aa645-f88a-4341-83c9-cfcef32a2d29/iimt_a_882449_f0004_b.jpg)
Exudative CD11chi/CD11bhi macrophages are the most prevalent macrophage population in lungs of WT and SRA KO mice following repetitive ODE exposure and following a 1-week recovery
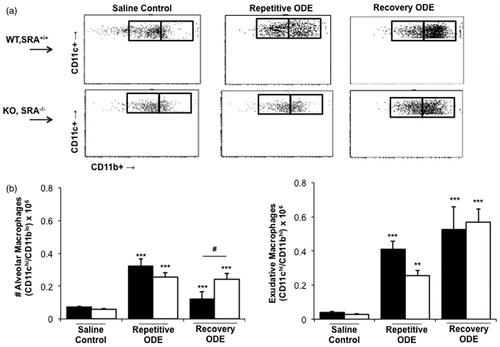
In previous studies, we found that repetitive ODE treatment induces the influx of lung exudative CD11chi/CD11bhi macrophages (Poole et al., Citation2012b). Because SRA was initially described as a macrophage receptor, we had hypothesized that it would play an important role in ODE-induced lung inflammation via its macrophage immunomodulatory properties. Consistent with that prior work, repetitive ODE treatment resulted in increased levels of lung exudative CD11chi/CD11bhi macrophages; however, in this current study, we report that the exudative macrophages remain significantly increased following a 1-week recovery (). Further, there were no significant differences in the number of exudative CD11chi/CD11bhi macrophages between the WT and SRA ODE repetitive and recovery treated groups. The numbers of alveolar macrophages (CD11chi/CD11blo macrophages) were decreased in WT animals as compared to SRA KO mice after the 1-week recovery. We also investigated cell surface expression of MHC Class II (murine I-Ab) and co-stimulatory CD80 and CD86 molecules on these alveolar and exudative macrophages (Supplemental Figure 2; the numbers of exudative CD11chi/CD11bhi macrophages in saline-treated animals were unsurprisingly too low for adequate flow cytometric analysis). Consistent with prior studies, repetitive ODE treatment was associated with increased CD80, CD86, and I-Ab cell surface expression on exudative CD11chi/CD11bhi macrophages in WT animals (data not shown), and at 1-week recovery CD80, CD86, and I-Ab expression remains enhanced (Supplemental Figure 2). Although there were subtle differences in I-Ab expression (less robust cell surface expression in SRA KO mice), there were no significant differences overall in CD80, CD86, and I-Ab surface expression on exudative CD11chi/CD11bhi macrophages between the WT and SRA ODE repetitive and recovery treatment groups. Collectively, these data did not support our hypothesis that the macrophage was significantly dysregulated in SRA KO animals as an explanation for the enhanced lung inflammation these hosts displayed following ODE treatments ( and ).
Figure 5. Exudative CD11chi/CD11bhi macrophages in lungs of WT and SRA KO mice after repetitive ODE exposure and following the 1 week recovery. Mice were intra-nasally treated with saline control or ODE daily for 3 weeks (repetitive ODE) or treated daily for 3 weeks followed by no treatment for 1 week (recovery ODE) whereupon mice were euthanized, lavage fluid removed, pulmonary vasculature perfused, and lung cells dissociated and analyzed by flow cytometry. Initial gating on CD45+ lung leukocytes excludes debris, lymphocytes, and neutrophils, and a plot identified CD11c versus FSC identifies CD11c+ cells (see Supplemental Figure 1). Next, a plot of CD11c versus CD11b distinguishes alveolar macrophages and exudative macrophages (top panel, a). (b, bottom panel) Numbers of macrophages were calculated by multiplying the percentage of macrophages in respective gate (percentage of all CD45+ cells, as analyzed by FACS) multiplied by total lung cells (determined using a hemocytometer) for each mouse. Data shown are means ± SEM of 5–8 mice/group. Statistically significant (**p < 0.01, ***p < 0.001) versus saline. #p < 0.05: significant difference between SRA deficient mice (1-way ANOVA with Tukey’s post-hoc test).
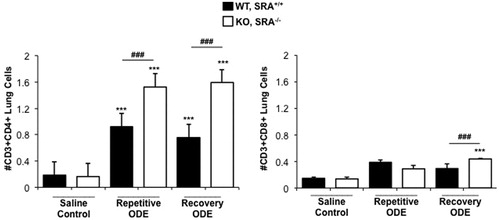
CD4+ lymphocyte recruitment is increased in SRA KO mice following repetitive ODE exposure and remains elevated following a 1-week recovery
Because histopathology demonstrated enhanced lymphoid aggregates ( and ) following ODE treatments, infiltrating lung T-cell phenotypes were investigated by flow cytometry. Consistent with prior reports (Ivanov et al., Citation2005; Poole et al., Citation2012a), repetitive ODE treatments resulted in increased levels of CD3+CD4+ T-cells; moreover, levels of these cells remained increased after 1 week of recovery (). Interestingly, SRA KO mice demonstrated significantly increased numbers of CD3+CD4+ T-cells following ODE repetitive and recovery treatment periods as compared to WT animals (). Although CD3+CD8+ T-cells were slightly increased following ODE as compared to saline treatment, this did not reach statistical significance in WT mice. There were more CD3+CD8+ T-cells in SRA KO mice following the 1week recovery as compared to in the WT mice. These studies suggest that infiltrating CD4+ T-cells may play an important role in explaining the enhanced lung inflammatory response in SRA KO mice following ODE treatment.
Figure 6. CD4+ lymphocyte recruitment in SRA KO mice following repetitive ODE exposure and after the 1 week recovery. Mice were treated and processed as outlined in the legend. Lymphocytes identified by CD45+ leukocytes excluding debris and characteristic FSC and SSC properties of lymphocytes (see Supplemental Figure 1) followed by staining for CD3, CD4, and CD8. Total numbers of each lymphocyte population were determined by multiplying the frequency of CD3+CD4+ cells or CD3+CD8+ cells (among the CD45+ leukocytes) by the total number of cells (determined by hemocytometer) for each mouse. Data shown are means ± SEM of 5–8 mice/group. Statistically significant (***p < 0.001) versus saline. ###p < 0.001: significant difference between SRA KO and WT mice (1-way ANOVA with Tukey’s post-hoc test).
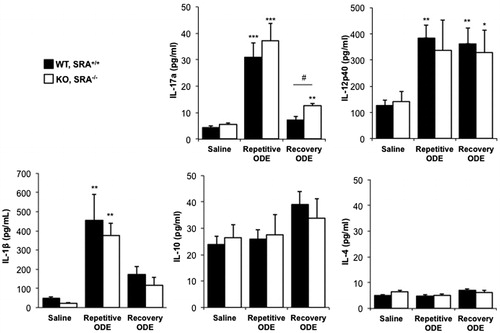
ODE exposure induces a TH17/TH1-polarized cytokine response in the whole lung
Next we investigated the cytokine milieu in lung homogenates, and found evidence of both a T-helper (TH)-17 and TH1 cell responses induced by repetitive ODE treatment in both WT and SRA KO animals (). There were no significant differences in the cytokine milieu between groups, with the exception that IL-17A remained significantly elevated in SRA KO mice as compared to WT following 1 week recovery from ODE treatments ().
Figure 7. ODE exposure-induced TH17/TH1-polarized cytokine response in lung. Mice were intra-nasally treated with saline or ODE daily for 3 weeks (repetitive ODE) or treated daily for 3 weeks followed by no treatment for 1 week (recovery ODE), whereupon they were euthanized, lavage fluid removed, pulmonary vasculature perfused, and half of their lung tissue was homogenized. Cytokine profiles (associated with T-cell sub-sets) were analyzed from cell-free supernatants of the homogenates using protein multiplex immunoassay and ELISA. Results shown are means ± SEM of 5–8 mice/group. #p < 0.05: significant difference between SRA-deficient and WT mice (1-way ANOVA with Tukey’s post-hoc test). Statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001) versus saline.
Discussion
Our studies demonstrate that SRA plays a significant role in regulating complex organic dust extract (ODE)-induced lung injury, and that SRA KO mice recover less effectively as compared to control animals, implicating a role for SRA in post-inflammatory homeostasis. SRA KO mice repetitively treated with ODE demonstrated increased lung histopathology changes marked by increased lymphoid aggregates and CD4+ T-cell infiltration. This lung inflammatory process remained significantly increased in SRA KO mice following 1 week of recovery post-exposure. The T-cell polarization, consistent with a TH1/TH17 skewing, was similar between SRA and WT animals following the ODE treatments. Collectively, these are the first studies to establish a potential protective functional role for SRA in repetitive ODE-induced airway inflammation, and, moreover, propose its role in regulating post-inflammatory recovery.
Recent studies of occupational/agricultural organic dusts and dust extract exposure-induced airway disease have led to an appreciation for the presence of a wide diversity of microbial components, particularly Gram-positive as well as Gram-negative bacterial cell wall components (May et al., Citation2012). Although most studies have focused on swine confinement facility environments, other industrial animal farm environments such as dairy farming have also been associated with atmospheres bearing a high predominance of Gram-positive and Gram-negative bacterial cell wall components (Poole et al., Citation2010). Roles for TLR2 that recognizes lipotechoic acids and peptidoglycans and TLR4 that recognizes endotoxin have been described (Charavaryamath et al., Citation2008; Gao et al., Citation2013; Poole et al., Citation2011; Senthilselvan et al., Citation2009). Namely, TLR2 and TLR4 KO mice have blunted airway inflammatory responses following acute and sub-chronic swine facility dust/dust extract exposures (Bauer et al., Citation2013; Charavaryamath et al., Citation2008; Poole et al., Citation2011), and polymorphisms in the TLR2 and TLR4 have been associated with lung function consequences in exposed swine workers (Gao et al., Citation2013; Senthilselvan et al., Citation2009). However, the ODE-induced airway inflammatory response is not completely abrogated in TLR2 and TLR4 KO mice (Bauer et al., Citation2013; Charavaryamath et al., Citation2008; Poole et al., Citation2011), and, moreover, direct comparisons with lipopolysaccharide at concentrations found in ODE did not replicate the magnitude of lung inflammatory responses induced by ODE (Dusad et al., Citation2013). Thus, there may not be one critical ligand responsible for eliciting complex organic dust extract-induced disease. Indeed, MyD88, the common adaptor protein for all TLR pathway signaling responses (except TLR3), appears to be the central pathway mediating ODE-induced airway inflammation because there was a near absent airway response following acute dust challenge in MyD88 KO mice (Bauer et al., Citation2013). However, targeting MyD88 may not be a viable potential preventative or therapeutic option due to its critical role in overall host defense.
Thus, due to its broad ligand binding affinity, SRA might be an important pattern recognition receptor to target in organic dust-induced airway inflammatory diseases because the dust is noted to contain an abundance of pathogen-associated molecular patterns (PAMPs) (Canton et al., Citation2013). Although there are reports that knocking out SRA results in improved outcomes following polymicrobial sepsis (Ozment et al., Citation2012) and Cryptococcus neoformans lung infection (Qiu et al., Citation2013), several studies have suggested the opposite. Namely, several studies have reported that SRA acts as an immunosuppressive molecule because infectious consequences were worsened in the absence of SRA (Areschoug & Gordon, Citation2009; Ohnishi et al., Citation2011; Seimon et al., Citation2006; Yi et al., Citation2009; Yu et al., Citation2011, Citation2012). The data here are entirely consistent with these later observations in that SRA may play an important role in the limiting airway inflammatory responses. We interpret our findings to be mixed in comparison to results demonstrated in a non-infectious, silica exposure model (Beamer & Holian, Citation2005). Specifically, absence of SRA was protective against silica-induced lung fibrosis development but not against inflammatory cell infiltration. It is likely that these differences are not explained by one critical ligand, but instead by the complexity of occupational/agricultural organic dusts and dust extracts that contain a wide diversity of PAMPs and particulate matter.
The remarkable finding was the enhanced histopathologic changes following repetitive ODE exposures that remained elevated after a 1 week recovery phase in the SRA KO mice. The dominant cell type to explain this difference was CD4+ T-cells (). Yi et al. (Citation2012) also showed that SRA KO mice developed more robust CD4+ T-cell responses after OVA immunization, with a concomitant increase in IL-12 production. Lung CD4+ T-cell accumulation has also been demonstrated following cryptococcal lung infections in SRA KO animals (Qiu et al., Citation2013). Our group has previously reported that repetitive ODE exposure results in a cytokine milieu consistent with a TH1/TH17 polarized lung response, and, in this study, a TH1/TH17 response was confirmed in the WT animals and also extended to the SRA KO mice, but no predominant difference in the lung cytokine milieu was found between groups. Neutrophil infiltration was not enhanced in SRA KO mice, but, rather, neutrophil recruitment was diminished. Others have observed no effect on neutrophil recruitment in SRA KO mice challenged with infectious insults (Ozment et al., Citation2012). SRA is not expressed on neutrophils (Yamamoto et al., Citation1999), and, therefore, regulation of neutrophil functions are likely not directly dependent on SRA.
Exudative macrophages (i.e. CD11chiCD11bhi) (Osterholzer et al., Citation2009, Citation2011) with an increased expression of co-stimulatory molecules (CD80 and CD86) and MHC Class II expression are normal, protective responses following infectious and organic dust-induced inflammatory insults in the lung (Poole et al., Citation2012b). Others have found that expression markers (co-stimulatory molecules) are up-regulated on classically activated lung macrophages following lung infections as a potential explanation for improved host defense in SRA KO mice (Ozment et al., Citation2012). Here, the number of lung exudative macrophages did not differ significantly in SRA KO mice treated with ODE as compared to WT animals, and the pattern of expression markers also did not differ. These results might suggest that the mechanism by which the macrophage SRA/CD204 receptor alters organic dust-induced inflammatory responses is indirectly through its modulation of T-cell recruitment instead of direct changes to the lung macrophage response.
Prior research investigating effects of occupational exposure to agricultural organic dusts or bioaerosols has focused on acute and repetitive dust exposure scenarios. In contrast, information regarding post-inflammatory recovery events is lacking but represents a novel area to potentially intervene, as symptomatic workers are more apt to seek medical care after disease has established. For these reasons, we investigated the normative post-inflammatory response to repetitive ODE exposures in WT mice and compared that response to SRA KO animals. At 1 week after the repetitive exposures, the inflammatory lung consequences in WT mice persisted, marked by significant lung histopathology and increased infiltration of CD4+ T-cells and exudative CD11chiCD11bhi macrophages. However, there was clear evidence of a resolving neutrophil response, and IL-17 (but not IL-12) levels were also diminishing at 1 week post-exposure. This association of IL-17 and neutrophil responses might be expected because TH17 responses have been closely associated with neutrophilic inflammation (Korn et al., Citation2009). Although subtle, it is noted that neutrophil response was slightly lower in the ODE-treated SRA KO mice, despite higher levels of IL-17A ( and ), suggesting that the IL-17 response is not the sole driver of neutrophil inflammation following ODE exposures. The inflammatory differences between WT and SRA KO mice appeared stronger in the post-recovery experiments. In the SRA KO mice, resolution of neutrophil infiltration was observed, but levels of CD4+ T-cells, CD8+ T-cells, and macrophages remained strikingly elevated. These data support the role of involvement of SRA in immune homeostasis.
In summary, this study demonstrated several new features important in understanding organic dust-induced airway inflammation. First, SRA appeared to serve a functional role in regulating repetitive organic dust-induced airway inflammation, as evident by worsening lung histopathology in the absence of SRA. As opposed to an overall global increase in inflammatory cellular infiltration; the enhanced response was associated with a robust CD4+ T-cell influx. Even more pronounced was the enhanced inflammatory response following a post-exposure, 1-week recovery in SRA KO mice. Collectively, SRA/CD204 is a functionally diverse pattern recognition receptor that might be exploited to impact health consequences in organic dust-exposed workers. There also may be merit in future studies to examine if genetic polymorphisms in SRA contribute to airway responses in exposed workers.
Declaration of interest
Study supported by grants from the National Institute of Environmental Health Sciences (R01ES019325 to JAP), National Institute of Alcohol Abuse and Alcoholism (R01AA017993 to TAW), and National Institute of Occupational Safety Health (R01OH008539 to DJR and U54OH010162 to JAP and TAW). This work was supported in part by the Central States Center for Agricultural Safety and Health (CS-CASH). This material is the result of work supported with resources and the use of the VA Nebraska-Western Iowa Health Care System.
| Abbreviations | ||
| AHR, | = | airway hyper-responsiveness; |
| BALF, | = | bronchoalveolar lavage fluid; |
| COPD, | = | chronic obstructive pulmonary disease; |
| IL, | = | interleukin; |
| KO, | = | knockout; |
| ODE, | = | swine confinement organic dust extract; |
| PAMP, | = | pathogen-associated molecular pattern; |
| SRA, | = | scavenger receptor A, CD204; |
| TLR, | = | toll-like receptor; |
| TNFα, | = | tumor necrosis factor-α; |
| WT, | = | wild-type |
| Abbreviations | ||
| AHR, | = | airway hyper-responsiveness; |
| BALF, | = | bronchoalveolar lavage fluid; |
| COPD, | = | chronic obstructive pulmonary disease; |
| IL, | = | interleukin; |
| KO, | = | knockout; |
| ODE, | = | swine confinement organic dust extract; |
| PAMP, | = | pathogen-associated molecular pattern; |
| SRA, | = | scavenger receptor A, CD204; |
| TLR, | = | toll-like receptor; |
| TNFα, | = | tumor necrosis factor-α; |
| WT, | = | wild-type |
Supplementary Figures 1-2
Download PDF (168 KB)Acknowledgements
The authors wish to thank Jane DeVasure, Jackie Pavlik, and Christopher Bauer for technical assistance, and David Wert, Facility Supervisor, Pathology and Microbiology Department in the UNMC Tissue Science Facility for assistance with lung tissue processing, sectioning, H&E staining, and assistance with digital microscopy images prepared for the manuscript. We also thank Charles A. Kuszynski, PhD (Scientific Director) and Victoria B. Smith (Cytometer Operator) with the UNMC Cell Analysis Facility for assistance with flow cytometry analysis, and Lisa Chudomelka for assistance in the manuscript preparation.
References
- Areschoug, T., and Gordon, S. 2009. Scavenger receptors: Role in innate immunity and microbial pathogenesis. Cell. Microbiol. 11:1160–1169
- Arredouani, M. S., Franco, F., Imrich, A., et al. 2007. Scavenger Receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J. Immunol. 178:5912–5920
- Bauer, C., Kielian, T., Wyatt, T. A., et al. 2013. MyD88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am. J. Respir. Cell. Mol. Biol. 48:781–789
- Beamer, C. A., and Holian, A. 2005. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am. J. Physiol. 289:L186–L195
- Canton, J., Neculai, D., and Grinstein, S. 2013. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 13:621–634
- Charavaryamath, C., Juneau, V., Suri, S. S., et al. 2008. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp. Lung Res. 34:19–35
- de Winther, M. P., van Dijk, K. W., Havekes, L. M., and Hofker, M. H. 2000. Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 20:290–297
- Dieudonne, A., Torres, D., Blanchard, S., et al. 2012. Scavenger receptors in human airway epithelial cells: Role in response to double-stranded RNA. PLoS One 7:e41952
- Dusad, A., Thiele, G. M., Klassen, L. W., et al. 2013. Organic dust, lipopolysaccharide and peptidoglycan inhalant exposures result in bone loss/disease. Am. J. Respir. Cell Mol. Biol. 49:829–836
- Gao, Z., Dosman, J. A., Rennie, D. C., et al. 2013. Association of Toll-like receptor 2 gene polymorphisms with lung function in workers in swine operations. Ann. Allergy Asthma Immunol. 110:44–50.e1
- Ishiguro, T., Naito, M., Yamamoto, T., et al. 2001. Role of macrophage scavenger receptors in response to Listeria monocytogenes infection in mice. Am. J. Pathol. 158:179–188
- Ivanov, S., Palmberg, L., Venge, P., et al. 2005. IL-17A mRNA and protein expression within cells from the human bronchoalveolar space after exposure to organic dust. Respir. Res. 6:44
- Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V. K. 2009. IL-17 and TH17 cells. Annu. Rev. Immunol. 27:485–517
- May, S., Romberger, D. J., and Poole, J. A. 2012. Respiratory health effects of large animal farming environments. J. Toxicol. Environ. Health B Crit. Rev. 15:524–541
- Ohnishi, K., Komohara, Y., Fujiwara, Y., et al. 2011. Suppression of TLR4-mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem. Biophys. Res. Commun. 411:516–522
- Osterholzer, J. J., Chen, G. H., Olszewski, M. A., et al. 2009. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J. Immunol. 183:8044–8053
- Osterholzer, J. J., Chen, G. H., Olszewski, M. A., et al. 2011. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am. J. Pathol. 178:198–211
- Ozment, T. R., Ha, T., Breuel, K. F., et al. 2012. Scavenger receptor class a plays a central role in mediating mortality and the development of the pro-inflammatory phenotype in poly-microbial sepsis. PLoS Pathog. 8:e1002967
- Pluddemann, A., Hoe, J. C., Makepeace, K., et al. 2009. The macrophage scavenger receptor A is host-protective in experimental meningococcal septicemia. PLoS Pathog. 5:e1000297
- Poole, J. A., and Romberger, D. J. 2012. Immunological and inflammatory responses to organic dust in agriculture. Curr. Opin. Allergy Clin. Immunol. 12:126–132
- Poole, J. A., Dooley, G. P., Saito, R., et al. 2010. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J. Toxicol. Environ. Health 73:684–700
- Poole, J. A., Gleason, A. M., Bauer, C., et al. 2012a. αβ T-cells and a mixed TH1/TH17 response are important in organic dust-induced airway disease. Ann. Allergy Asthma Immunol. 109:266–273.e2
- Poole, J. A., Gleason, A. M., Bauer, C., et al. 2012b. CD11c+/CD11b+ cells are critical for organic dust-elicited murine lung inflammation. Am. J. Respir. Cell. Mol. Biol. 47:652–659
- Poole, J. A., Wyatt, T. A., Kielian, T., et al. 2011. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am. J. Respir. Cell. Mol. Biol. 45:711–719
- Poole, J. A., Wyatt, T. A., Oldenburg, P. J., et al. 2009. Intra-nasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am. J. Physiol. 296:L1085–L1095
- Qiu, Y., Dayrit, J. K., Davis, M. J., et al. 2013. Scavenger receptor A modulates the immune response to pulmonary Cryptococcus neoformans infection. J. Immunol. 191:238–248
- Seimon, T. A., Obstfeld, A., Moore, K. J., et al. 2006. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. USA 103:19794–19799
- Senthilselvan, A., Dosman, J. A., Chenard, L., et al. 2009. Toll-like receptor 4 variants reduce airway response in human subjects at high endotoxin levels in a swine facility. J. Allergy Clin. Immunol. 123:1034–1040.e1–2
- Thakur, S. A., Hamilton, R. F. Jr., and Holian A. 2008. Role of scavenger receptor a family in lung inflammation from exposure to environmental particles. J. Immunotoxicol. 5:151–157
- Wyatt, T. A., Kharbanda, K. K., Tuma, D. J., et al. 2005. Malondialdehyde-acetaldehyde adducts decrease bronchial epithelial wound repair. Alcohol 36:31–40
- Yamamoto, T., Ebe, Y., Hasegawa, G., et al. 1999. Expression of scavenger receptor class A and CD14 in lipopolysaccharide-induced lung injury. Pathol. Int. 49:983–992
- Yi, H., Yu, X., Gao, P., et al. 2009. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood 113:5819–5828
- Yi, H., Zuo, D., Yu, X., et al. 2012. Suppression of antigen-specific CD4+ T-cell activation by SRA/CD204 through reducing the immunostimulatory capability of antigen-presenting cell. J. Mol. Med. (Berlin) 90:413–426
- Yu, H., Ha, T., Liu, L., et al. 2012. Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim. Biophys. Acta 1823:1192–1198
- Yu, X., Yi, H., Guo, C., et al. 2011. Pattern recognition scavenger receptor CD204 attenuates Toll-like receptor 4-induced NF-κB activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J. Biol. Chem. 286:18795–18806

