Abstract
Previous studies have shown that occupational lead (Pb) exposure might influence human T-lymphocyte function, including such as changes in T-cell receptor (TCR) Vβ and Vγ repertoire and in expression of the TCRζ gene. Thus, the study here further investigated expression of TCRζ-related factors and the FcɛRIγ gene (whose product has a functional role complementary to the TCRζ chain) and the Elf-1 gene whose product is involved in regulation of TCR expression. Quantitative real-time RT-PCR was used to measure expression of TCRζ, FcɛRIγ, and Elf-1 genes in peripheral blood mononuclear cells (PBMC) isolated from 17 Pb-exposed workers. Samples were collected before and after the workers had undergone chelation therapy regimens. Twenty-three healthy individuals served as controls. The results showed that TCRζ, FcɛRIγ, and Elf-1 gene expression in Pb-exposed workers before chelation therapy was significantly lower than in PBMC from healthy individuals. After chelation therapy, expression of TCRζ appeared to trend toward normal levels; in comparison, lower expressions of FcɛRIγ and Elf-1 persisted. In conclusion, the previously-documented impairment of T-lymphocyte functions and T- lymphocyte-mediated immune responses seen previously in response to occupational Pb exposure might be attributable, in part, to effects on TCR signaling pathways - including those related to TCRζ and FcɛRIγ - and to any down-regulation of membrane TCRζ expression/activity that might be associated with Pb-induced effects on Elf-1 expression.
Introduction
The heavy metal lead (Pb) is a widespread environmental pollutant. Pb poisoning occurring in humans either due to occupation or environment has become a great public problem. Pb is toxic to many organ systems including the immune system. The toxicity of Pb leading to immuno-suppression/-dysregulation may consequently cause allergy or autoimmunity (Fischbein et al., Citation1993; Sata et al., Citation1998). In experimental animals, Pb has been shown to target macrophages and T-lymphocytes with low–moderate levels of exposure (Chen et al., Citation1977; Guo et al., Citation1996; Hsiao et al., Citation2011; McCabe & Lawrence, Citation1991). In occupational Pb-exposed workers, Pb has been reported to lead to changes in T-lymphocyte sub-sets and the response to T-lymphocyte mitogens (Mishra et al., Citation2003; Pinkerton et al., Citation1998; Undeger et al., Citation1996). Our previous studies also showed that occupational Pb exposure has an influence on human T-lymphocyte functions such as changes in the T-cell receptor (TCR) Vβ and Vγ repertoire and the expression level of the genes coding for CD3ζ, T-bet, and GATA-3 (Liu et al., Citation2009, Citation2011, Citation2012; Niu et al., Citation2012).
When a T-lymphocyte encounters antigens via the TCR, information about the quantity and quality of antigen engagement is relayed to intracellular signal transduction machinery. The TCR has a short intracellular sequence that lacks signaling capacity, and the CD3 molecule contains immuno-receptor tyrosine-based activation motifs (ITAM) that couple the TCR to the signal transduction mechanism (Call & Wucherpfenning, Citation2005; Gouaillard et al., Citation2001). TCR signal transduction is mediated by ITAMs. A total of 10 ITAMs are distributed in the TCR/CD3 complex in two distinct signaling modules termed the CD3ζζ (also referred to as TCRζ) and CD3γɛ/δɛ (Call et al., Citation2004; Clevers et al., Citation1988). TCRζζ plays a central role in TCR signal transmission. The Fcɛ receptor type Iγ (FcɛRIγ) chain is a member of the TCRζ chain protein family, and it is a component of the high-affinity IgE receptor FcɛRI. There is evidence that the FcɛRIγ chain can replace a functionally deficient TCRζ chain and facilitate TCR/CD3 complex-mediated signaling (Enyedy et al., Citation2001; Koyasu et al., Citation1992).
Abnormalities in the transcription of TCRζ may be related to its transcription factors such as E74-like factor 1 (Elf-1). Elf-1 is a transcription factor that belongs to the Ets family. In hematopoietic cells, Elf-1 mediates the induction of various genes, including those for CD4, GM-CSF, IgH enhancer, TCRα, and TCRζ (Juang et al., Citation2002; Tsokos et al., Citation2003). However, little is known about the expression pattern of the FcɛRIγ gene and the molecular mechanism of the aberrant expression of TCRζ in workers occupationally exposed to Pb. Based on our previous finding that TCRζ gene expression level was changed after occupational Pb exposure, we investigated in the studies reported here the expression level of FcɛRIγ and Elf-1. Because research has indicated that FcɛRIγ mRNA expression correlates well with expression of the protein (Butkiewicz et al., Citation2005; Enyedy et al., Citation2001), we believe monitoring of the gene itself will reflect upon the status of the receptor expression on cells of the exposed worker. We believe that analysis of these factors in Pb-exposed workers (before and after chelation therapy) might be useful in helping to identify other potential Pb-mediated immunotoxicities that might occur in situ that are ultimately related to changes in the products of these genes.
Materials and methods
Samples
All samples in this study were collected at Guangzhou 12th Municipal People’s Hospital (Guangzhou Occupational Disease Hospital). All procedures were performed according to guidelines of the Medical Ethics Committee of the Health Bureau of Guangdong Province. All subjects in this study certified they were not taking any medications known to affect the immune system nor suffering from any acute/chronic disease, as these may impact on the immune system. For these studies, 23 healthy subjects (15 male, eight female; median age = 30 years, range = 21–57 years) who received an annual check-up at Guangzhou Hospital served as the control group. The characteristics of the populations examined are provided in . There were no significant differences in age and sex between the control and the PE and PT groups. Subjects in the control group had no occupational exposure to Pb and (background) Pb levels were not detectable.
Table 1. Characteristics of the study control, PE (and PT) groups.
Blood and urine samples from all Pb-exposed workers (PE group) were collected to obtain clinical verification of abnormal blood (BPb) and urinary (UPb) Pb levels. In each case, blood (3–5 ml) was collected into heparin-coated tubes at 8 am (fasting blood sample). Urine was collected as 24-h samples. BPb and UPb levels were then determined using graphite furnace atomic absorption. A diagnosis of Pb exposure was based on the Diagnostic Criteria of Occupational Lead Poisoning (GB: Z37-2002). Specifically, a Pb-exposed worker had to have had prolonged occupational exposure to Pb, as well as (a) high BPb (i.e., ≥2.9 μmol Pb/L)/UPb (≥0.58 μmol Pb/L) levels, (b) blood zinc protoporphyrin (ZPP) levels ≥2.91 μmol/L, (c) urinary δ-aminolevulinic acid (ALA) levels ≥61 μmol/L, or (d) overt clinical manifestations such as abdominal pain or distension, and/or constipation. Measures of ZPP were performed using a ZPP-3800 blood zinc protoporphyrin hematofluorometer (Kangda Company, Guangdong, China) and of ALA using a 7230G spectrophotometer (Jinghua Instruments, Shanghai, China). Apart from using these criteria, only workers with a UPb ≥0.34 μmol Pb/L in a 24-h period on each of 3 consecutive days were selected for chelation therapy. Clinical data and information about the final PE population of this study are provided in .
Table 2. Clinical data for Pb-exposed workers before and after chelation.
For the chelation therapy, each of these workers underwent a course of intravenous treatment with 1 g calcium-EDTA/day over a period of 3 days; if another round of therapy was needed (based on urinary Pb measures), this occurred after a 4-day gap. Each Pb-exposed worker (now deemed member of PT group) underwent a round of chelation therapy until clinical indices reflected an obvious improvement. In this study, there was no stand-alone non-therapy cohort (i.e., to assess effects of time-alone/time-away from exposure).
PBMC RNA extraction and cDNA synthesis
From the blood collected prior to/after the final chelation treatment, peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Ficoll-Hypaque, ρ = 1.077 g/ml). The isolated PBMC (107 cells/subject) were then processed with TRIzol agent (according to manufacturer protocols; Invitrogen, Carlsbad, CA) to yield their total RNA. Purity of the isolated material was confirmed by electrophoresis of a representative amount of material (0.5 μg/sample) over a 0.8% agarose gel subsequently stained with ethidium bromide. First-strand cDNA was then synthesized using random hexamers and reverse transcriptase in a Super-script II Kit (PowerScript Reverse, BD, San Jose, CA), according to manufacturer instructions. cDNA quality was confirmed by RT-PCR for amplification of the β2-microglobulin (β2 M) gene.
TCRζ, FcɛRIγ, and Elf-1 gene quantification by real-time RT-PCR
Real-time RT-PCR using a SYBR Green I method was employed to examine TCRζ, FcɛRIγ, and Elf-1 gene expression levels in PBMC cDNA generated from subject cells. Primer sequences and PCR conditions were as described in Li et al. (Citation2012). Briefly, a PCR reaction (total volume = 25 μl) was performed with ∼1 μl cDNA, 0.5 μM each primer pair, and 11.25 μl of 2.5X RealMasterMix (Tiangen, Beijing, China). After initial denaturation at 95 °C for 2 min, 45 cycles consisting of the following were performed using a MJ Research DNA Engine Opticon 2 PCR cycler (BioRad, Hercules, CA): 15 s at 95 °C; 1 min at 58.9 °C for β2 M, 60 °C for TCRζ and FcɛRIγ, 62 °C for Elf-1; and 1 s at 82 °C for plate reading. The 2−ΔCT × 100% method was used to represent expression of the gene of interest relative to that of internal control gene (%).
Statistical analysis
An independent samples t test was performed to compare mean gene expression levels between the PE/PT group and control groups. Paired sample t tests were performed to compare mean gene expression levels between the PE and PT groups. All data are expressed as the mean ± SD. A p value < 0.05 was considered statistically significant.
Results
Blood (BPb) and urine (UPb) lead levels
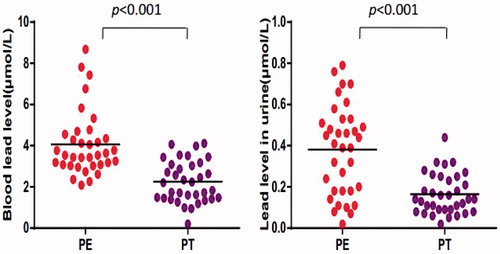
Prior to chelation therapy, blood and urine lead levels in the exposed workers were seen to be 4.06 ± 1.57 (BPb) and 0.38 ± 0.22 (UPb) μmol Pb/L. Following the entire course of chelation therapy/therapies, there were significant decreases in both values, i.e., BPb was now 2.25 ± 1.00) μmol Pb/L and UPb was (0.16 ± 0.10) μmol Pb/L (). BPb and UPb values for the control subjects were each no more than, respectively, 0.97 and 0.12 μmol Pb/L.
Expression of TCRζ, FcɛRIγ, and Elf-1 genes in PBMC
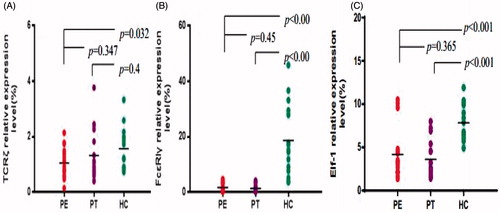
TCRζ, FcɛRIγ, and Elf-1 gene expression levels in PBMC from the Pb-exposed workers, both before after chelation therapy - as well as in cells from healthy controls - are shown in . In the PE cells, expression of TCRζ (1.05 ± 0.53), FcɛRIγ (1.73 ± 1.43), and Elf-1 (4.19 ± 2.97%) genes was significantly decreased in comparison with that in cells from the healthy controls (TCRζ = 1.57 ± 0.75, FcɛRIγ = 18.65 ± 13.06, and Elf-1 = 7.83 ± 2.04%). Chelation therapy led to increased TCRζ and decreased FcɛRIγ and Elf-1 expression levels compared with the PE values, but these changes were not significant. Even with chelation, the expression levels of FcɛRIγ (1.37 ± 1.33) and Elf-1 (3.61 ± 2.13%) remained significantly decreased in comparison to levels in control host PBMC. In contrast, expression of TCRζ (1.32 ± 0.93%) now no longer significantly differed from control PBMC values.
Effect of number of chelation rounds on TCRζ, FcɛRIγ, and Elf-1 gene expression in PBMC
All Pb-exposed workers underwent chelation therapy until their clinical indices had obvious improvement. A comparison of the above-noted outcomes in the context of the different numbers of courses of chelation therapies had little-to-no impact on expression of the measured genes. In workers who underwent two courses, expression of TCRζ in the PE and PT cells were decreased in comparison with that in cells from healthy controls, but these changes were not significant (). In Pb-exposed workers, only expression of TCRζ in the PE cells was significantly decreased compared to in cells from controls. In all cases, chelation therapy led to increased TCRζ expression levels compared with pre-therapy PE values, albeit the changes were not significant. After chelation, expression levels of TCRζ no longer significantly differed from control levels. In the case of the single individual that underwent five rounds of therapy, the highly unusual PT values was most likely a result of individual variation in response to the therapy (as the PE value was in line with those in all other Pb-exposed workers).
Table 3. Relative TCRζ expression level (%) in PE/PT PBMC as a function of number of therapy rounds.
In the workers who underwent two or three courses of therapy, even though their clinical indices had reached normal levels, expression of FcɛRIγ and Elf-1 genes was still significantly decreased in comparison with that in cells from the healthy controls ( and ). As a function of the numbers of courses of chelation therapy, FcɛRIγ and Elf-1 expression levels were not significantly impacted and expression levels of each remained significantly decreased compared to levels in control PBMC. Again, in the case of the single individual that underwent five therapy rounds, the highly unusual PT Elf-1 value was most likely a result of individual variation in response to the chelation therapy; in the case of FcɛRIγ, both sets of values were in line with the other Pb-exposed workers.
Table 4. Relative Elf-1 expression level (%) in PE/PT PBMC as a function of number of therapy rounds.
Table 5. Relative FcɛRIγ expression level (%) in PE/PT PBMC as a function of number of therapy rounds.
Correlation among TCRζ, FcɛRIγ, and Elf-1 expression levels in PBMC
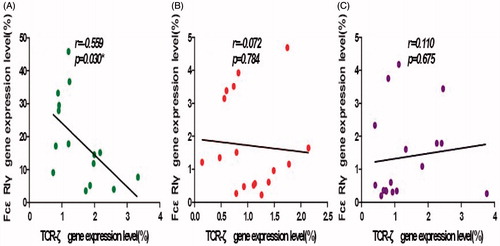
A significant negative-correlation was observed between expression levels of TCRζ and FcɛRIγ genes in cells isolated from the healthy control subjects (r = −0.559, p = 0.03, ). A negative correlation was found with the cells from the Pb-exposed workers before chelation therapy, though the difference was not statistically significant (r = −0.072, p = 0.784, ). This negative correlation was no longer apparent following chelation therapy (r = 0.110, p = 0.675, ).
Figure 3. Correlation analyses for TCRζ and FcɛRIγ expression levels. (A) HC, (B) PE, and (C) PT groups. A significant negative correlation was found for the HC group and a non-significant negative correlation was found for the PE group. There was no correlation for the PT group.
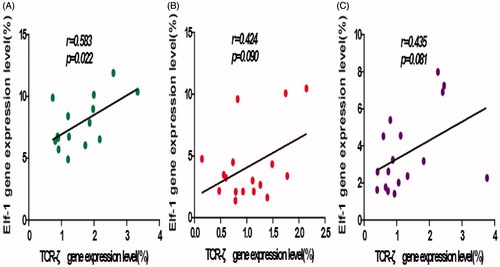
A significant positive correlation between TCRζ and Elf-1 gene expression levels was found for the PBMC of the healthy control hosts (r = 0.583, p < 0.022, ). Although a positive correlation was noted for in PBMC TCRζ and Elf-1 expression for Pb-exposed workers both before and after chelation therapy, the differences were not statistically significant (r = 0.424, p = 0.090; ; and r = 0.435, p = 0.081; ).
Figure 4. Correlation analyses for TCRζ and Elf-1 expression levels. (A) HC, (B) PE, and (C) PT groups. A significant positive correlation was found in the HC group; non-significant positive correlations were found for the PE and PT groups.
In the context of the clinical measurements, there was no correlation between blood Pb levels and TCRζ (r = −0.208, p = 0.238), Elf-1 (r = −0.071, p = 0.691), or FcɛR1γ (r = −0.236, p = 0.178) values for the PE subjects. Similarly, there was no correlation between urine Pb levels and TCRζ (r = −0.053, p = 0.764), Elf-1 (r = −0.255, p = 0.146), or FcɛR1γ (r = −0.226, p = 0.198) values.
Discussion
The TCR/CD3 complex plays a central role in T-lymphocyte activation; alterations of any sub-units in the complex may impact on T-lymphocyte activation levels (Chen et al., Citation2009; Fischer et al., Citation2005). Decreased TCRζ gene expression has been described in Pb-exposed workers (Liu et al., Citation2009). Absence of the TCRζ chain not only influences TCR expression on the cells and the number of single-positive circulating T-lymphocytes it also impairs proliferative responses and mature T-lymphocyte activation (Li, Citation2008; Mozaffari et al., Citation2004). It may be interesting to examine whether TCRζ expression could recover in Pb-exposed workers after chelation therapy. In this study, the TCRζ gene expression was compared among 17 Pb-exposed workers before and after chelation therapy and against those in PBMC from healthy individuals. Similar to what was seen in our previous study (Liu et al., Citation2009), it was seen here that TCRζ gene expression in cells from Pb-exposed workers before chelation treatments was significantly lower than in healthy control cells, but was increased as a result of chelation.
It is known that FcɛRIγ, which is unlike the TCRζ chain, mediates signaling through ZAP-70 by associating with the phosphorylated protein kinase Syk (Enyedy et al., Citation2001; Shiue et al., Citation1995; Taylor et al., Citation1997) that can substitute when there are TCRζ deficiencies. In the study here, FcɛRIγ gene expression and its correlation with TCRζ expression was analyzed in Pb-exposed workers before and after chelation therapy. Expression of FcɛRIγ was significantly lower (relative to in control subject cells) in Pb-exposed workers, pre- and post-therapy. This finding suggested to us that, in the case of TCRζ down-regulation, FcɛRIγ expression in the Pb-exposed workers was incapable of being up-regulated to contribute to transduction of TCR signaling in a manner similar to that used by a conserved functional motif ITAM. The present study also analyzed the correlation between TCRζ and FcɛRIγ gene expression; FcɛRIγ expression lost its negative correlation with TCRζ expression in the Pb-exposed workers, both before and after chelation therapy. This suggested that apparent defects in T-lymphocyte-mediated immunity were involved in the immuno-dysregulation seen in Pb-exposed workers (Liu et al., Citation2009, Citation2011, Citation2012; Niu et al., Citation2012; Valentino et al., Citation2007).
The mechanism of abnormal of TCRζ gene expression in T-lymphocytes of Pb-exposed workers is unclear. To gain insight into the molecular mechanism of the lower TCRζ gene expression, expression levels of Elf-1, which could bind to the TCRζ promoter and is involved in transcriptional regulation of the TCRζ gene and promotes its transcription in T-lymphocytes, were assessed (Juang et al., Citation2008; Kulkarni et al., Citation2009). The data showed a significantly lower expression of Elf-1 in Pb-exposed workers before and after chelation therapy. Moreover, the loss of Elf-1 expression was positively correlated with the change in TCRζ expression level in the PBMC from these workers (both before and after the therapy). It is, therefore, possible that decreased Elf-1 expression contributed to down-regulation of TCRζ expression in Pb-exposed workers.
Conclusions
We believe this to be the first study to describe TCRζ-related genes, i.e., FcɛRIγ and Elf-1, in Pb-exposed workers. Our findings suggest that the T-lymphocyte immune impairment caused by occupational lead exposure might be associated, at least in part, with abnormal TCR signaling pathways. This impairment is mainly related to the down-regulation of downstream molecules in TCR signaling pathways, e.g., TCRζ and FcɛRIγ, and down-regulation of TCRζ might be mainly associated with changes in expression of Elf-1. Although there was an improvement in the clinical indices of the Pb-exposed workers after chelation therapy, further investigation is needed to follow-up on functional outcomes/immune cell recovery, including on T-cell activation, TCR signaling, alterations in Th1/Th2 balance, etc. in these workers following chelation therapy. In addition, our future studies need to include a time-alone (time-away from Pb exposure) group to confirm that the outcomes we measured here were in fact due solely to the chelation therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was sponsored by grants from the National Natural Science Foundation of China (No. 81370605), the Natural Science Foundation of Guangdong Province (No. S2012010008794), the Fundamental Research Funds for the Central Universities (No. 21612425), and the Post-doctoral Science Foundation Project of China (No. 20070410840).
References
- Butkiewicz, L., Duriagin, S., Laddach, R., et al. 2005. Prevalence of FcγR chain expression in CD4+ T-cells of patients with systemic lupus erythematosus. Scand. J. Rheumatol. 34:216–219
- Call, M. E., and Wucherpfenning, K. W. 2005. The T-cell receptor: Critical role of the membrane environment in receptor assembly and function. Annu. Rev. Immunol. 23:101–125
- Call, M. E., Pyrdol, J., and Wucherpfenning, K. W. 2004. Stoichiometry of T-cell receptor-CD3 complex and key intermediates assembled in the endoplasmic reticulum. EMBO. J. 23:2348–2357
- Chen, S., Miller, T. E., Golemboski, K. A., and Dietert, R. R. 1977. Suppression of macrophage metabolite production by lead glutamate in vitro is reversed by meso-2,3-dimercaptosuccunic acid (DMSA). In Vitro Toxicol. 10:351–357
- Chen, S., Yang, L., Chen, S., and Li, Y. 2009. TCRζ chain expression in T-cells from patients with CML. Hematology 14:95–100
- Clevers, H., Alarcon, B., Wileman, T., and Terhorst, C. 1988. The T-cell receptor/CD3 complex: A dynamic protein ensemble. Annu. Rev. Immunol. 6:629–662
- Enyedy, E. J., Nambiar, M. P., Liossis, S. N., et al. 2001. FcɛRIγ chain replaces deficient T-cell receptor ζ chain in T-cells of patients with systemic lupus erythematosus. Arthritis Rheum. 44:1114–1121
- Fischbein, A., Tsang, P., Luo, J. C., et al. 1993. Phenotypic aberrations of CD3+ and CD4+ cells and functional impairments of lymphocytes at low-level occupational exposure to lead. Clin. Immunol. Immunopathol. 66:163–168
- Fischer, A., de Saint Basile, G., and Le Deist, F. 2005. CD3 deficiencies. Curr. Opin. Allergy Clin. Immunol. 5:491–495
- Guo, T. L., Mudzinski, S. P., and Lawrence, D. A. 1996. The heavy metal lead modulates the expression of both TNFα and TNFα receptors in LPS-activated human peripheral blood mononuclear cells. J. Leukocyte Biol. 59:932–939
- Gouaillard, C., Huchen-Champagne, A., Arnaud, J., et al. 2001. Evolution of T-cell receptor (TCR) heterodimer assembly with the CD3 complex. Eur. J. Immunol. 31:3798–3805
- Hsiao, C. L., Wu, K. H., and Wan, K. S. 2011. Effects of environmental lead exposure on T-helper cell-specific cytokines in children. J. Immunotoxicol. 8:284–287
- Juang, Y. T., Solomou, E. E., Rellahan, B., and Tsokos, G. C. 2002. Phosphorylation and O-linked glycosylation of Elf-1 leads to its translocation to nucleus and binding to promoter of the TCRζ-chain. J. Immunol. 168:2865–2871
- Juang, Y. T., Wang, Y., Jiang, G., et al. 2008. PP2A dephosphorylates Elf-1 and determines expression of CD3ζ and FcRγ in human systemic lupus erythematosus T-cells. J. Immunol. 181:3658–3664
- Koyasu, S., d’Adamio, L., Arulanandam, A. R., et al. 1992. T-Cell receptor complexes containing FcɛRIγ homodimers in lieu of CD3ζ and CD3ɛ components: A novel isoform expressed on large granular lymphocytes. J. Exp. Med. 175:203–209
- Kulkarni, D. P., Wadia, P. P., Pradhan, T. N., et al. 2009. Mechanisms involved in the down-regulation of TCRζ chain in tumor vs. peripheral blood of oral cancer patients. Int. J. Cancer 124:1605–1613
- Li, B., Liu, S., Niu, Y., et al. 2012. Altered expression of the TCR signaling related genes CD3 and FcɛRIγ in patients with aplastic anemia. J. Hematol. Oncol. 5:6
- Li, Y. 2008. Alterations in the expression pattern of TCRζ chain in T-cells from patients with hematological diseases. Hematology 13:267–275
- Liu, S., Li, B., Niu, Y., et al. 2011. Change in pattern of peripheral blood TCR Vβ gene repertoire in occupational lead-exposed workers. Zhong Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 27:1322–1324
- Liu, S., Pan, H., Li, B., et al. 2012. Change of TCR Vγ gene expression in PBMC from lead exposure and poisoning workers. Zhong Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 28:304–305
- Liu, S., Pan, H., Xu, Y., et al. 2009. Expression feature of CD3ζ gene in peripheral T-cells from lead exposure and poisoning workers. Zhong Di San Jun Yi Da Xue Xue Bao 31:1399–1340
- McCabe, M. J., and Lawrence, D. A. 1991. Lead, a major environmental pollutant, is immune-modulatory by its differential effects on CD4+ T-cell subsets. Toxicol. Appl. Pharmacol. 111:13–23
- Mishra, K. P., Singh, V. K., Rani, R., et al. 2003. Effect of lead exposure on immune response of some occupationally-exposed individuals. Toxicology 188:251–259
- Mozaffari, F., Hansson, L., Kiaii, S., et al. 2004. Signaling molecules and cytokine production in T-cells of multiple myeloma-increased abnormalities with advancing stage. Br. J. Hematol. 124:315–324
- Niu, Y., Li, B., Liu, W., et al. 2012. Change of T-bet and GATA-3 gene expression levels in PBMC from lead exposure workers. Zhong Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 30:379
- Pinkerton, L. E., Biagini, R. E., Ward, E. M., et al. 1998. Immunologic findings among lead-exposed workers. Am. J. Ind. Med. 33:400–408
- Sata, F., Araki, S., Tanigwa, T., et al. 1998. Changes in T-cell sub-populations in lead workers. Environ. Res. 76:61–64
- Shiue, L., Zoller, M. J., and Brugge, J. S. 1995. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J. Biol. Chem. 270:10498–10502
- Taylor, N., Jahn, T., Smith, S., et al. 1997. Differential activation of the tyrosine kinases ZAP-70 and Syk after FcγRI stimulation. Blood 89:388–396
- Tsokos, G. C., Nambiar, M. P., and Juang, Y. T. 2003. Activation of the Ets transcription factor Elf-1 requires phosphorylation and glycosylation: Defective expression of activated Elf-1 is involved in the decreased TCRζ chain gene expression in patients with systemic lupus erythematosus. Ann. N.Y. Acad. Sci. 987:240–245
- Undeger, U., Başaran, N., Canpinar, H., and Kansu, E. 1996. Immune alterations in lead-exposed workers. Toxicology 109:167–172
- Valentino, M., Rapisarda, V., Santarelli, L., et al. 2007. Effect of lead on the levels of some immunoregulatory cytokines in occupationally-exposed workers. Human Exp. Toxicol. 26:551–556