Abstract
A Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) case was maintained in remission with the use of chemo-immunotherapy. The latter involved sibling bone marrow transplant (BMT) (three procedures) followed by intravenous (IV) donor lymphocyte infusion (DLI). The third relapse responded to routine chemotherapy and again DLI was employed. During hematological and molecular remission verified at the level of iliac crest aspiration, extra-medullary relapse in the bones was apparent. A novel procedure of donor lymphocyte injection to the bone leukemic lesions was developed and employed. A dose of 106 donor lymphocytes/kg body weight (BW) of the recipient were each time injected to the plane of the right and left tibia, the head of the humerus, and the calcaneus, which resulted in healing of the destructive process. In consequence of this novel approach, in addition to the healing of bone lesions, an accumulation of cytotoxic activated T-cells in the marrow was documented, which was mirrored by an increase in the number of transcripts for interferon (IFN)-γ, interleukin (IL)-17, as well as RORγt. The local administration of DLI directly to the leukemic lesions requires a lower dose that diminishes the toxicity due to the general immune system activation.
Introduction
The pioneering work of Hans Kolb showed that infusion of donor lymphocytes (DLI) to patients relapsing with chronic myelogenous leukemia (CML) after allogeneic hematopoietic stem cell transplantation (alloHSCT) has a curative potential (Kolb et al., Citation1995; van Rhee & Kolb, Citation1995). This was interpreted as a graft versus leukemia (GvL) effect. In CML cases cells with a hybrid gene (bcr/abl) product p210 positive effect of DLI were frequently observed. The GvL effect is also sometimes seen in acute myeloid leukemia (Schmid et al., Citation2007), in which recognized epitope(s) may differ from case to case. Infused lymphocytes in addition to leukemia-associated antigens may recognize normal body constituents, which makes this approach toxic and the array of the activity depends on the dose used (Blair et al., Citation2005; Kircher et al., Citation2002). Consequently, a life-threatening acute graft versus host disease (aGvHD) may be clinically apparent.
The present study was based on a Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) (Ph+ ALL) case with a previous history of a positive response to DLI during relapses after alloHSCT. The patient presented with leukemic bone infiltrations and received donor lymphocytes directly to the leukemic bone lesions. The aim of this approach was to facilitate direct contact between donor lymphocytes and host leukemic cells, with two aims: (i) to increase the rate of cytotoxic lymphocyte attack on leukemia, and (ii) to propagate in vivo specific cells recognizing leukemia-associated antigen(s). For that, a bone infusion gun was employed to deliver the donor lymphocytes at the site in bones that were affected by leukemia. This novel approach appeared to be effective in a local anti-leukemic effect and resulted in the presence of a high number of activated cytotoxic lymphocytes in the marrow.
Materials and methods
Evaluation of effectiveness of intra-bone (IB) DLI
To evaluate the effectiveness of the intra-bone (IB) DLI protocol, the following methods were used. To localize leukemic infiltrations, positron emission tomography and magnetic resonance imaging (detailed in ) was employed. To phenotype marrow cell populations, 3-color flow cytometry (FACSCalibur, BD, Mountain View, CA) after staining with monoclonal antibodies (mAb) to human CD45, CD10, CD19, CD3, CD28, HLADR, CD56, and/or Perforin A (all BD, Erembodegen, Belgium) was performed using routine procedures (Dlubek et al., Citation2006). In addition, trephine biopsy staining was used for determination of lymphocyte sub-sets (CD8, CD56, and CD3) and for blast cells including Ki67 positivity as was detailed elsewhere (Klimczak & Lange, Citation2000). Molecular karyotyping, fluorescence in situ hybridization (FISH) analysis (9;22 translocation), and detailed enumeration of e1a2 bcr/abl transcripts were done using a standardized RT-PCR approach, as outlined in Baccarani et al. (Citation2009) and Guilhot et al. (Citation2012). Marrow cell gene expression analysis of interferon (IFN)-γ, interleukin (IL)-17, RORγt, and Notch1 was performed using real-time PCR (primers and probes were purchased from Life Technologies, Foster City, CA); all expression levels were normalized with respect to four housekeeping genes (i.e. β-actin, ABL, HPRT, and β2M) and further normalized to reference marrow cell RNA (Clontech, Mountain View, CA).
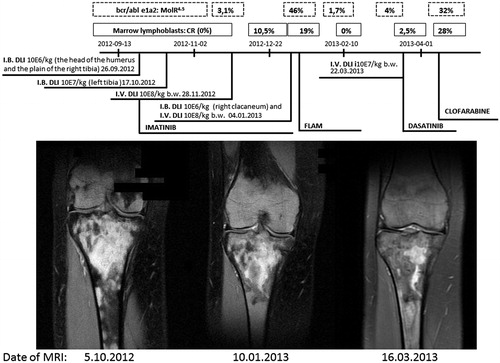
Figure 1. Magnetic resonance imaging: coronal proton density fat saturation image (Cor PD-fs FSE) (normal bone is grey while that infiltrated and vascularized becomes bright; fat signal suppression improves the contrast in fat-rich tissues). Note the nodular pattern of signal and decrease in signal intensity along intra-bone and intravenous DLI procedure (106 MNC/kg BW separated from a primary HSCT donor) using a Cobe Spectra continuous flow separator (COBE BCT inc., Blood Component Technology, Lakewood, CO). Above, the clinical data are provided.
Patient
A 14-year-old girl was referred to the Lower Silesian Center for Cellular Transplantation with National Bone Marrow Donor Registry (Wroclaw) for alloHSCT as consolidation of remission of her ALL. At the diagnosis, she was Philadelphia chromosome positive (Ph+). A more-detailed analysis performed in 2005 revealed a mosaic karyotype: i.e. 46,XX,t(9;22)(q34;q11.2)[27]/51,XX,+X,+5,t(9;22)(q34;q11.2),+?del(10)(q22),+20,+21[23]. A FISH analysis showed 63% bcr/abl positive inter-phase nuclei. Prior to the first HSCT procedure (in 2006), the patient presented with 4% lymphoblasts in the marrow with a phenotype consistent with a common ALL (CD10+, CD19+ with CD33 positivity as an aberrant expression).
Past history of leukemia
After 2 years, a hematological relapse was noted that resulted in a second alloHSCT (in 2008) from the same sibling donor. Due to a presence of 3% lymphoblasts and a lack of any symptoms of aGvHD to substantiate the results of alloHSCT, 3-times donor lymphocytes were intravenously (IV) infused in, respectively, doses of 106, 107, and 108 cells/kg BW, at 1-month intervals. In addition, Imatinib was administered in a daily dose of 600 mg; this approach resulted in eradication of lymphoblasts. Notably, the second complete remission lasted for 2 years 3 months. By the end of this period, a routine molecular surveillance of molecular residual disease revealed that bcr/abl e1/a2 transcripts rose to 2.6% and soon thereafter to 38%, with a concomitant hematological relapse (53% blast cells in marrow). Chemotherapy was applied, i.e. vincristine (1.5 mg/m2) and epirubicin (50 mg/m2), followed by three intra-thecal infusions of methotrexate (15 mg), cytosine arabinoside (40 mg), and dexamethasone (4 mg). Molecular remission was achieved (bcr/abl e1/a2 MolR4.5). Chemotherapy was followed by the second course of three IV DLI given in 1-month intervals. DLI started at 4% blasts in the marrow and resulted in complete eradication of blasts and achievement of complete molecular remission, which was stable at the marrow level for 11 months thereafter.
Intra-bone (IB) DLI
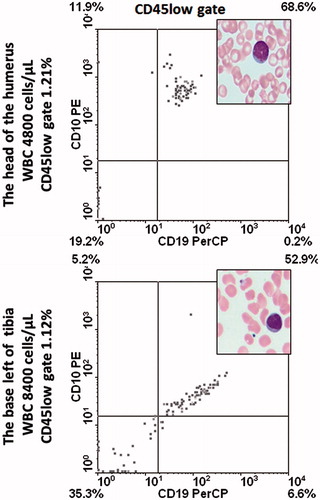
During hematological remission (normal blood and marrow morphology and smears), the subject began to complain of bone pain in both legs and in the right humerus. Positron emission tomography showed a high standardized uptake value (SUV) in the head of the right humerus (left) and in the right tibial base. Having the marrow in remission, the idea arose to deliver donor lymphocytes directly into the areas of the bone lesions. For that, a Bone Injection Gun (15-G, 33.3 mm [1.31’ Trocar/Luer Lock Needle]; Waismed, Herzliya, Israel) was used. At first, after obtaining written consent from the patient, and approval of the procedure by the Institutional Ethics Committee, donor lymphocytes were to be infused into the right tibia plane and the head of the humerus via a needle shot. A pre-treatment aspiration biopsy taken from the targeted bones revealed a presence of blast cells (). Donor cells (at 106/kg BW) were then infused to the plane of the right tibia and to the head of the right humerus. The effect was promptly noted—pain ceased. Further, an iliac crest aspiration biopsy was negative for blasts and bcr/abl e1a2 transcripts.
Results
Morphology
The effects of the treatment regimens were followed by sequential MRI scans (). MRI scans taken at the beginning of the treatment revealed bone destructive lesions, widespread vascularized areas, and local edema. After completion of the treatment, a substantial improvement was documented, with only a few small spots of vascularization and edema being evident at the same sites. Similarly, healing of bone destruction was seen in the head of the right humerus after the local IB DLI. The patient was still in hematological remission. After 1 month, the same process was done with the left tibia and then the right calcaneus. During a 4-month period, four IB DLI were performed, each time with a Bone Injection Gun, with clinical improvement and without any side-effects, in particular aGvHD symptoms. At that time, two courses of IV DLI were also employed (between and after the last IB DLI).
Leukemia progresses in the marrow
In spite of the relief of bone symptoms (i.e. the patient was no longer prevented from walking due to pain), the leukemia progressed in the marrow. This ultimately led to death of the patient; she did not survive the third alloHSCT attempt in 2013.
Immunological observations during IB DLI
Phenotype analyses
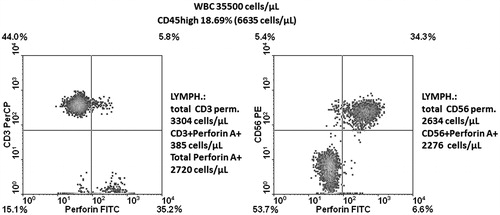
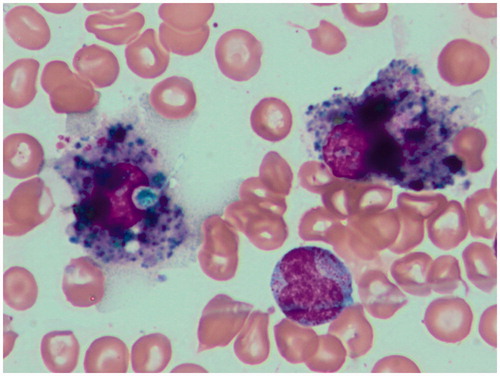
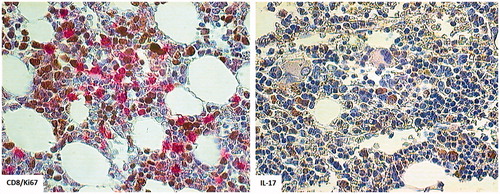
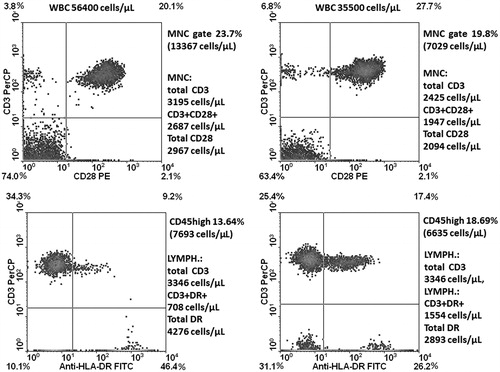
Proportions of lymphocytes in the marrow along the observation time since achieving CR (years 2011/2012 after the second course of three IV DLI) exceeded in all marrow aspirates (median cellularity: 49 400 cells/µl) 15% (median: 20.12%). It was also at the time of IB DLI delivery. A lymphocyte sub-population study showed that the contribution of total CD3+ as well as those with activation markers increased in the time span covering the IB DLI program (CD3+ = 23.9–34.5%, CD3+DR+ = 9.2–17.4%, CD3+CD28+ = 20.1–27.7%; ). At that time, the disease was brought under molecular and hematological remission. The second population in the marrow lymphocyte compartment comprised CD56+ cells that were in the vast majority (86%) perforin+ (). Altogether 40.1% of lymphocytes were perforin+ at 4 months after initiation of the IB DL infusions. Routine May-Grunwald/Giemsa staining of bone marrow smears performed after two IB DLI revealed an increased proportion of lymphocytes and macrophages, some of which appeared to have been actively phagocytizing cellular debris (). Trephine biopsy taken at the relapse starting coinciding with the last i.b. DLI bone injection showed CD8+ lymphocyte cell clusters and numerous CD56+ cells in close proximity to Ki67+ cells (). Notably, IL-17-producing cells were also observed; this finding was substantiated by the presence of an increase in IL-17 gene transcripts in the marrow (see below for details).
Figure 3. Marrow cell profiling for CD3+ lymphocytes and those CD28+ or HLADR+. Left panels represent analysis made at the time of IB DLI; right ones after completion of this therapy, just prior to the relapse.
Molecular analyses
Basic values of IFNγ, IL-17, RORγt, and Notch1 transcripts in patient marrow (collected at remission seen after 4 months free of chemotherapy, and expressed as normalized relative values [NRV]) were as follows: IFNγ: 0.842, RORγt: 0.323, CXCL10: 0.029, Notch1: 0.572, close to the reference preparation of bone marrow total RNA. There was a lack of IL-17 transcripts at that time. The next determination made 1 month after two IB DLI showed an increase in the number of IFNγ mRNA (NRV: 2.363). One week after completion of DLI (three IB and one IV), the number of IFNγ transcripts decreased, only to become elevated again 8 weeks later (NRV: 0.262 versus 1.358) and IL-17 transcripts for the first time appeared (NRV: 0.166), which was associated with an increase of RORγt mRNA (NRV: 0.310 versus 0.478). Notably, the highest values of IFNγ (NRV: 2.690), CXCL10 (NRV: 0.185) and IL-17 transcripts (NRV: 0.452) and those of RORγt (NRV: 3.022) were noted at the time of an overt hematological relapse. Notch1 transcripts, being in reference value range at the remission, increased at the relapse (NRV: 0.438 versus 1.694).
Discussion
Surveillance of neoplastic cells by the immune system keeps the proliferation down, provided there is (i) recognition of the cancer cell associated antigen by the immune system, and (ii) prevalence of pro-inflammatory over immunosuppressive cytokines and cellular milieu at the site. Hematopoietic stem cell transplantation from alloreactive donors facilitates the recognition of cancer cells if they harbor recipient epitopes that can be targeted by donor lymphocytes.
In this regard, the current study dealt with a patient having Ph+ acute lymphoblastic leukemia (Ph+ ALL). It is known that 9;22 translocation results in unlimited activation of tyrosine kinase activity, promoting cell proliferation (Kurzrock et al., Citation2003); as we learned from the CML study, Ph+ leukemic clones may be sensitive to IV DLI given post-transplant. In this study, these two characteristic features were considered during the planning of the patient’s treatment. The patient was on imatinib and, when she relapsed, on dasatinib (Mustjoki et al., Citation2013); DLI was also given. Notably, it was seen that an IV DLI after re-inducing remission chemotherapy was associated with a 2-year+ remission. A recent study showed that the positive effect of IV DLI was significantly associated with a presence of chronic GvHD (Terwey et al., Citation2013). This was not the case in the current single case observation. Here, the patient lacked any GvHD, which supports the notion that these two reactivities can be split. Remission induced by chemotherapy and IV DLI lasted 4 years. After this period, an extra-medullary relapse in the patient became clinically apparent. This is a rather rare situation but has been reported in the literature (see Kamimura et al., Citation2012; Yan et al., Citation2013).
The use of PET and MRI helped to detail localization of the lesions. Having these data in hand, it was possible to deliver donor lymphocytes directly to leukemic infiltrations so as to confront donor lymphocytes with the leukemic cells. For that, donor lymphocytes were infused directly into the bone lesions via needle shot to the site using a Bone Injection Gun, a safe procedure. The Bone Injection Gun is an accepted tool that enables infusions into the marrow cavity and so was used here as a novel approach for a local DLI. The effect of the treatment was prompt; the patient became pain-free and could walk. MRI analyses documented that the destructive vascularized and edematous lesions in the region regressed strikingly. The same outcome was seen in the tibia as well as in the head of the humerus. In both these localizations, leukemic cells were identified. Therefore, donor lymphocytes already known to react with the recipient’s leukemic cells (long-lasting IV DLI-induced remission) and being exposed to blasts could expand locally. This assumption was in line with the recent report of Warren & Deeg (Citation2013) suggesting that the GvL effect can be augmented by specific stimulation of cells directed against leukemia.
The patient enjoyed on two occasions a long-lasting remission when the chemotherapy was followed by DLI. This treatment was not accompanied by any aGvHD symptoms. The patient was bcr/abl-positive. The positive effect of DLI in Ph+ ALL was recently documented by Eefting et al. (Citation2014). DLI effectivity is usually accompanied by aGvHD, but this was not the case in our patient and also in some CML patients (Kolb et al., Citation1995). This might be due to the presence of Minor Histocompatibility Antigen (MiHA) exclusively on hematopoietic cells but not other cells usually targeted during aGvHD (van der Zouwen et al., Citation2012). In general, the risk of developing aGvHD after sequential infusion of donor lymphocytes frequently increases. The germinal studies of the Ikehara group (Fukui et al., Citation2007; Ikehara, Citation2007; Miyake et al., Citation2009) documented that IB DLI was associated with a lower risk of aGvHD but still having the potential to evoke GvL responses. From the present study we cannot judge whether IV or IB DLI was more effective in healing bone lesions. A recent paper by Maldonado et al. (Citation2014) showed that intramuscular vaccination against HPV antigens led to proliferation of T-cells, due to the recognition of cognate antigens used for vaccination, at the site of neoplastic lesions caused by HPV. Our observations indicated that lymphocytes injected at the site of leukemia infiltration favored direct cell-to-cell contact, facilitating leukemia cell-associated antigen(s) recognition by CD8+ cells, followed by proliferation.
Based on the findings, it was our understanding that T-cells injected into the bone lesions responded to the leukemia and – after being activated – could migrate after local expansion (T-cells respond to an antigen they recognize in an autonomous fashion) to marrow cavities, including that in the iliac crest when confrontation between the leukemic cells and the immune system takes place. In the marrow, CXCL10 transcripts (whose presence confirms local IFNγ production) were seen in the cells of the study patient. The marrow became colonized by cytotoxic perforin+ CD8+ lymphocytes and an increase of IFNγ transcripts was found. Notably, the highest number of IFNγ and of IL-17 transcripts, as well as a dense infiltration into the marrow with CD8+ and IL-17-producing cells were evident at the overt hematological relapse. Cytotoxic lymphocytes were also observed to be in cell-to-cell contact (). Marrow smears revealed macrophages phagocytizing cellular remnants (). The local immune response was confirmed as well using molecular and phenotype analyses. The results here were similar to those described by van der Voort et al. (Citation2013), who found that CD8+ effector T-cells expressing inflammatory homing receptors, after DLI, were recruited to multiple myeloma-bearing bone marrow (BM) in murine hosts.
Conclusions
In this study, we presented for the first time the process of DLI directly into leukemic lesions in a human. This attempt was preceded by an experimental study by the Ikehara group (Fukui et al., Citation2007; Ikehara, Citation2007) that showed that IB DLI was superior to an IV approach and yielded a lower risk of aGvHD – while preserving an anti-tumor effect (Miyake et al., Citation2009; Suzuki et al., Citation2005). In the approach used here, direct contact between lymphocytes and the target blast cells was made possible. Lymphocyte-blast cell interactions resulted in healing of bone destructive processes and lymphocytes responding to the leukemia likely expanded at the site and showed inflammatory-guided migration. In addition, the cells found in the marrow were in close proximity to the leukemia. Thus, the targeted IB DLI approach presented in this study, if further exploited, could both provide new information and open new possibilities to expand, in vivo, T-cells specific against leukemia, ultimately boosting a host’s level of immunosurveillance against this particular malignancy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was supported by the National Centre for Research grant N N402 430039 and the National Centre for Research and Development grant ‘CellsTherapy’.
References
- Baccarani, M., Cortes, J., Pane, F., et al; European LeukemiaNet. 2009. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 27:6041–6051
- Blair, A., Goulden, N. J., Libri, N. A., et al. 2005. Immunotherapeutic strategies in acute lymphoblastic leukemia relapsing after stem cell transplantation. Blood Rev. 19:289–300
- Dlubek, D., Drabczak-Skrzypek, D., and Lange, A. 2006. Low CXCR4 membrane expression on CD34+ cells characterizes cells mobilized to blood. Bone Marrow Transplant. 37:19–23
- Eefting, M., Halkes, C. J., de Wreede, L. C., et al. 2014. Myeloablative T-cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. 49:287–291
- Fukui, J., Inaba, M., Ueda, Y., et al. 2007. Prevention of graft-versus-host disease by intra-bone marrow injection of donor T-cells. Stem Cells 25:1595–1601
- Guilhot, J., Baccarani, M., Clark, R. E., et al. 2012. Definitions, methodological, and statistical issues for Phase 3 clinical trials in chronic myeloid leukemia: A proposal by the European Leukemia Net. Blood 119:5963–5971
- Ikehara, S. 2007. Innovative BMT methods for intractable diseases. Immunol. Res. 38:251–260
- Kamimura, T., Miyamoto, T., Kawano, N., et al. 2012. Successful treatment by donor lymphocyte infusion of adult T-cell leukemia/lymphoma relapse following allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 95:725–730
- Kircher, B., Stevanovic, S., Urbanek, M., et al. 2002. Induction of HA-1-specific cytotoxic T-cell clones parallels the therapeutic effect of donor lymphocyte infusion. Br. J. Hematol. 117:935–939
- Klimczak, A., and Lange, A. 2000. Apoptosis of keratinocytes is associated with infiltration of CD8+ lymphocytes and accumulation of Ki67 antigen. Bone Marrow Transplant. 26:1077–1082
- Kolb, H. J., Schattenberg, A., Goldman, J. M., et al; European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. 1995. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow-grafted patients 1995. Blood 86:2041–2050
- Kurzrock, R., Kantarjian, H. M., Druker, B. J., and Talpaz, M. 2003. Philadelphia chromosome-positive leukemias: From basic mechanisms to molecular therapeutics. Ann. Intern. Med. 138:819–830
- Maldonado, L., Teague, J. E., Morrow, M. P., et al. 2014. Intramuscular therapeutic vaccination targeting HPV16 induces T-cell responses that localize in mucosal lesions. Sci. Transl. Med. 29:6:221ra13
- Miyake, T., Hosaka, N., Cui, W., et al. 2009. Adult thymus transplantation with allogeneic intra-bone marrow-bone marrow transplantation from same donor induces high thymopoiesis, mild graft-versus-host reaction, and strong graft-versus-tumor effects. Immunology 126:552–564
- Mustjoki, S., Auvinen, K., Kreutzman, A., et al. 2013. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia 27:914–924
- Schmid, C., Labopin, M., Nagler, A., et al; EBMT Acute Leukemia Working Party. 2007. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. 25:4938–4945
- Suzuki, Y., Adachi, Y., Minamino, K., et al. 2005. A new strategy for treatment of malignant tumor: Intra-bone marrow-bone marrow transplantation plus CD4- donor lymphocyte infusion. Stem Cells 23:365–370
- Terwey, T. H., Le Duc, T. M., Hemmati, P. G., et al. 2013. NIH-defined graft-versus-host disease and evidence for a potent graft-versus-leukemia effect in patients with acute lymphoblastic leukemia. Ann. Oncol. 24:1363–1370
- van der Voort, R., Volman, T. J., Verweij, V., et al. 2013. Homing characteristics of donor T-cells after experimental allogeneic bone marrow transplantation and post-transplantation therapy for multiple myeloma. Biol. Blood Marrow Transplant. 19:378–386
- van der Zouwen, B., Kruisselbrink, A. B., Jordanova, E. S., et al. 2012. Alloreactive effector T-cells require the local formation of a pro-inflammatory environment to allow crosstalk and high avidity interaction with non-hematopoietic tissues to induce GVHD reactivity. Biol. Blood Marrow Transplant. 18:1353–1367
- van Rhee, F., and Kolb, H. J. 1995. Donor leukocyte transfusions for leukemic relapse. Curr. Opin. Hematol. 2:423–430
- Warren, E. H., and Deeg, H. J. 2013. Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies. Tissue Antigens 81:183–193
- Yan, C. H., Wang, J. Z., Liu, D. H., et al. 2013. Chemotherapy followed by modified donor lymphocyte infusion as a treatment for relapsed acute leukemia after haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: Superior outcomes compared with chemotherapy alone and an analysis of prognostic factors. Eur. J. Hematol. 91:304–314