Abstract
Pteridium aquilinum (bracken fern), one of the most important toxic plants in the world, contains the toxic norsequiterpene ptaquiloside that induces cancers in humans and farm animals. Previous studies in the laboratory demonstrated immunotoxic effects produced by ptaquiloside, which are characterized by suppression of natural killer (NK) cell activity (i.e. cytotoxicity and interferon [IFN]-γ production). However, it is unknown whether these immunosuppressive effects could contribute to carcinogenesis in situ in general because of the important function of NK cells in innate killing of tumor cells. This study assessed the impact of P. aquilinum-induced immunosuppression on urethane-induced lung cancer in C57BL/6 mice. Adult mice were treated with an extract of P. aquilinum (30 g/kg/day) by gavage once daily for 14 days, followed by gavage (5 days/week) during an 11-week period that was accompanied by treatment with urethane (1 g/kg) via once-weekly intraperitoneal injection; 20 weeks after the end of the treatment period, all lungs were evaluated. The results indicated there was a significant increase in lung nodule number as well as in multiplicity of lesions in mice treated with both P. aquilinum and urethane (PU group) compared to values in mice treated only with the urethane (U group). In addition, histologic evaluation revealed a 76% increase in the rate of lung adenomas and a 41% increase in rate of bronchiolization of alveoli in the mice from the PU group compared to levels seen in mice within the U group. Taken together, the results here show for the first time that immunosuppressive effects of P. aquilinum could increase the risk of cancer formation in exposed hosts.
Introduction
Immunosuppression is a complex process characterized by the reduction of immune cells or their function that results in increased susceptibility to cancer development and infectious diseases (Colosio et al., Citation2004; Drela, Citation2006). In innate immunity, natural killer (NK) cells play a central role in killing tumoral and infected cells (Brandstadter & Yang, Citation2011; Diefenbach & Raulet, Citation2002). It is well known that NK cells have an important role in cancer immunosurveillance and that the suppression of their activities can increase cancer metastasis and consequently decrease host survival (Whiteside, Citation2006). In fact, it was demonstrated that mice with lung cancer and high NK activity developed less metastasis than immunosuppressed mice (Trinchieri, Citation1989).
Pteridium aquilinum, commonly known as the bracken fern, is one of the most important toxic plants in the world. The plant is known to produce distinct syndromes in livestock such as thiamine deficiency, acute hemorrhagic disease, and bright blindness (Smith, Citation2004). This fern is also well established as a carcinogen in humans and livestock through ingestion of plant crosiers (Abnet, Citation2007; Gil da Costa et al., Citation2012; Sugimura, Citation2000). Humans can be exposed to the plant indirectly through consumption of milk and meat from animals grazing on bracken fern (Alonso-Amelot & Avendano, Citation2002; Fletcher et al., Citation2011; Shahin et al., Citation1999) or through ingestion of contaminated water (Rasmussen et al., Citation2003). Previous studies conducted in this laboratory have also demonstrated (in mice) that ptaquiloside produces immunosuppressive effects, including direct toxic effects on NK cells (Latorre et al., Citation2009). The toxin principally responsible for P. aquilinum-induced carcinogenic and immunosuppressive effects is ptaquiloside, a norsesquiterpene glucoside that acts as a DNA-alkylating agent (Hirono, Citation1986; Latorre et al., Citation2009; Yamada et al., Citation2007).
The present study was undertaken to ascertain if these documented immunosuppressive effects of P. aquilinum could be associated with its subsequent/concurrent carcinogenic effects. To achieve this, studies using a model carcinogen (i.e. urethane) in P. aquilinum-treated mice were performed and effects on tumor induction and spread (among several endpoints) assessed.
Materials and methods
Mice
A total of 119 60-day-old female C57BL/6 mice that were bred at the Department of Pathology at the School of Veterinary Medicine and Animal Sciences of the University of Sao Paulo (Sao Paulo, Brazil) were used in these experiments. The mice were maintained under controlled conditions of temperature (22–25 °C), relative humidity (50–65%), and lighting (12 h light/dark cycle). Drinking water and standard diet (Nuvilab-CR1®, Nuvital Nutrientes LTDA, Colombo, Parana, Brazil) were accessible ad libitum. All procedures using mice were performed following the Guidelines for the Care and Use of Laboratory Animals, NIH publication N° 85–23 (http://www.nap.edu/readingroom/books/labrats/) and were reviewed and approved by the Bio-ethics Committee of the FMVZ-USP (Process # 1511/2008).
Chemicals and reagents
Thiamine (vitamin B1) was obtained from Labsynth (Sao Paulo). EDTA was acquired from Merck (Darmstadt, Germany). RPMI-1640 medium, fetal bovine serum (FBS), and trypan blue were purchased from Gibco (Carlsbad, CA). Urethane and propidium iodide (PI) were purchased from Sigma Chemical Co. (St. Louis, MO). Dopalen (ketamine) and Anasedan (xylazine) were obtained from Vetbrands™ (Sao Paulo, Brazil). 5- (and 6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Molecular Probes (Carlsbad, CA).
Pteridium aquilinum
A voucher specimen (#MH 513) was deposited at the Laboratory of Chemistry of Natural Products, Center of Animal Health, Biological Institute of Sao Paulo. Pteridium aquilinum var. arachnoideum was identified by Dr. Jefferson Prado, a specialist in Pteridophyta identification from the Botanical Institute of Sao Paulo. P. aquilinum crosiers were collected in Pirassununga (Sao Paulo) in February 2007 and January 2009. They were frozen at −80 °C until utilized for extract preparation. The extract was prepared as previously described by Latorre et al. (Citation2009).
Urethane
Urethane is a carcinogen to rodents classified as Group 2A (i.e. most likely carcinogenic to humans) by the International Agency for Research on Cancer (IARC, Citation2010). It was previously demonstrated that repeated intraperitoneal (IP) administration of urethane (at 1 g/kg) to adult C57BL/6 mice resulted in significant increases in lung adenomas (Miller et al., Citation2003). Thus, here, the mice were treated once weekly by IP administration of urethane (at 1 g/kg) for 11 weeks to induce lung lesions.
Treatment protocol
Mice were allocated into four groups: control (Co), P. aquilinum (P), P. aquilinum and urethane (PU), and urethane (U). For suppression of NK cell activity, P. aquilinum extract (30 g/kg) was administered by daily gavage for 14 days. Mice in the Co and U groups received only water in the 14-day period. Thereafter, lung carcinogenesis was induced in mice in the U and PU groups by once-weekly IP injection of urethane (1 g/kg) for 11 weeks; mice in the Co and P groups were injected with PBS only. For mice in the P and PU groups, P. aquilinum was also administered in this period (daily gavage, 5 days/week) (); U and Co group mice only received daily gavages of PBS in this period. The same regimens were used in studies to analyze the development of lung lesions, except that a 20 week post-treatment period was adopted to permit observations to be made.
Figure 1. Treatment schedule. (A) Extract of P. aquilinum (30 g/kg/day) was administered by gavage once daily for 14 days, and then daily (5 days/week) during an 11-week period in which some mice were also treated with urethane (1 g/kg) once weekly via IP injection. (B) The same regimens were used for assessment of lung lesion development, except that an additional 20 week post-treatment period was adopted (one that contained no treatments in it) to permit observations to be made.
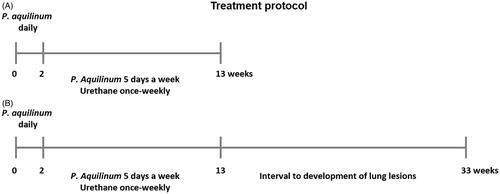
The dose of P. aquilinum extract (30 g/kg/day) used in this experiment was based on a previous study performed by our group (Latorre et al., Citation2009) and corresponded to 5.3 mg/kg of ptaquiloside as quantified in crosiers collected at the same time and place that we used in another study (Latorre et al., Citation2011). All mice were supplemented with vitamin B1 in their drinking water (10 mg/ml; as proposed by Schacham et al., Citation1970) throughout the treatment period to prevent the effects of thiaminase 1, one toxic component of P. aquilinum (Fenwick, Citation1988). Throughout the various exposures, the body weight of each mouse was measured once a week to permit dose adjustment. Food and water consumption were also measured weekly.
NK cell activity assay
At the end of the 13-week treatment period, sets of control and experimental mice (distinct from those used for the lung evaluation studies; ) were euthanized to prepare splenic cell suspensions and non-adherent splenic cell populations as previously described (Latorre et al., Citation2009). Non-adherent splenic cells were used as effector cells and YAC-1 (ATCC TIB-160, Manassas, VA) cells (mouse lymphoma cell line) as targets in the NK assays. Briefly, triplicate cell cultures for each mouse (E:T ratio = 100:1) were prepared in 96-well round-bottom plates; each well contained 5 × 105 effectors cells and 5 × 103 target cells pre-stained with CFSE. The plates were all cultured 4 h at 37 °C in a humidified atmosphere containing 5% CO2. Spontaneous target death was determined by incubating YAC-1 cells in complete RPMI medium. Propidium iodide (PI; 50 µg/ml, 2.5 µl/well) was then added to all wells to help detect dead cells, and all samples were then subjected to flow cytometry in a BD FACSCalibur system (BD Biosciences, San Jose, CA). A minimum of 5000 target cells/sample was acquired. All data were analyzed using FlowJo® software (Tree Star, Inc., Ashland, KY). The level of NK cell cytotoxicity was expressed as: Cytotoxicity (%) = 100 ×(dead targets in samples [%] − spontaneously-dead targets [%])/(100 − spontaneously-dead targets [%]).
Lung evaluation
On Week 33 of the experiment, all mice () were euthanized by IP overdosing with a combination of Dopalen (100 mg/kg) and Anasedan (10 mg/kg). Thereafter, the lungs were exposed and carefully inflated with metacarn (60% methanol, 30% chloroform, 10% acetic acid), and the nodules immediately measured and counted to calculate incidence, tumor number, and multiplicity (number of nodules/mouse that had lung nodules) per mouse. The lungs were fixed in metacarn for a maximum of 8 h and routinely processed for paraffin embedding for further analysis. The histopathological analyses were performed on representative lung sections of each lobe (5 µm) that had been stained with hematoxylin and eosin (HE). Classification of pulmonary lesions was performed in accordance with Nikitin et al. (Citation2004).
Statistical analysis
All data were analyzed using Prism 5.00® software (GraphPad Software, Inc., San Diego, CA) by one-way analysis of variance (ANOVA), followed by a Tukey’s test for multiple comparisons. The incidence of lung nodules in control and experimental mice was compared using a Fisher’s exact test. Data with two variables were analyzed by two-way ANOVA, followed by a Bonferroni’s test. Percentage data from three or more groups were compared using a Kruskal-Wallis test followed by a Dunn’s test; percentage data from two groups were compared using a Mann-Whitney test. Data were expressed as the mean [±SD] or median with range values. Differences were considered to be significant at p < 0.05.
Results
Effects of P. aquilinum and urethane treatments on weight gain as well as food and water consumption
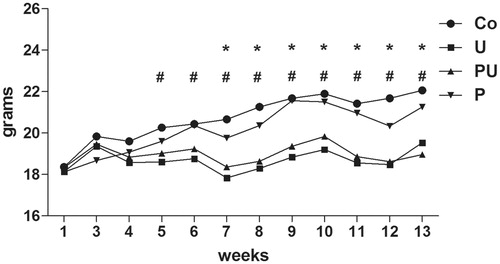
All mice treated with urethane (U and PU groups) displayed reduced body weight gain compared with the other groups as treatment duration increased (p < 0.0001 for treatment, p < 0.0001 for time and p < 0.0001 for interaction, two-way ANOVA; p < 0.05 for the Co versus U groups during Weeks 5 and 6, p < 0.001 for the Co versus U and Co versus PU groups during Weeks 7–13, Bonferroni’s post-hoc test) (). However, food consumption during the same period was not altered among groups (data not shown). On the other hand, a reduction in water consumption was noted in all mice treated with urethane (p = 0.0013, Kruskal-Wallis test; p < 0.05 for the Co versus U and p < 0.001 for the Co versus PU, Dunn’s post-hoc test) (data not shown).
Figure 2. Body weight gain of mice treated with P. aquilinum (P) (30 g/kg/day) by gavage once daily for 14 days, followed by gavage 5 dys/week during an 11-week period that was accompanied by treatment with urethane (U) (1 g/kg) via once-weekly IP injection. #p < 0.001 versus Co group, *p < 0.001 versus P group, Bonferroni’s post-hoc test. n = 5 mice/group.
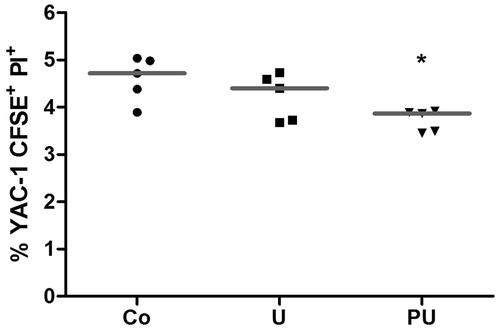
Effects of urethane treatment on NK cell cytotoxicity
To verify the effect of urethane on NK cell cytotoxicity, mice were submitted to a 13-week treatment and then splenic NK cell activities were immediately evaluated thereafter. The data illustrate that treatment with urethane did not significantly alter splenic NK cell cytotoxicity (). However, NK cell cytotoxicity was suppressed in mice treated with both P. aquilinum and urethane (PU groups) (p = 0.046, Kruskal-Wallis test; p < 0.05 for the Co versus PU, Dunn’s post-hoc test). Data from our previous studies (see Caniceiro et al., Citation2008; Latorre et al., Citation2009, Citation2011) showed that use of this same treatment regimen resulted in reductions (i.e. 60%) in NK cell cytotoxic activity to a level of 2%. As such, those particular P. aquilinum-alone studies for this one endpoint were not repeated/reported in the current study. It remains to be determined as to why there was no similar final level of NK activity (i.e. ≈2%) for the PU hosts; clearly, exposure to urethane alone was not stimulatory of NK activity.
Figure 3. Splenic NK cell cytotoxicity (median) of mice treated with P. aquilinum (P) (30 g/kg/day) by gavage once daily for 14 days, followed by gavage 5 days/week during an 11-week period that was accompanied by treatment with urethane (U) (1 g/kg) or PBS (Co) via once-weekly IP injection. *p < 0.05 versus Co group, Dunn’s post-hoc test. n = 5 mice/group.
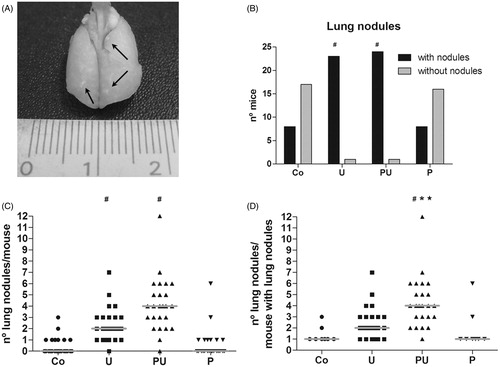
Effects of NK suppression caused by P. aquilinum on urethane-induced lung carcinogenesis
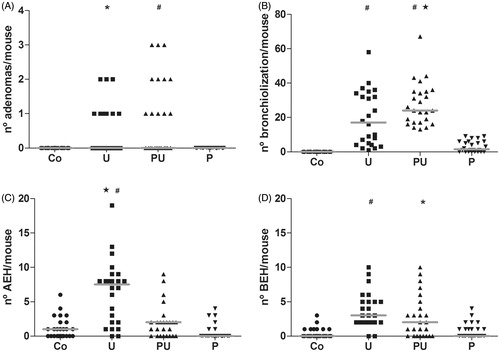
The effects of any immunosuppression induced by P. aquilinum treatment on urethane-induced lung carcinogenesis were analyzed 20 weeks after the end of the treatment period. Lesions were counted in all lung lobes upon gross examination (). A 96% incidence of lung nodules was noted in the groups of mice treated with urethane (U and PU groups); relative risk to develop lung nodules was 3-times higher in these mice than in those in the control group (p < 0.0001 and RR = 3, Fisher’s exact test; ). Unsurprisingly, the number of lung nodules was higher in mice from the group that was treated with P. aquilinum and urethane (PU groups) (; p < 0.0001, Kruskal-Wallis test; p < 0.001 for the Co versus U, Co versus PU, P versus U, and P versus PU, Dunn’s test). Multiplicity was also higher in this group (p < 0.0001, Kruskal-Wallis test; p < 0.001 for Co versus PU, p < 0.05 P versus PU, p < 0.05 U versus PU, Dunn’s test) ().
Figure 4. Nodules were observed upon gross examination of the lungs from mice that were treated with P. aquilinum (P) (30 g/kg/day) by gavage once daily for 14 days, followed by gavage 5 days/week during an 11-week period that was accompanied by treatment with urethane (U) (1 g/kg) via once-weekly IP injection. (A) Photo of representative lung with nodules (arrows). (B) Incidence of lung nodules (#p < 0.0001 and RR = 3 versus Co group, Fisher’s exact test). (C) Number of lung nodules/mouse and medians (#p < 0.001 versus Co and P groups, Dunn’s post-hoc test). (D) Multiplicity and medians (#p < 0.001 versus Co group, *p < 0.01 versus P group, and p < 0.05 versus U group, Dunn’s test). Number of mice per group was: Co = 25, U = 25, PU = 25, and P = 24.
Induction of lung carcinogenesis with urethane resulted in pre-neoplastic lesions classified as bronchiolization of alveoli (bronchiolar subvariant), bronchial epithelial hyperplasia (BEH), as well as alveolar epithelial hyperplasia (AEH) () and benign tumors (including papillary and mixed adenomas; ). Treatment with P. aquilinum associated with urethane increased the occurrence of both benign tumors and bronchiolization, which was the main pre-neoplastic lung lesion seen in the present study (). Mice in the PU group displayed a 76% increase in rate of adenomas compared with mice in the U group (p < 0.0001, one-way ANOVA; p < 0.001 for Co versus PU and P versus PU, p < 0.05 for Co versus U, and P versus U, Tukey’s post-hoc test; ), and a 41% increase in rate of bronchiolization (p < 0.0001, one-way ANOVA; p < 0.001 for Co versus U, Co versus PU, P versus U, and P versus PU, p < 0.05 for U versus PU, Tukey’s test; ). There was no significant difference in incidence of papillary and mixed sub-type adenomas (data not shown). The rate of AEH was higher in mice from the U group than in mice in the PU group (p < 0.0001, Kruskal-Wallis test; p < 0.001 for the Co versus U and P versus U, p < 0.05 for U versus PU, Dunn’s test), and the rate of BEH did not differ between the U and PU groups (p < 0.0001, Kruskal-Wallis test; p < 0.001 for Co versus U, Co versus PU and P versus U, as assessed using Dunn’s test), as shown in , respectively.
Figure 5. Urethane-induced pre-neoplastic lung lesions in mice treated during an 11-week period with urethane (1 kg/g) via once-weekly IP injection. (A) Bronchiolization (bronchiolar subvariant) (arrow). (B) Alveolar epithelial hyperplasia (arrow). (C) Bronchial epithelial hyperplasia (arrow). Bar = 50 µm.
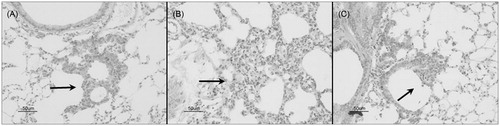
Figure 6. Urethane-induced benign lung tumors in mice treated during 11-week period with urethane (1 mg/g), via once-weekly IP injection. (A) Mixed sub-types adenoma. Bar = 50 µm. (B) Papillary adenoma. Bar = 100 µm.
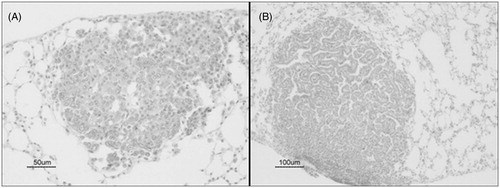
Figure 7. Urethane-induced lung lesions of mice treated with P. aquilinum (P) (30 g/kg/day) by gavage once daily for 14 days, followed by gavage 5 days/week during an 11-week period that was accompanied by treatment with urethane (U) (1 g/kg) via once-weekly IP injection. (A) Adenomas (#p < 0.001 versus Co and P groups, *p < 0.05 versus Co and P groups, Tukey’s post-hoc test) (B) Bronchiolization (#p < 0.001 versus Co and P groups and p < 0.05 versus the U group, Tukey’s test). (C) Alveolar epithelial hyperplasia (#p < 0.001 versus Co and P groups and p < 0.05 versus U group, Dunn’s post-hoc test). (D) Bronchial epithelial hyperplasia (#p < 0.001 versus Co and P groups and *p < 0.01 versus Co group, Dunn’s test). Data are presented as median and range values. Number of mice per group was: Co = 25, U = 24, PU = 25, and P = 24.
Discussion
Pteridium aquilinum is considered one of the most important toxic plants in the world as it is responsible for a variety of toxic syndromes in herbivores and cancer development in animals and humans. Experimentally, the reduction of natural killer (NK) cell cytotoxicity and interferon [IFN]-γ production in mice was also verified (Gil da Costa et al., Citation2012). It is well established that ptaquiloside is the main bracken carcinogen (Hirono, Citation1986), producing this effect via initial DNA damage (Yamada et al., Citation2007). Likewise, this compound can suppress some aspects of the cellular immune response (Latorre et al., Citation2009) and, as such, dampening immunosurveillance against cancers (Momburg et al., Citation2013). In the present work, we demonstrate that suppression of NK cell cytotoxicity caused by P. aquilinum resulted in enhanced susceptibility to cancer development – as indicated by a higher number of benign tumors and pre-neoplastic lung lesions in mice treated with both plant and carcinogen (urethane) than in mice given only urethane.
NK cells play an important role in innate immunity against infectious diseases and in cancer immunosurveillance by killing, respectively, infected and cancerous cells (Momburg et al., Citation2013). Suppression of NK cell activity has already been related to the escape of tumoral cells and, consequently, cancer growth (Zitvogel et al., Citation2006). Thus, it is plausible to assume that any P. aquilinum-induced suppression of NK cell cytotoxicity might increase a host’s risk for cancer development. To address this hypothesis, urethane (1 g/kg) was injected IP into test mice once weekly for 11 weeks, based on a murine model of induction of lung cancer (Miller et al., Citation2003). In keeping with that model, we observed a relative risk to develop lung nodules that was 3-times higher in urethane-treated mice compared to in control mice.
Urethane is classified as a Group 2A possible human carcinogen IARC (Citation2010) and is used in laboratory animals as an anesthetic (Kotanidou et al., Citation1996; Norlén et al., Citation2000). Thus, it is likely that the alterations noted in weight gain and water consumption among the urethane-treated mice here were a result of this anesthetic effect. Urethane is also a known immunosuppressant. Indeed, it was previously demonstrated that daily administration of urethane at 1, 2, or 4 g/kg for 14 days led to reduced NK cell cytotoxicity in female B6C3F1 mice (Luster et al., Citation1982). Therefore, to verify if urethane caused reductions in NK cytotoxicity in our C57BL/6 mice, an outcome that could ultimately be a synergistic effect to that from P. aquilinum, NK activity was evaluated after an 11-week urethane treatment period (1 g/kg; once-weekly, IP). We observed that urethane at this dose and after this period of treatment did not reduce NK cytotoxicity. As such, urethane is a proper model for evaluating what contributions any immunosuppressive effects of P. aquilinum could impart on carcinogenesis processes in situ.
At the end of the 33-week experimental period, when macroscopic evaluations of the lungs were conducted, it was seen that there were a greater number of macroscopic lung lesions and in tumor multiplicity in the group that had been treated with P. aquilinum and urethane (PU group). This is what we had expected based on the immunosuppressive effects of this plant on NK cell cytotoxicity (Caniceiro et al., Citation2008; Latorre et al., Citation2009, Citation2011). In those earlier studies (not repeated here), it was seen that treatment with P. aquilinum extract (at 30 g/kg/day) alone for up to 13 weeks led to significant reductions (i.e. 60%) in NK cell activity among splenocytes of treated hosts.
Corroborating our hypothesis here, a recent study conducted by Kreisel et al. (Citation2012) indicated that only the suppression of NK cell activity contributes to an increase in incidence of urethane-induced lung lesions in the C57BL/6 mouse strain, the strain used here. Furthermore, an association was also demonstrated between suppression of NK cell activities and a higher risk of developing head and neck cancers in humans, as well as with a higher incidence of 3-methylcholanthrene-induced sarcomas in mice (Accomando et al., Citation2012; O’Sullivan et al., Citation2012).
When histologic analyses of the lungs were performed here, it was noted that mice treated with both urethane and P. aquilinum displayed higher rates of adenoma and bronchiolization of their alveoli than did mice treated only with urethane. The bronchiolization may indicate an early stage of adenoma formation (Nikitin et al., Citation2004); this highlights the important role that NK cells have in cancer immunoediting and in host protection against cancers.
Conclusions
In summary, the present study provided evidence that P. aquilinum-induced suppression of NK cytotoxicity increases host susceptibility to urethane-induced lung carcinogenesis in C57BL/6 mice. To our knowledge, this is the first study to support the idea that immunosuppressive effects of P. aquilinum could increase the risk of cancer formation in carcinogen-exposed hosts. It remains to be determined if this is also the case for cancers that could be induced by the plant itself (i.e. due to ptaquiloside).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work was supported by grants #2008/50073-6 to B.D.C. and #2007/50313-4 to A.O. L., São Paulo Research Foundation (FAPESP).
| Abbreviations | ||
| ANOVA, | = | analyses of variance; |
| CFSE, | = | 5-(and 6-)-carboxyfluorescein diacetate succinimidyl ester; |
| EDTA, | = | Ethylenediamine tetraacetic acid; |
| FBS, | = | fetal bovine serum; |
| HE, | = | hematoxylin and eosin; |
| NK, | = | natural killer; |
| PBS, | = | phosphate-buffered saline; |
| PI, | = | propidium iodide |
Acknowledgements
We thank Harry Leo Wysocki Jr. (Biological Institute of Sao Paulo, Sao Paulo, SP, Brazil) and Priscila Sales Maldonado (School of Animal Sciences and Food Engineering, University of São Paulo, Pirassununga, SP, Brazil) for their collaboration.
References
- Abnet, C. C. 2007. Carcinogenic food contaminants. Cancer Invest. 25:189–196
- Accomando, W. P., Wiencke, J. K., Houseman, E. A., et al. 2012. Decreased NK cells in patients with head and neck cancer determined in archival DNA. Clin. Cancer Res. 18:6147–6154
- Alonso-Amelot, M. E., and Avendano, M. 2002. Human carcinogenesis and bracken fern: A review of the evidence. Curr. Med. Chem. 9:675–686
- Brandstadter, J. D., and Yang, Y. 2011. Natural killer cell responses to viral infection. J. Innate Immun. 3:274–279
- Caniceiro, B. D., Latorre, A. O., Fukumasu, H., et al. 2008. Study of the immunotoxicity induced by Pteridium aquilinum during pulmonary carcinogenesis induced in mice. In: Proceedings of the XVII Semana Científica do Departamento de Patologia, São Paulo, Brazil, October 2008
- Colosio, C., Birindelli, S., Corsini, E., et al. 2004. Low-level exposure to chemicals and immune system. Toxicol. Appl. Pharmacol. 197:146–147
- Diefenbach, A., and Raulet, D. H. 2002. The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol. Rev. 188:9–21
- Drela, N. 2006. Xenobiotic-induced alterations in thymocyte development. APMIS 114:399–419
- Fenwick, G. R. 1988. Bracken (Pteridium aquilinum) - Toxic effects and toxic constituents. J. Sci. Food Agric. 46:147–173
- Fletcher, M. T., Reichmann, K. G., Brock, I. J., et al. 2011. Residue potential of norsesquiterpene glycosides in tissues of cattle fed austral bracken (Pteridium esculentum). J. Agric. Food Chem. 59:8518–8523
- Gil da Costa, R. M., Bastos, M. M., Oliveira, P. A., and Lopes, C. 2012. Bracken-associated human and animal health hazards: Chemical, biological and pathological evidence. J. Haz. Mater. 203:1–12
- Hirono, I. 1986. Carcinogenic principles isolated from bracken fern. Crit. Rev. Toxicol. 17:1–22
- IARC (International Agency for Research on Cancer). 2010. Ethyl carbamate (urethane). IARC monographs on the evaluation of carcinogenic risks to humans. Available online at: http://monographs.iarc.fr/ENG/Monographs/vol96/mono96.pdf, accessed 2010
- Kotanidou, A., Choi, A. M., Winchurch, R. A., et al. 1996. Urethane anesthesia protects rats against lethal endotoxemia and reduces TNFα release. J. Appl. Physiol. 81:2305–2311
- Kreisel, D., Gelman, A. E., Higashikubo, R., et al. 2012. Strain-specific variation in murine natural killer gene complex contributes to differences in immunosurveillance for urethane-induced lung cancer. Cancer Res. 72:4311–4317
- Latorre, A. O., Canciero, B. D., Wysocki, H. L. Jr., et al. 2011. Selenium reverses Pteridium aquilinum-induced immunotoxic effects. Food Chem. Toxicol. 49:464–470
- Latorre, A. O., Furlan, M. S., Sakai, M., et al. 2009. Immunomodulatory effects of Pteridium aquilinum on natural killer cell activity and select aspects of the cellular immune response of mice. J. Immunotoxicol. 6:104–114
- Luster, M. I., Dean, J. H., Boorman, G. A., et al. 1982. Immune functions in methyl and ethyl carbamate-treated mice. Clin. Exp. Immunol. 50:223–230
- Miller, Y. E., Dwyer-Nield, L. D., et al. 2003. Induction of a high incidence of lung tumors in C57BL/6 mice with multiple ethyl carbamate injections. Cancer Lett. 198:139–144
- Momburg, F., Watzl, C., and Cerwenka, A. 2013. NK cells - versatile tools for viral defense and cancer treatment. Eur. J. Immunol. 43:860–863
- Nikitin, A. Y., Alcaraz, A., Anver, M. R., et al. 2004. Classification of proliferative pulmonary lesions of the mouse: Recommendations of the mouse models of human cancers consortium. Cancer Res. 64:2307–2316
- Norlen, P., Kitano, M., Lindstrom, E., and Hakanson, R. 2000. Anesthetic agents inhibit gastrin-stimulated but not basal histamine release from rat stomach ECL cells. Br. J. Pharmacol. 130:725–730
- O'Sullivan, T., Saddawi-Konefka, R., Vermi, W., et al. 2012. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med. 209:1869–1882
- Rasmussen, L. H., Kroghsbo, S., Frisvad, J. C., and Hansen, H. C. B. 2003. Occurrence of the carcinogenic bracken constituent ptaquiloside in fronds, topsoils, and organic soil layers in Denmark. Chemosphere 51:117–127
- Schacham, P., Philp, R. B., and Gowdey, C. W. 1970. Anti-hematopoietic and carcinogenic effects of bracken fern (Pteridium aquilinum) in rats. Am. J. Vet. Res. 31:191–197
- Shahin, M., Smith, B. L., and Prakash, A. S. 1999. Bracken carcinogens in the human diet. Mutat. Res. 443:69–79
- Smith, B. L. 2004. Bracken Fern (genus Pteridium) Toxicity - a Global Problem. In: Poisonous Plants and Related Toxins (Acamovic, T., Stewart, C. S., and Pennycott, T. W., Eds.). Cambridge, UK: CAB International, pp. 227–240
- Sugimura, T. 2000. Nutrition and dietary carcinogens. Carcinogenesis 21:387–395
- Trinchieri, G. 1989. Biology of natural killer cells. Adv. Immunol. 47:187–376
- Whiteside, T. L. 2006. Immune suppression in cancer: Effects on immune cells, mechanisms, and future therapeutic intervention. Semin. Cancer Biol. 16:3–15
- Yamada, K., Ojika, M., and Kigoshi, H. 2007. Ptaquiloside, the major toxin of bracken, and related terpene glycosides: Chemistry, biology and ecology. Nat. Prod. Rep. 24:798–813
- Zitvogel, L., Tesniere, A., and Kroemer, G. 2006. Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nat. Rev. Immunol. 6:715–727

