Abstract
Artemisia species are important medicinal plants throughout the world. The present in vitro study, using a sesquiterpene lactone-bearing fraction prepared from Artemisia khorassanica (SLAK), sought to investigate immunomodulatory/anti-inflammatory properties of this plant and elucidate potential underlying mechanisms for the actions. Effects of the SLAK on mitogen-induced murine splenocyte proliferation and interleukin (IL)-4 and interferon (IFN)-γ secretion were evaluated. To assess anti-inflammatory activities, levels of inducible of nitric oxide (NO) and prostaglandin E2 (PGE2), as well as expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), in peritoneal macrophages was examined. The results showed that SLAK noticeably was capable of suppressing PHA/LPS-stimulated splenocyte proliferation and of up-regulating production of the T-helper (TH)-2 cell cytokine IL-4 while down-regulating formation of TH1 IFNγ. In addition, while SLAK caused negligible proliferation inhibition, peritoneal macrophages displayed considerable decrease in NO and PGE2 production along with iNOS and COX-2 expression. The current experiment shows Artemisia khorasanica – a traditionally used herb – may have immunomodulatory and anti-inflammatory effects. It is anticipated that the ingredients may be employed as therapeutic candidates in the regulation of some immune responses implicated in various conditions and ailments.
Introduction
Natural compounds derived from medicinal plants have been traditionally used to treat various diseases or to promote general health; nowadays, they are increasingly being exploited for pharmacologic purposes. For example, plant-based immunomodulants are one natural way to treat immune dysfunction. Accordingly, numerous investigations have been done to identify plant-derived natural products that can function as immunomodulatory mediators to control aberrant immune responses (Tapsell et al., Citation2006; Craig, Citation1999).
Artemisia L. (Astraceae, Compositae) is a large heterogeneous genus of evergreen and deciduous shrubs, perennials, and annuals, distributed mainly in the temperate zones of Europe, Asia, and North America. Artemisia consist of ≈550 species; 43 are found in Iran, with two used in traditional medicine (Emami & Aghazari, Citation2011; Podlech, Citation1986). Different classes of chemicals, e.g., coumarins, terpenes, sesquiterpenes, flavonoids, aromatics, dipeptides, phenolics, esterols, germacranolides, secoguaianolides, and several polysaccharides have been isolated from Artemisia species (Valless et al., Citation2011). In recent years, increasing evidence has shown that sesquiterpene lactones are one of the constituents that impart a wide-range of pharmacological effects, including anti-cancer and immunomodulatory actions (Choi et al., Citation2011; Kim et al., Citation2002; Lu et al., Citation2009; Yang et al., Citation2012).
Among the few studies that evaluated the spectrum of immunomodulatory effects of Artemisia, few have assessed the impact on immune cell (including lymphocyte and macrophage) functions. Some studies reported that some constituents of Artemisia exerted stimulatory effects on T-cell-mediated immune responses (Chang et al., Citation2009; Noori et al., Citation2010; Sun et al., Citation2010; Yin et al., Citation2011). In a previous study, we reported the anti-inflammatory effects of a sesquiterpene lactone-bearing fraction isolated from Artemisia khorassanica (SLAK); those studies showed that SLAK impacted on lipopolysaccharide (LPS)-induced release of nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α and interleukin (IL)-1β release, as well as the expression of both iNOS and COX-2 enzymes, by the J774A.1 macrophage cell line (Emami et al., Citation2010). To expand our understanding of how the products of Artemisia might act as immunomodulants, the present study sought to clarify the effects of SLAK in vitro in freshly-isolated murine cells involved in immune responses in situ.
Materials and methods
Plant materials
Aerial parts of Artemisia khorassanica were collected from Chovailly-Bajgiranin in Northeast Iran (23 December 2004). Dr V. Mozaffarian (Research Institute of Forest and Rangelands, Ministry of Jahad Keshavarzi, Iran) confirmed the plant identity and a voucher specimen was deposited in the herbarium at the National Botanical Garden of Iran (TARI, Teheran, Iran).
Preparation of sesquiterpene lactone-bearing fraction
The terpenoide fraction was prepared from the isolated samples as previously described (Emami et al., Citation2010; Iranshahi et al., Citation2007). The Herz-Högenauer technique was used to remove chlorophyll and phenolics (by lead (Π) acetate precipitation) and to prepare a crude sesquiterpene sample for further chromatographic/spectral investigation. In brief, dried ground plant material (20 g) was soaked in dichloromethane (DCM; ≈100 ml) overnight and the slurry product then filtered and evaporated in vacuo. The gummy residue was dissolved in 96% ethanol (≈50 ml) and heated to improve solubility. Aqueous 5% lead acetate solution was added to precipitate fatty acids, phenolics, and chlorophyll present. The precipitate was removed by filtration through a pad of silica gel (230–400 mesh, Merck, Darmstadt, Germany). The filtrate was concentrated in a waterbath (40–50 °C) until a viscous mass developed. An IR spectrum was recorded (as KBr disks) in CH2Cl2 on a Unicam dp 110 spectrometer (Shimidzu Scientific, Tokyo, Japan). 1H-NMR (500 MHz) spectra of the sample was assessed in deuterated chloroform (CDCl3, Sigma-Aldrich, Berlin, Germany) using a DRX 500 spectrometer (Bruker, Bremen, Germany). SLAK was dissolved in dimethyl sulfoxide (DMSO) and the final concentration of DMSO was < 0.1% in the cell culture medium; this level does not have any effect on the experiments carried out in the present study.
Mice
Balb/c mice (male, 4–6-weeks-old) were purchased from the Pasteur Institute (Tehran) and housed under standard laboratory conditions (25 [±2]°C and 40–70% relative humidity) with a 12-h day/night lighting cycle throughout the experiment. The mice were kept in large spacious polypropylene cages and provided ad libitum access to standard rodent chow and filtered water. The Ethnic Council of Mashhad University of Medical Sciences (Mashhad, Iran) approved all protocols involving the mice.
Isolation of splenocytes and peritoneal macrophages
To isolate splenocytes for the experiments, naïve mice were euthanized by cervical dislocation and their spleens removed under aseptic conditions. Splenocytes were isolated by gently passing RPMI 1640 medium (GlutaMAX, Gibco, Detroit, MI) from a syringe through the spleen. The resulting single cell suspensions then underwent Ficoll-Hypaque (Sigma, St. Louis, MO) density centrifugation (700 × g, 20 min, 4 °C) to permit isolation of splenic mononuclear cells (MNC). The MNC present at the interface layer were collected, washed twice with ice-cold phosphate-buffered saline (PBS, pH 7.5) by centrifugation (400 × g, 10 min at 4 °C), and then suspended in complete RPMI 1640 medium (RPMI supplemented with 10% heat-inactivated fetal bovine serum [FBS], 100 streptomycin μg/ml, 100 U penicillin/ml; all Gibco), and incubated in a 95% air/5% CO2 humidified atmosphere at 37 °C. Trypan blue exclusion was used to determine concentration/viability of the isolated MNC. Sample viability was routinely >90%.
Macrophages were isolated from peritoneal cavities of the mice by loading the cavity twice with RPMI 1640 (≈5 ml/injection) and aspirating each peritoneal liquid under sterile conditions. The resulting cell suspension was centrifuged (10 min, 300 × g, 4 °C) and the cell pellet collected. The macrophages were re-suspended in complete RPMI 1640, seeded into 24-well dishes at 5 × 105 cells/well, and incubated for 3 h at 37 °C to permit adherence. The cultures were washed with medium to remove all non-adherent cells prior to their use in experiments.
Assay of splenic MNC proliferation
The effect of SLAK on splenic MNC proliferation was evaluated using an MTT [3,(4,5-dimethylthiazal-2-yl)-2,5-diphenyl tetrazolium bromide] colorimetric assay (Mosmann, Citation1983; Zamani Taghizadeh Rabe et al., 2011). In brief, splenocytes were dispensed into 96-well microtiter plates at 4 × 105 cells/well (100 μl per well), and then SLAK (0.5–20 μg/ml) was added in fixed volumes so a total volume/well was 200 μl. These doses were selected for analysis based on our previous study (Emami et al., Citation2010). Non-treated control wells received medium in place of the SLAK. PHA was then added (5 μl, at final concentration of 5 ng PHA/ml/well); unstimulated cultures received complete RPMI 1640 in place of PHA. To assess potential effects of SLAK on B-lymphocyte proliferation capacity, LPS (at 1 μg/ml) was used in place of PHA. After incubation at 37 °C for 3 days, the medium was removed from each well and replaced with fresh medium; this step mitigated any potential for the SLAK to interfere with the MTT. Thereafter, MTT solution (5 mg/ml; 20 μl) was added to each well and the plate incubated a further 4 h. The plate was then centrifuged, supernatant from each well was removed, and the cells then lysed by addition of 150 μl of 100% DMSO (dimethyl sulfoxide; Merck) to dissolve the formazan crystals that had been produced in the viable cells. The optical density in each well was then measured at 545 nm in a Convergys EL reader (Convergent Technologies, Marburg, Germany). Cell proliferation was presented as stimulation index (SI), where SI = OD of PHA (or LPS)-treated cells/OD of non-treated cells.
Measurement of MNC cytokine formation
To evaluate IFNγ and IL-4 production by the splenic MNC cells, cells (106/well) were incubated in the presence of PHA (5 μg/ml) and SLAK (5, 10, 15, or 20 μg/ml) for 3 days at 37 °C. These doses were selected for analysis based on data from our previous study (Emami et al., Citation2010). Thereafter, the supernatant from each well was collected (after centrifugation of the plate) and held at −70 °C until analyzed in ELISA kits (R & D, Minneapolis, MN) and following manufacturer instructions. The kit levels of sensitivity for IFNγ and IL-4 were each 2 pg/ml.
Evaluation of anti-inflammatory properties of SLAK
SLAK toxicity in peritoneal macrophages was determined using the MTT assay. Non-toxic doses of SLAK were selected for use in all subsequent assessments of anti-inflammatory effects. For this, the macrophages were seeded into 24-well plates (5 × 105 cell/well; 500 μl/well) and incubated for 3 h at 37 °C. The cells were then treated with SLAK (5–l00 μg/ml; 500 μl/well) and incubated for 24 h. These doses were selected for analysis based on our previous study (Emami et al., Citation2010). The medium was then removed from each well and replaced with fresh medium to mitigate any potential for SLAK to interfere with the MTT. Thereafter, the samples were processed as above for measures of formazan levels and estimation of cell survival.
For assays of formation of indicators of inflammation by the cells (e.g., nitric oxide [NO], prostaglandin [PGE]-2 [PGE2]), macrophages were seeded into 24-well plates (5 × 105 cell/well; 500 μl/well) and incubated for 3 h at 37 °C. After 3 h, the cells were treated with SLAK (5–100 μg/ml; 500 μl/well) in the presence/absence of LPS (10 ng/ml) + IFNγ (100 U/ml) and incubated a further 24 h. Nitrite accumulation (index of NO synthesis) in the culture medium was subsequently measured using Griess reagent (Zamani Taghizadeh Rabe et al., 2014). The supernatants were also assessed for prostaglandin (PGE)-2 (PGE2) content using an enzyme immunoassay (EIA) kit (Assay Designs, Farmingdale, NY) and following manufacturer instruction. For the nitrite analyses, an equal amount of supernatant was mixed with Griess reagent [equal volume 1% (w/v) sulphanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylenediamine] and incubated at room temperature for 20 min. The optical density in each sample was then measured at 545 nm in the Convergys EL system. The nitrite level (μM) in each sample was calculated by extrapolation from a standard curve generated in parallel using sodium nitrite.
Western blot analyses were used to assess expression of iNOS and COX-2 proteins in cell extracts using primary (rabbit anti-mouse iNOS and anti-COX-2 polyclonal antibodies; Panomics Inc., Redwood City, CA) and secondary (goat anti-rabbit-IgG; KOMA Biotech, Seoul, Korea) antibodies. For this, 106 cells/treatment were harvested, placed in lysis buffer [10 mM HEPES; pH 7.5, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiotheritol (DTT), 0.5% IGEPAL along with the protease inhibitor cocktail], and allowed to swell on ice for 10 min. After determining the protein concentration in the lysate using a Bradford assay, equal amounts of lysate proteins (50 μg/lane) from each sample were separated over a 12.5% polyacrylamide gel and these materials then electrotransferred to a polyvinylidene difluoride (PVDF) membrane. Acting as a gel loading control, β-actin protein in each lane was also assessed (using appropriate antibody for detection). After blocking non-specific binding by incubation in a 2% BSA/PBS solution overnight at 4 °C, the membranes were probed with each primary antibody (anti-iNOS at 1:200 in PBS/1% BSA solution OR anti-COX-2 at 1:500 in PBS/1% BSA) overnight at 4 °C. After washing away unbound antibody, the membranes were then incubated in a solution containing horseradish peroxidase-conjugated secondary antibody (1:500 in PBS/1% BSA) at room temperature for 2 h. Each membrane was then extensively washed with PBS, and bound antibody detected using enhanced chemiluminescence (ECL) detection reagent (Pierce, Rockford, IL). Signals were quantified using a Syngene documentation system (Cambridge, UK).
Statistical analysis
The significance of differences was evaluated by one-way analysis of variance (ANOVA) and a Bonferroni’s post-hoc test using SPSS 11.0 software (Chicago, IL). All results were expressed as mean ± SD. A p-value <0.05 was considered statistically significant.
Results
Analysis of Artemisia Khorasanica fraction
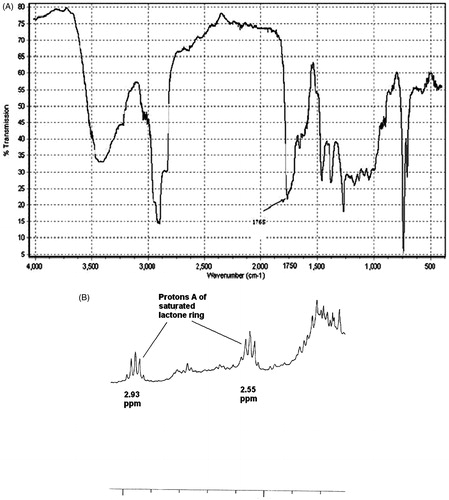
In IR spectra, a sample showing a strong absorption between ν 1730 and ν 1780 indicated the presence of a carbonyl moiety. Samples with absorptions above ν 1760 were considered to contain a γ-lactone moiety. The A. khorassanica extract here displayed a strong absorption peak in ν 1765, indicating a high content of sesquiterpene lactones in this terpenoid extract (). In general, diagnostic features in 1H-NMR spectra of sesquiterpenoid α-methylene γ-lactones are (unsaturated sesquiterpene lactones) two doublets (JB,C = 1–4 Hz) which appear above and below 6 ppm. On the other hand, a diagnostic feature in the 1H-NMR spectra of sesquiterpenoid α-methyl γ-lactones (saturated sesquiterpene lactones) is a quintet between 2.0–3.0 ppm (Proton B). As all diagnostic features in the 1H-NMR spectra were not usually obscured with other signals in the terpenoid extract, the noted signals could be used to determine the type of γ-lactone present. Overall, the results gleaned from the 1H-NMR spectra of the sample were also almost in agreement with those from the results of the IR analyses. The main types of lactone in the A. khorassanica terpenoid extract were saturated sesquiterpene lactones. Specifically, the 1H-NMR spectra of this sample showed there was a quintet signal (Proton A) between 2.0–3.0 ppm (); exocyclic methylene protons peaks at 5.5 and 6.2 ppm, indicating α-methylene γ-lactone, were not observed.
Effect of SLAK on splenic MNC proliferation
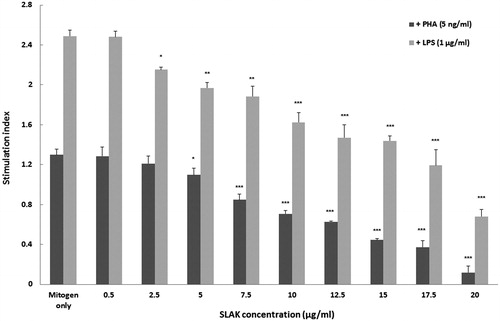
Proliferation—as reflected in terms of stimulation index (SI)—was evaluated among the cells (). As expected, PHA (5 ng/ml) or LPS (1 μg/ml) alone enhanced splenocyte proliferation. The results also clearly indicated that SLAK at doses >5 μg/ml significantly decreased the SI in comparison with cells that only received PHA. Comparatively, it appeared SLAK was able to decrease PHA-stimulated proliferation of splenocytes moreso than that due to LPS.
Effect of SLAK on any shift in patterns of IFNγ and IL-4 formation by MNC
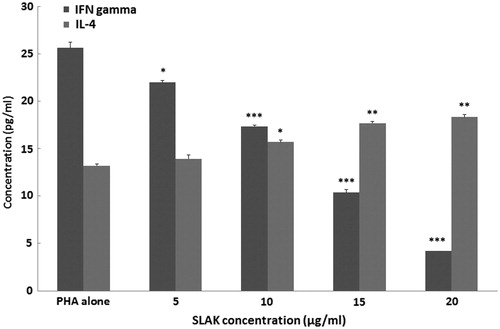
To evaluate the effects of SLAK on splenic T-helper (TH)-1 and TH2 cell cytokine formation, pro- (IFNγ) and anti-inflammatory (IL-4) cytokine release into culture medium by the MNC was measured. Here, MNC were treated with PHA (5 ng/ml) and SLAK (5, 10, 15 and 20 μg/ml) and the concentration of cytokines in the medium compared against that in cultures of MNC that received only PHA (). Levels of IFNγ and IL-4 produced by the MNC after stimulation with PHA rose to 26.6 [± 0.6] and 13.2 [± 0.2] pg/ml, respectively, compared to barely detectable background levels in MNC receiving medium only (data not shown). MNC co-treated with SLAK showed remarkable decreases in IFNγ production. In contrast, IL-4 production by these cells was significantly elevated by concentrations of SLAK >10 μg/ml.
Anti-inflammatory property of SLAK in peritoneal macrophages
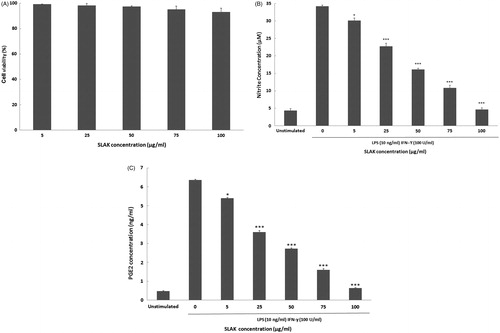
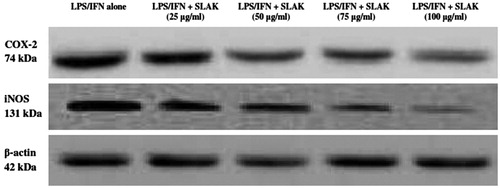
The survival of peritoneal macrophages in the presence of SLAK (5–100 μg/ml) was assessed. None of these concentrations of SLAK caused significant cytotoxicity (). To evaluate any inhibitory effect of SLAK on nitric oxide (NO) production by the macrophages, the cells were stimulated with LPS (10 ng/ml) + IFNγ (100 U/ml) and then co-treated with SLAK before nitrite (index of NO) in the culture medium was measured. The results show that NO levels were markedly increased (≈8-fold) by LPS + IFNγ stimulation (change from 4.3 [± 0.5] μM to 34.2 [± 0.3] μM). However, pre-incubation with concentrations ≥ 5 μg SLAK/ml before challenge with LPS/IFNγ resulted in a dose-related reduction in NO formation by the cells. NO produced by macrophages treated with 100 μg SLAK/ml approximated that by unstimulated control cells. In the analyses of any modulatory effect of SLAK on stimulated production of PGE2, the data revealed that, while treatment with the two stimulators alone caused an appreciable increase in PGE2 level (6.4 [± 0.05] ng/ml) from unstimulated levels (0.5 [± 0.03] ng/ml), SLAK remarkably inhibited this induction PGE2 at the tested concentrations (). To determine if SLAK might also modulate LPS+IFNγ-induced iNOS and COX-2 expression, Western blot analyses were undertaken. The results indicated that treatment with SLAK significantly inhibited any induced both iNOS and COX-2 protein expression in these peritoneal macrophages ().
Figure 4. Effect of SLAK on (A) cell viability, (B) NO production, and (C) PGE2 release from peritoneal macrophages after 24 h treatment. Results are shown as mean ± SD. Value significantly different vs control at *p < 0.05, **p < 0.01, or ***p < 0.001.
Figures 5. Effect of SLAK on LPS/IFN-induced iNOS and COX-2 expression levels. Lysates were prepared from peritoneal macrophages that were incubated for 24 h with 10 ng LPS/ml + 100 U IFNγ/ml alone or in combination with SLAK (25, 50, 75, and 100 μg/ml). β-actin is shown for housekeeping/gel loading purposes.
Discussion
The Artemisia genus has been used in folk medicine for many years; numerous reports have indicated a vast range of biological effects associated with different Artemisia species (Abad Martínez et al., Citation2012; Choi et al., Citation2013). Among the various chemical agents found in these plants, sesquiterpene lactones have been proven to impart immunomodulatory and anti-cancer effects (Cho et al., Citation2012; Firestone & Sundar, Citation2009; Tan et al., Citation1998). Because effects from a sesquiterpene lactone-bearing fraction (SLAK) isolated from Artemisia khorassanica were seen against LPS-induced NO, PGE2, TNFα, and IL-1β release and iNOS and COX-2 expression by J774A.1 macrophages (in part, through inhibition of NF-κB [p65]) activity, the present study sought to build on those findings and clarify the effects of SLAK in vitro in freshly-isolated murine cells involved in immune responses in situ.
In general, the balance between TH1- vs TH2-type lymphocytes – and their released cytokines – influences the phenotype and progression of several diseases (Boehm et al., Citation1997; Heikamp & Powell, Citation2012; Saenz et al., Citation2010). Numerous experiments have reported various types of plant-derived compounds capable of altering the pattern of produced cytokines in vitro. The results with splenocyte cultures here illustrate that SLAK could play an important role in regulating mechanisms (including cytokine formation) that underlay T-cell-based immune responses in situ. These results are especially of high importance if one were to consider potential therapeutic uses for SLAK as an immunomodulant used in treatments for inflammatory diseases. A few studies have shown some sesquiterpene lactones from other Artemisia species have immunomodulatory effect on lymphocytes (Chang et al., Citation2009; Li et al., Citation2012; Wang et al., Citation2005; Yin et al., Citation2010, Citation2011). In this regard, SLAK behaved in a manner similar to cirsilineol, jaceosidin, and artemisinin isolated from these plants, i.e., each suppressed splenocyte proliferation and led to skewed TH2-type cytokine formation (Li et al., Citation2012; Yin et al., Citation2010, Citation2011).
Like lymphocytes, macrophages play central roles in both native and adaptive immune responses. Nitric oxide (NO) and prostaglandin E2 (PGE2) in macrophages are key products released by macrophages during responses to pathogens, are critical to onset/degree of inflammation, and can also auto-regulate cell activity. Accordingly, modulation of NO and PGE2 production (and also iNOS and COX-2 expression) has often been a target in the treatment of various inflammatory and/or autoimmune-based ailments (Saenz et al., Citation2010; Stow et al., Citation2009).
The present in vitro study demonstrated that SLAK suppressed the function of peritoneal macrophages as evaluated through the end-points of NO and PGE2 produced and iNOS and COX-2 expression in LPS-stimulated cells. The concentration of SLAK that altered splenocyte proliferation and cytokine secretion were ∼5-times lower than concentrations that decreased NO and PGE2 production. To our knowledge, this is the first study that has examined the effect of SLAK on the function of mouse peritoneal macrophages. These results were in agreement with previous studies that showed that SLAK exerted an anti-inflammatory effect in J774A.1 macrophages through the inhibition of NO and PGE2 production as well iNOS and COX-2 expression (Emami et al., Citation2010). Moreover, the overall impact of these changes also reflect results of previous studies that demonstrated that various individual sesquiterpene compounds from Artemisia exert anti-inflammatory effects that may be effective in treating some types of experimental inflammation/autoimmune conditions (Choi et al., Citation2011; Ho et al., Citation2014; Kim et al., Citation2002; Lu et al., Citation2009; Omer et al., Citation2007; Sun et al., Citation2010; Yang et al., Citation2012).
Conclusion
A sesquiterpene lactone fraction derived from the aerial portions of Artemisia khorassanica (SLAK) did not only impart considerable immunomodulatory effects on cells that can partake in immune response. We anticipate that constituents of the A. khorassanica might be useful in the development of therapeutic approaches toward prevention/treatment of various immune conditions. This study also provides a basis for the potential use of SLAK as a component in some immunosuppressive therapy. However, further investigations are required to elucidate the precise component responsible for the observed biological activities, and effects in vivo need to also be evaluated so that the potential broader utility of the SLAK can be more precisely determined.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This research was supported by the Research Council of Mashhad University of Medical Sciences (MUMS) vice president (Grant # 89401).
References
- Abad Martínez, M. J., Bedoya del Olmo, L. M., Ticona, L. A., and Bermejo Benito, P. 2012. Artemisia L. genus: A review of bioactive sesquiterpene lactones. Stud. Nat. Prod. Chem. 37:43–65
- Boehm, U., Klamp, T., Groot, M., and Howard, J. C. 1997. Cellular responses to IFNγ. Annu. Rev. Immunol. 15:749–795
- Chang, S. H., Jung, E. J., Park, Y. H., et al. 2009. Anti-inflammatory effects of Artemisia princeps in antigen-stimulated T-cells and regulatory Tccells. J. Pharm. Pharmacol. 61:1043–1050
- Cho, Y. C., Lee, S. H., Lee, M., et al. 2012. Enhanced IL-12p40 production in LPS-stimulated macrophages by inhibiting JNK activation by artemisinin. Arch. Pharm. Res. 35:1961–1968
- Choi, E., Park, H., Lee, J., and Kim, G. 2013. Anti-cancer, anti-obesity, and anti-inflammatory activity of Artemisia species in vitro. J. Trad. Chinese Med. 33:92–97
- Choi, E. J., Lee, S., Chae, J. R., et al. 2011. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 20:1121–1126
- Craig, W. J. 1999. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 70:491–499
- Emami, S. A., and Aghazari, F., (Eds.). (2011). Endemic Phanerogams in the Flora of Iran. Teheran, Iran: Teheran University Publications, pp. 450–451
- Emami, S. A., Zamanai Taghizadeh Rabe, S., Iranshahi, M., et al. 2010. Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression via inactivation of NF-κB. Immunopharmacol. Immunotoxicol. 32:688–695
- Firestone, G. L., and Sundar, S. N. 2009. Anti-cancer activities of artemisinin and its bioactive derivatives. Expert. Rev. Mol. Med. 11:1–15
- Heikamp, E. B., and Powell, J. D. 2012. Sensing the immune microenvironment to coordinate T-cell metabolism, differentiation, and function. Sem. Immunol. 24:414–420
- Ho, W. E., Peh, H. Y., Chan, T. K., and Wong, W. S. 2014. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 142:126–139
- Iranshahi, M., Emami, S. A., and Mahmoud-Soltani, M. 2007. Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. IJBMS. 10:183–188
- Kim, J. H., Kim, H. K., Kwon Kang, S., et al. 2002. New sesquiterpene-monoterpene lactone, artemisolide, isolated from Artemisia argyi. Tetrahedron. Lett. 43:6205–6208
- Li, T., Chen, H., Wei, N., et al. 2012. Anti-inflammatory and immunomodulatory mechanisms of artemisinin on contact hypersensitivity. Int. Immunopharmacol. 12:144–150
- Lu, Y. Y., Chen, T. S., Qu, J. L., et al. 2009. Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J. Biomed. Sci. 16:16–31
- Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation cytotoxicity assay. J. Immunol. Meth. 65:55–63
- Noori, S., Taghikhani, M., Hassan, Z. M., et al. 2010. Tehranolide molecule modulates the immune response, reduce regulatory T-cells, and inhibits tumor growth in vivo. Mol. Immunol. 47:1579–1584
- Omer, B., Krebs, S., Omer, H., and Noor, T. O. 2007. Steroid-sparing effect of wormwood (Aremisia absinthium) in Crohn's disease: A double-blind placebo-controlled study. Phytomedicine. 14:87–95
- Podlech, D. (1986). Compositae VI Anthemideae. In: Flora Iranica (Rechinger, K. H., Ed.). Graz, Germany: Akademische Druck-u Verlagsansalt, pp. 159–223
- Saenz, S. A., Noti, M., and Artis, D. 2010. Innate immune cell populations function as initiators and effectors in TH2 cytokine responses. Trends Immunol. 31:407–413
- Stow Jennifer, L., Low, P. C., Offenhauser, C., and Sangermani, D. 2009. Cytokine secretion in macrophages and other cells: Pathways and mediators. Immunobiology. 214:601–612
- Sun, Y., Wu, X. X., Yin, Y., et al. 2010. Novel immunomodulatory properties of cirsilineol through selective inhibition of IFNγ signaling in a murine model of inflammatory bowel disease. Biochem. Pharmacol. 79:229–238
- Tan, R. V., Zheng, W. F., and Tang, H. Q. 1998. Biologically active substances from genus Artemisia. Planta. Med. 64:295–302
- Tapsell, L. C., Hemphill, I., Cobiac, L., et al. 2006. Health benefits of herbs and spices: The past, the present, the future. Med. J. Australia. 185:4–24
- Valless, J., Garcia, S., Hidalgo, O., et al. 2011. Biology, Genome Evolution, Biotechnological issues and research including applied perspectives in Artemisia (Asteraceae). Adv. Botan. Res. 60:349–419
- Wang, J., Sun, Y., Li, Y., and Xu, Q. 2005. Aqueous extract from aerial parts of Artemisia vestita, a traditional Tibetan medicine, reduces contact sensitivity in mice by down regulating the activation, adhesion and metalloproteinase production of T-lymphocytes. Int. Immuno-pharmacol. 5:407–415
- Yang, Z., Ding, J., Yang, C., et al. 2012. Immunomodulatory and anti-inflammatory properties of Artesunate in experimental colitis. Curr. Med. Chem. 19:4541–4551
- Yin, Y., Gong, F. Y., Tian-Tian Cai, Y. S., et al. 2010. Novel immunomodulatory properties of cirsilineol through selective inhibition of IFNγ signaling in a murine model of inflammatory bowel disease. Biochem. Pharmacol. 79:229–238
- Yin, Y., Sun, Y., Gu, L., et al. 2011. Jaceosidin inhibits contact hypersensitivity in mice via down-regulating IFNγ/STAT1/T-bet signaling in T-cells. Eur. J. Pharmacol. 651:205–211
- Zamanai Taghizadeh Rabe, S., Mahmoudi, M., Ahi, A., and Emami, S. A. 2011. Anti-proliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm. Biol. 49:962–969
- Zamani Taghizadeh Rabe, S., Mousavi, S. H., Tabasi, N., et al. (2014). Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages. J. Immunotoxicol. (Epub ahead of print)