Abstract
Methoxychlor, an organochlorine insecticide developed to replace DDT (dichlorodiphenyltrichloroethane), has been reported to induce mast cell degranulation and to enhance IgE-mediated allergic responses. However, the mechanisms underlying these effects are not clear. To clarify potential mechanisms, the effects of methoxychlor on degranulation of mast cells were examined. Degranulation responses were evaluated using RBL-2H3 cells and mouse bone marrow-derived mast cells with either the antigen-induced or calcium ionophore-induced stimulation. Phosphorylation of enzymes related to signaling events associated with mast cell degranulation was analyzed by immunoblotting. Effects on vascular permeability in the passive cutaneous anaphylaxis reaction were evaluated following oral administration of methoxychlor to BALB/c mice. The results indicated that methoxychlor caused increased mast cell degranulation in the presence of antigen, whereas it had no effect on calcium ionophore-induced degranulation of RBL-2H3 cells. Immunoblot analyses demonstrated that the phosphorylation level of phosphoinositide 3-kinase (which plays a central role in mast cell signaling) was increased by methoxychlor during antigen-induced degranulation. In addition, methoxychlor activated the signaling pathway via the high-affinity IgE receptor by inducing phosphorylation of Syk and PLCγ1/2, which transfer the signal for degranulation downstream. Lastly, oral administration of methoxychlor exhibited a tendency to promote vascular permeability in passive cutaneous anaphylaxis model mice. Taken together, the results here suggested that methoxychlor enhanced degranulation through FcϵRI-mediated signaling and promoted allergenic symptoms involved in mast cell degranulation.
Introduction
Immediate-type allergy is a disorder of the immune system provoked by an allergen bound to immunoglobulin E (IgE) on mast cells. Mast cells are a critical effector involved in the inflammatory reaction and allergic diseases including asthma, pollinosis, and atopic dermatitis. After stimulation of the high-affinity IgE receptor (FcϵRI) by antigen via IgE, mast cells initiate the release of chemical mediators such as histamine and several cytokines inducing inflammatory reaction and vasodilation. In recent years, a number of papers have suggested that allergic diseases are becoming more common worldwide (Yamaki et al., Citation2007) and that the exposure to environmental chemicals may affect the occurrence and/or severity of allergic diseases (Mizota & Ueda, Citation2006).
Methoxychlor (2,2-bis(4-methoxyphenyl)-1,1,1-trichloroethane) is an organochlorine insecticide developed to replace the potent chlorinated pesticide dichlorodiphenyltrichloroethane (DDT) and had been widely used until 2004 (Stuchal et al., Citation2006). Because methoxychlor had been reported to be problematic in terms of environmental contamination and toxicity (Li & Kupfer, Citation1998), the usage of methoxychlor was not approved at the pesticide re-registration process by the Environmental Protection Agency in 2004 in the US (Stuchal et al., Citation2006). Methoxychlor is thought to be a non-steroidal estrogen and has been reported to adversely affect development and reproduction in rats (Chapin et al., Citation1997; Cummings, Citation1997). One of the metabolites of methoxychlor, 2,2-bis(4-hydroxyphenyl)-1,1,1-trichloroethane, converted by cytochrome P450s in the liver, is more estrogenic than methoxychlor (Akgul et al., Citation2011; Cummings, Citation1997).
To date, methoxychlor and its metabolites, which display estrogenic activity both in vitro and in vivo, have drawn attention as environmental pollutants or endocrine-disrupting chemicals. Some papers have noted that exposure to methoxychlor may lead to increased concern regarding fertility, pregnancy, and/or development of organisms (Basavarajappa et al., Citation2012; Vaithinathan et al., Citation2010; Versonnen et al., Citation2004). From an immunological perspective, some environmental estrogens have been reported to induce mast cell degranulation and to enhance IgE-mediated release of allergic mediators; this has been shown in studies using a human mast cell line and mouse primary bone marrow-derived mast cells (BMMC) (Narita et al., Citation2007). However, although some organochlorine pesticides affect immunity based on their estrogenic activity (Sobel et al., Citation2005), the precise mechanism underlying the effects of methoxychlor on allergic responses remains to be defined.
In this study, whether methoxychlor enhanced antigen-induced degranulation in mast cells and affected immediate-type allergic reactions in mice was investigated. Underlying mechanisms whereby the methoxychlor might promote degranulation were also investigated. The results herein indicated to us that methoxychlor—acting like an environmental estrogen—has a capacity to regulate mast cell signaling. This investigation could be of some help to investigators seeking to verify the effects of other environmental pollutants on the incidence and/or magnitude of allergic diseases in exposed human populations.
Materials and methods
Reagents
Methoxychlor (>98% purity), wortmannin, and 4-nitrophenyl 2-acetamido-2-deoxy-β-d- glucopyranoside were purchased from Wako Pure Chemical Industries (Osaka, Japan). Methoxychlor was dissolved in ethanol to prepare a 10 mM solution for use in the experiments below. Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640 medium, penicillin, streptomycin, bovine serum albumin (BSA), mouse anti-dinitrophenol (DNP) monoclonal IgE, DNP-human serum albumin (HSA), calcium ionophore A23187, Triton X-100, Evans Blue dye, and mineral oil were bought from Sigma (St. Louis, MO). Goat anti-actin antibody (sc-1616) and horseradish peroxidase (HRP)-labeled anti-goat IgG antibody (sc-2020) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphoinositide 3-kinase (PI3K) p85 antibody (#4257), anti-phosphorylated PI3K p85/p55 antibody (#4228), a B-cell signaling antibody sampler kit (#9768) including antibodies against Syk, phosphorylated (p)-Syk, Lyn, p-Lyn, Btk, and p-Btk, a phospholipase C (PLC)-γ antibody sampler kit (#3860) including antibodies against PLCγ1, phosphorylated PLCγ1, PLCγ2, and phosphorylated PLCγ2, and HRP-labeled anti-rabbit IgG antibody (#7074) were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals were purchased from Wako Pure Chemical Industries or Nacalai Tesque (Kyoto, Japan), unless otherwise noted.
Cells and cell culture
RBL-2H3 cells were obtained from American Type Culture Collection (Rockville, MD) and cultured in DMEM supplemented with 100 U of penicillin/ml, 100 µg streptomycin/ml, and 10% fetal bovine serum (FBS, SAFC Biosciences, Lenexa, KS) at 37 °C under a humidified 5% CO2\95% air atmosphere.
BMMC were prepared as below. Five-week-old female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan) and kept in a temperature-controlled environment (24 °C) with a 12-h light/12-h dark cycle. Animals received standard chow and water ad libitum. After acclimatization to their housing environment for at least 1 week, BMMC were generated from the 6–8-week-old mice according to the method of Lee et al. (Citation2005) with minor modification. Bone marrow cells were cultured in RPMI 1640 medium supplemented with 100 U penicillin/ml, 100 µg streptomycin/ml, 10% FBS, and 10 ng recombinant murine IL-3/ml (PeproTech, Rocky Hill, NJ) for 3 weeks at 37 °C in a humidified 5% CO2–95% air system. Culture medium was replaced once a week. The BMMC obtained were used for the degranulation assays described below. The Laboratory Animal Care Committee of Ehime University approved all procedures. Mice were maintained in accordance with Guidelines for the Care and Use of Laboratory Animals at Ehime University.
Degranulation assay using RBL-2H3 cells and BMMCs
RBL-2H3 cells or BMMC were seeded, respectively, at 2.5 × 105 or 5.0 × 105 cells/well in 24-well culture plates (BD Falcon, Franklin Lakes, NJ) and cultured at 37 °C overnight. The cells were sensitized with anti-DNP IgE (50 ng/ml; diluted in medium) for 2 h. After washing with modified Tyrode’s buffer (TB; 20 mM HEPES, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 0.05% BSA [pH 7.4]), the cells were treated for 20 min with various concentrations of methoxychlor or with ethanol alone (control) diluted in modified TB (final ethanol concentration; 0.5%). The cells were subsequently stimulated with DNP-HSA (50 ng/ml) diluted in the modified TB for 30 min. After the supernatant was collected, the cells were sonicated in 500 μl of modified TB containing 0.1% Triton X-100 for 10 s on ice. β-Hexosaminidase activity in both the supernatant and cell lysate was measured using 4-nitrophenyl 2-acetamido-2-deoxy-β-d-glucopyranoside as described previously (Ishida et al., Citation2013). The absorbance was measured at 405 nm using a microplate reader (SH-8000Lab, Corona Electric, Ibaraki, Japan). A β-hexosaminidase release rate was calculated as 100 × [(Asupernatant − Ablank of supernatant)/{(Asupernatant − Ablank of supernatant) + (Acell lysate − Ablank of cell lysate)}], in which A is the absorbance of each well.
To assess the effect of methoxychlor on Ca2+ influx leading to degranulation, RBL-2H3 cells cultured in 24-well culture plates overnight as described above were simultaneously treated with various concentrations of A23187 and 20 μM methoxychlor diluted in modified TB for 30 min. Thereafter, β-hexosaminidase activity was measured.
To assess the effect of PI3K inhibition on degranulation events, RBL-2H3 cells were treated for 30 min with various concentrations of wortmannin diluted in modified TB after sensitization with anti-DNP IgE (50 ng/ml) for 2 h, as described above. The cells were subsequently treated with 20 µM methoxychlor diluted in modified TB for 20 min. The cells were then stimulated with DNP-HSA (50 ng/ml) for 30 min, and β-hexosaminidase activity then measured.
Measurement of cell viability
Cytotoxicity of methoxychlor in the RBL-2H3 cells was examined using a WST-8 assay kit (Kishida Chemical, Osaka) according to manufacturer instructions. RBL-2H3 cells sensitized with anti-DNP IgE as above were treated with various concentrations of methoxychlor or ethanol alone and then stimulated with DNP-HSA as above. After washing with phosphate-buffered saline (PBS, pH 7.4) once, 100 μl of medium containing 10 μl WST-8 solution was added to each well of the cell culture plate and the cells were incubated for 30 min at 37 °C. The absorbance was then measured at 450 nm using a microplate reader (SH-8000Lab, Corona Electric). The extent of any change in viability among the treated cells was compared to that of the cells that received vehicle only.
Immunoblot analysis
RBL-2H3 cells were seeded at 2.0 × 106 cells/dish in 100 mm culture dishes (BD Falcon) and cultured at 37 °C overnight. The cells were then sensitized with anti-DNP IgE (50 ng/ml), and cultured for 2 h. Afterward, the cells were washed with modified TB and treated with 20 µM methoxychlor or ethanol alone diluted in modified TB for 20 min. The cells were subsequently stimulated with DNP-HSA (50 ng/ml) for 30 min before preparation of total cell lysates and immunoblotted with various antibodies as previously described (Ishida et al., Citation2013).
Murine passive cutaneous anaphylaxis (PCA) model
The assay was performed by the method of Knoops et al. (Citation2005), with some modifications. Six-week-old BALB/c mice (Japan SLC) were kept in a temperature-controlled facility. After acclimatization for 1 week, PBS alone and 20 ng anti-DNP IgE diluted in PBS was injected intradermally into, respectively, the left and right ears using a 50 µl syringe (Hamilton, Reno, NV). After 24 h, 200 µg DNP-HSA diluted in 200 µl PBS containing 0.5% Evans Blue dye was injected into the tail vein using a 0.5 ml syringe. To assess the effect of methoxychlor, the mice were orally administered with 23 mg/kg body weight of methoxychlor diluted in 200 µl mineral oil or with 200 µl mineral oil alone (control) 1 h before injection of DNP-HSA. All mice were euthanized by cervical dislocation 30 min after the treatment with DNP-HSA, and each ear was excised. The extravasated Evans Blue dye was then extracted from each ear with 700 µl formamide at 63 °C overnight, and the absorbance of the dye was then measured at 620 nm using a Ultraspec 3000 spectrophotometer (Amersham Pharmacia Biotech, Uppsala, Sweden).
Statistical analysis
Data obtained were expressed as the mean ± SD. A Dunnett’s test or a Student’s t-test was used to assess the statistical significance of the difference against control; p-values < 0.05 were considered statistically significant.
Results
Effect of methoxychlor on antigen-dependent degranulation of mast cells
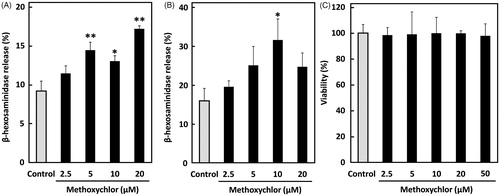
Whether methoxychlor affects degranulation of mast cells was first investigated using RBL-2H3 cells and BMMC, a primary mast cell model, in vitro. Mast cells provoke granule release through two events; sensitization with IgE and stimulation with antigen. Here, mast cells sensitized with IgE (anti-DNP IgE) were treated with methoxychlor and subsequently with antigen (DNP-HSA). The results showed that methoxychlor significantly enhanced β-hexosaminidase release from intracellular granules in the mast cells (). Release of the enzyme from the cells was increased by ≈70% compared with the control when cells were treated with 20 µM methoxychlor. Granule release from BMMC caused by the antigen-induced degranulation was also significantly increased by treating with methoxychlor (). Degranulation of the cells treated with 10 µM methoxychlor was increased 2-fold compared to that of the control. Cytotoxicity of methoxychlor in the RBL-2H3 cells was not noted at the lower concentrations (i.e. < 50 µM; ), indicating that the enhanced degranulation by the RBL-2H3 cells was not caused by cell death. Degranulation rates of BMMC were decreased with 20 µM methoxychlor. Unlike with the RBL-2H3 cells, this outcome could be due to a cytotoxic effect on the BMMC. It is likely that sensitivity to methoxychlor might be different between the RBL-2H3 cells and the BMMC.
Figure 1. Effect of methoxychlor on FcϵRI-mediated β-hexosaminidase release from mast cells. β-Hexosaminidase release by (A) RBL-2H3 cells and (B) BMMC. Anti-DNP IgE-sensitized mast cells were treated with ethanol alone (control) or various concentrations of methoxychlor for 20 min. The cells were subsequently stimulated with DNP-HSA for 30 min and then β-hexosaminidase release was measured. (C) Viability of RBL-2H3 cells. Viability of cells first sensitized with anti-DNP IgE, then treated with various concentrations of methoxychlor or ethanol, and then stimulated with DNP-HSA, was assessed using WST-8 solution. Control cells were treated with antigen, but not with methoxychlor. Data shown are mean ± SD (n = 3). A Dunnett’s test was used to assess significance of difference versus control; *p < 0.05, **p < 0.01.
Effects on calcium ionophore-induced degranulation of RBL-2H3 cells
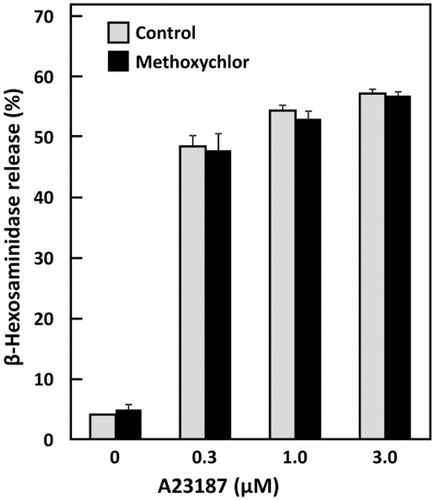
Increases in intracellular Ca2+ concentration are crucial for mast cell degranulation responses. Calcium ionophore A23187 enhances membrane permeability to Ca2+ and causes mast cell degranulation; thus, the effect of methoxychlor on Ca2+ influx was examined here by treating RBL-2H3 cells with A23187. Any influence of methoxychlor on the ionophore-induced degranulation was not observed (). This outcome suggested that any enhanced degranulation caused by methoxychlor depended on signaling from antigen-antibody reactions mediated by FcϵRI.
Figure 2. Effect of methoxychlor on calcium ionophore-induced β-hexosaminidase release from RBL-2H3 cells. RBL-2H3 cells were simultaneously treated with various concentrations of A23187 or with ethanol and with 20 μM methoxychlor for 30 min. β-Hexosaminidase release from the cells was then measured. Data shown are mean ± SD (n = 3). No statistical significance of the difference against the corresponding control was found when assessed using a Student’s t-test.
Effect of PI3K inhibition on enhanced degranulation induced by methoxychlor
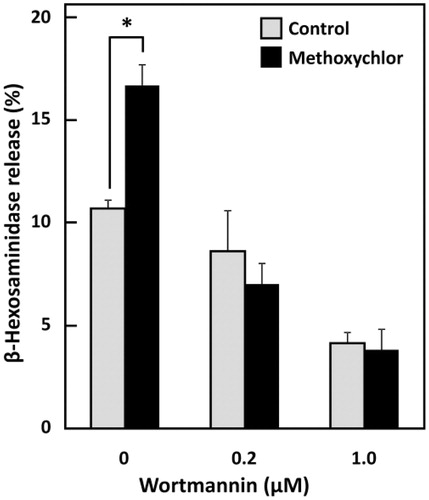
PI3K activated by phosphorylation performs some crucial functions on inducing FcϵRI-mediated degranulation in mast cells. Whether the influence of methoxychlor on mast cell degranulation is related to PI3K was examined using a PI3K inhibitor, wortmannin. The degranulation that had been enhanced by methoxychlor was abrogated by wortmannin (), indicating that PI3K enzyme activity was necessary for the enhanced degranulation induced by methoxychlor.
Figure 3. Effect of methoxychlor on PI3K inhibition by wortmannin in RBL-2H3 cells. Anti-DNP IgE-sensitized RBL-2H3 cells were incubated with various concentrations of wortmannin for 30 min. The cells were washed and treated with 20 µM methoxychlor for 20 min. The cells were subsequently stimulated with DNP-HSA for 30 min. Then, the β-hexosaminidase release from the cells was measured. Control cells were not treated with methoxychlor. Data shown are mean ± SD (n = 3). A Student’s t-test was used to assess the statistical significance of difference versus corresponding control; *p < 0.05.
Effects of methoxychlor on phosphorylation level of PI3K in RBL-2H3 cells
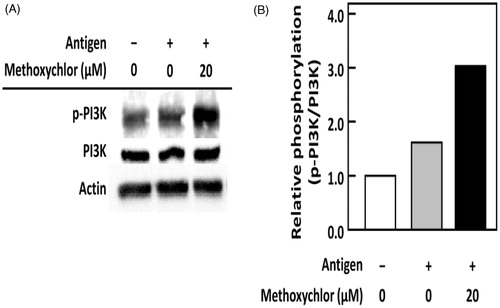
The effect of methoxychlor on PI3K phosphorylation in RBL-2H3 cells was examined by immunoblot analysis. As shown in , phosphorylation of PI3K was strongly increased by treating the RBL-2H3 cells with methoxychlor. This outcome suggested to us that the degranulation enhanced by methoxychlor was caused by a strongly activated PI3K by phosphorylation.
Figure 4. Effect of methoxychlor on phosphorylation of PI3K in RBL-2H3 cells. Anti-DNP IgE-sensitized RBL-2H3 cells were treated with or without 20 μM methoxychlor for 20 min. The cells were subsequently stimulated with or without DNP-HSA for 30 min. Cell lysates were then prepared, and immunoblot analyses performed. The p-PI3K represents phosphorylated PI3K. (A) Representative image of PI3K phosphorylation from two independent immunoblot analyses. (B) Ratio of p-PI3K/PI3K relative to values for intact cells.
Effects on signaling pathways involved in antigen-dependent degranulation of RBL-2H3 cells
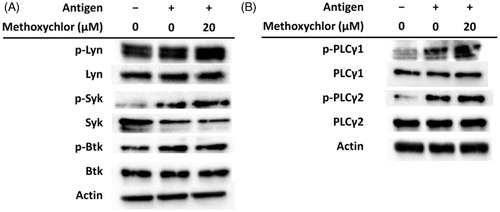
The effects of methoxychlor on phosphorylation of tyrosine kinases and PLCγ in signal pathways mediated by FcϵRI in RBL-2H3 cells were examined by immunoblot analysis. As seen in , methoxychlor did not affect phosphorylation of Lyn localizing on the immunoreceptor tyrosine-based activation motif (ITAM) under the β-chain of FcϵRI. On the other hand, phosphorylation of Syk, a tyrosine kinase phosphorylated by Lyn, was increased due to the methoxychlor. In addition, phosphorylation of PLCγ1/2 was also increased along with that of Syk (). Methoxychlor did not affect phosphorylation of Btk. These results suggested to us that methoxychlor enhanced FcϵRI-mediated degranulation in RBL-2H3 cells via activation of Syk and PLCγ cascades.
Figure 5. Effect of methoxychlor on phosphorylation of FcϵRI-mediated signaling factors in RBL-2H3 cells. Anti-DNP IgE-sensitized RBL-2H3 cells were treated with or without 20 μM methoxychlor for 20 min. Cells were subsequently treated with or without DNP-HSA for 30 min. Cell lysates were then prepared and immunoblot analyses performed. The p-Lyn, p-Syk, p-Btk, and p-PLC represent phosphorylated Lyn, Syk, Btk, and PLC, respectively. Representative image of phosphorylation of (A) tyrosine kinases and (B) PLCγ from three independent immunoblot analyses.
Effect of methoxychlor in the PCA mouse model
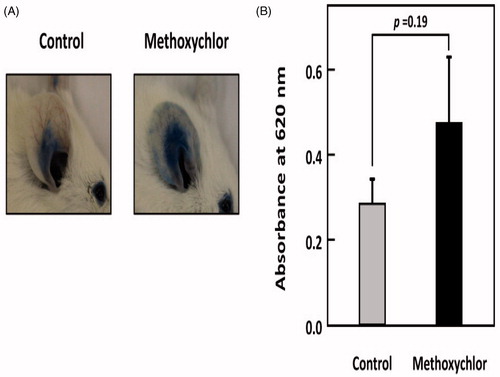
The effect of methoxychlor on IgE-mediated anaphylaxis was examined in vivo. showed there was a tendency to enhance the PCA reaction in mice after oral administration of methoxychlor at 23 mg/kg BW. The result indicated that there was an increased risk of worsening of immediate-type allergic symptoms in vivo due to methoxychlor or its metabolites.
Figure 6. Effect of methoxychlor on IgE-mediated PCA in mice. Seven-week-old female BALB/c mice were intravenously injected with 200 µg DNP-HSA containing 0.5% Evans Blue dye 24 h after intradermal administration of anti-DNP IgE (20 ng) in PBS and PBS alone into, respectively, their right and left ears. Methoxychlor (23 mg/kg BW) in mineral oil or mineral oil alone (control) was orally administered 1 h before antigen treatment. (A) Photographs of representative right ears from methoxychlor-treated and control mice 30 min after administration of DNP-HSA. (B) Evans Blue dye was extracted from each ear and the absorbance measured at 620 nm (n = 6). The absorbance of the dye extracted from the left ear was subtracted from that from the right ear. A Student’s t-test was used to assess the significance of the difference against control.
Discussion
The immediate allergic reaction is a life-threatening disease associated with the immune function of mast cells locating throughout the human body. Upon exposure to allergen, mast cells are stimulated via IgE on FcϵRI to release several chemical mediators such as histamine, serotonin, and heparin by degranulation, which is an important phase in the allergic reaction (Kemp & Lockey, Citation2002). In this study, methoxychlor was evaluated for the influence on allergic diseases and found to enhance the FcϵRI-mediated degranulation of both RBL-2H3 cells and BMMC. Because there is a possibility that RBL-2H3 cells might not represent the characteristics of mast cells (Passante et al., Citation2009), methoxychlor was evaluated using not only RBL-2H3 cells but also mouse BMMC. As a result, the influence of methoxychlor on BMMC was found to be highly similar to that on RBL-2H3 cells (), indicating that methoxychlor exacerbates degranulation provoked by allergen.
RBL-2H3 cells and BMMC are known to express estrogen receptor (ER)-α (Zaitsu et al., Citation2007). Narita et al. (Citation2007) reported that some environmental estrogens that affected degranulation of mast cells did so via effects on ERα. Those authors also demonstrated that degranulation of BMMC deficient in ERα was decreased when the cells were exposed to dichlorodiphenyldichloroethylene (a compound structurally similar to methoxychlor) or to other organochlorine pesticides. In addition, some estrogens increased intracellular Ca2+ levels in tumor cells via effects on membrane ERα (Wozniak et al., Citation2005). These facts indicated to us that methoxychlor might enhance degranulation responses of mast cells through not only effects on FcϵRI, but also upon ERα.
A calcium ionophore can induce a substantial granule release from RBL-2H3 cells by itself (Lo et al., Citation1987) and is, therefore, used as an investigative tool to decipher the signal transduction mechanism that regulates the downstream of calcium mobilization (Hanson & Ziegler, Citation2002). We examined the effect of methoxychlor on degranulation of RBL-2H3 cells induced by treating several doses of the calcium ionophore (). As a result, methoxychlor was found not to affect the calcium ionophore-induced degranulation of RBL-2H3 cells at all. Similarly, treatment of methoxychlor alone, without stimulation with antigen or calcium ionophore, had no practical impact on degranulation of mast cells (data not shown). Because Ca2+ influx results from the signaling induced by the antigen-antibody reaction on FcϵRI, as interpreted from results described above, methoxychlor seems to affect the early stage of the signaling pathways involved in degranulation, that is to say, in the intracellular signaling generated proximally to the plasma membrane where FcϵRI locates, but not in the downstream of signal transduction after calcium mobilization.
In mast cells, PI3K plays a central role in the FcϵRI signaling (Hirasawa et al., Citation1997). The activated PI3K catalyzes conversion of phosphatidylinositol-4,5-bisphosphate into phosphatidylinositol-3,4,5-trisphosphate that recruits several signaling factors regulating degranulation of mast cells and activates the subsequent cascades (Gilfillan & Tkaczyk, Citation2006; Kraft & Kinet, Citation2007). Therefore, the relationship between PI3K activation and the enhanced degranulation by methoxychlor was evaluated. We found that the enhancement of granule release by methoxychlor was significantly suppressed by the treatment of wortmannin, a PI3K-specific inhibitor (). In addition, the phosphorylation level of PI3K was increased by methoxychlor under the condition stimulated with antigen (). In the mast cell signaling, PI3K is phosphorylated through activation of Fyn and/or Syk (Gilfillan & Rivera, Citation2009). Hence, methoxychlor seems to directly promote PI3K phosphorylation or to affect other signaling factors locating upstream of PI3K.
After cross-linkage of antigen to IgE bound on the FcϵRI, complex, intracellular signaling cascades are initiated. In the mast cell signaling, many signaling factors such as tyrosine kinases that ultimately lead to the effector function including degranulation are concerned, as shown in earlier reviews (Gilfillan & Tkaczyk, Citation2006; Kraft & Kinet, Citation2007). Activation of Lyn, one of the Src family tyrosine kinases, is essential for phosphorylation of ITAM after the cross-linkage between allergen and IgE (Pribluda et al., Citation1994). The interaction between FcϵRI and Lyn is based on ITAM phosphorylation (Young et al., Citation2003) and initiated by recruitment of the FcϵRI subunits associated with aggregation of the receptors after the antigen stimulation. As observed by immunoblot analysis, the phosphorylation level of neither Btk nor Lyn was affected by treating with methoxychlor. The phosphorylation level of Syk was, on the other hand, increased by methoxychlor. Syk, which is essential for propagation of the FcϵRI-mediated signaling, is phosphorylated by the activated Lyn via phosphorylation of γ-chains of ITAM on the primary signaling pathway involved in degranulation of mast cells (Rivera & Gilfillan, Citation2006). Activation of Btk also depends on the activated Lyn (Iwaki et al., Citation2005). Thus, methoxychlor does not enhance phosphorylation of Lyn or Btk, but does phosphorylation of Syk for enhanced degranulation of RBL-2H3 cells.
The effect of methoxychlor on the PLCγ cascade was further examined by immunoblot analysis. In the mast cell signaling, PLCγ is phosphorylated and activated by the membrane recruitment of Btk induced by the phosphorylated PI3K through phosphatidylinositol-3,4,5-trisphosphate (Kraft & Kinet, Citation2007). PLCγ is also phosphorylated by recruitment of the phosphorylated Syk. Additionally, the activated PLCγ produces both diacylglycerol that activates protein kinase C and inositol-1,4,5-trisphosphate that induces Ca2+ release through the inositol-1,4,5-trisphosphate receptors on the endoplasmic reticulum, leading to degranulation (Gilfillan & Rivera, Citation2009). As shown here, phosphorylation of PLCγ1/2 was promoted as well as that of PI3K and Syk by the treatment with methoxychlor under stimulation with antigen, suggesting that methoxychlor causes activation of the PLCγ cascades which directly induce degranulation via the phosphorylation of Syk and PI3K.
We lastly investigated whether the outcomes of the in vitro studies might relate with those of in vivo studies. In a previous experiment, we found that oral administration of methoxychlor for 2 weeks (at 23 mg/kg BW corresponding to 1/20 of its LD50) enhanced IgE titers in the blood of mice with allergic contact dermatitis (data not shown). We therefore administered the same dose of methoxychlor (23 mg/kg BW) here to examine the effects on the immediate-type allergic reactions caused by the mast cell activation in vivo. Oral administration of methoxychlor had a tendency to promote the immediate-type allergic reaction in the mouse PCA model. These results in vivo most likely reflect a partial impact of methoxychlor on the degranulation of mast cells, as was noted in vitro as described above.
Enhancement of an allergic reaction in vivo by chemicals is affected not only by the level of dosage, but also by the length of exposure, time since exposure, any degree of accumulation of the toxicant in the body, and/or the generation of toxicologically-active metabolites. In general, orally-ingested chemicals are metabolized mainly by various forms of cytochrome P450 in the intestine and liver. Specifically, methoxychlor is metabolized to 2,2-bis-(4-hydroxyphenyl)-1,1,1-trichloroethane (HPTE); this latter compound, in turn, can act as an ERα agonist and an ERβ antagonist in several cell types (Gaido et al., Citation2000). There are reports that the mechanisms of mast cell activation by environmental estrogens are similar to those induced by estradiol which, alone, can enhance degranulation of and Ca2+ influx into RBL-2H3 cells (Zaitsu et al., Citation2007). In the in vivo studies here, as the single administration of methoxychlor had a relatively small effect on rapid allergic reactions, it is possible that methoxychlor might be impacting on mast cell degranulation not just through FcϵRI pathways, but potentially through changes induced in ER-related pathways as well.
Conclusions
In this study, methoxychlor was shown to promote the FcϵRI-mediated degranulation of RBL-2H3 cells and BMMC, a primary immature mast cell model, in vitro, and to tend to enhance the immediate-type allergic reaction in PCA model mice in vivo. In addition, the enhanced degranulation by methoxychlor was demonstrated to depend in a large part on the signaling pathway of RBL-2H3 cells via the cross-linkage of antigen to IgE on FcϵRI. Some signaling factors, including PI3K and Syk, were activated after treating the mast cells with methoxychlor. Overall, these results suggest to us that the exposure of methoxychlor might worsen immediate allergic diseases.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank members of the Integrated Center for Sciences, Ehime University for the use of the animal facility.
References
- Akgul, Y., Derk, R. C., Meighan, T., et al. 2011. The methoxychlor metabolite, HPTE, inhibits rat luteal cell progesterone production. Reprod. Toxicol. 32:77–84
- Basavarajappa, M. S., Hernández-Ochoa, I., Wang, W., and Flaws, J. A. 2012. Methoxychlor inhibits growth and induces atresia through the aryl hydrocarbon receptor pathway in mouse ovarian antral follicles. Reprod. Toxicol. 34:16–21
- Chapin, R. E., Harris, M. W., Davis, B. J., et al. 1997. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam. Appl. Toxicol. 40:138–157
- Cummings, A. M. 1997. Methoxychlor as a model for environmental estrogens. Crit. Rev. Toxicol. 27:367–379
- Gaido, K. W., Maness, S. C., McDonnell, D. P., et al. 2000. Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: Structure-activity studies. Mol. Pharmacol. 58:852–858
- Gilfillan, A. M., and Rivera, J. 2009. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 228:149–169
- Gilfillan, A. M., and Tkaczyk, C. 2006. Integrated signaling pathways for mast-cell activation. Nat. Rev. Immunol. 6:218–230
- Hanson, D. A., and Ziegler, S. F. 2002. Regulation of ionomycin-mediated granule release from rat basophil leukemia cells. Mol. Immunol. 38:1329–1335
- Hirasawa, N., Sato, Y., Yomogida, S., et al. 1997. Role of phosphatidylinositol 3-kinase in degranulation induced by IgE-dependent and -independent mechanisms in rat basophilic RBL-2H3 (ml) cells. Cell Signal. 9:305–310
- Ishida, M., Nishi, K., Watanabe, H., and Sugahara, T. 2013. Inhibitory effect of aqueous spinach extract on degranulation of RBL-2H3 cells. Food Chem. 136:322–327
- Iwaki, S., Tkaczyk, C., Satterthwaite, A. B., et al. 2005. Btk plays a crucial role in the amplification of FcϵRI-mediated mast cell activation by kit. J. Biol. Chem. 280:40261–40270
- Kemp, S. F., and Lockey, R. F. 2002. Anaphylaxis: A review of causes and mechanisms. J. Allergy Clin. Immunol. 110:341–348
- Knoops, L., Louahed, J., van Snick, J., and Renauld, J. C. 2005. IL-9 promotes but is not necessary for systemic anaphylaxis. J. Immunol. 175:335–341
- Kraft, S., and Kinet, J. P. 2007. New developments in FcϵRI regulation, function and inhibition. Nat. Rev. Immunol. 7:365–378
- Lee, E., Yook, J., Haa, K., and Chang, H. W. 2005. Induction of Ym1/2 in mouse bone marrow-derived mast cells by IL-4 and identification of Ym1/2 in connective tissue type-like mast cells derived from bone marrow cells cultured with IL-4 and stem cell factor. Immunol. Cell Biol. 83:468–474
- Li, H. C., and Kupfer, D. 1998. Mechanism of induction of rat hepatic CYP2B and 3A by the pesticide methoxychlor. J. Biochem. Mol. Toxicol. 12:315–323
- Lo, T. N., Saul, W., and Beaven, M. A. 1987. The actions of Ca2+ ionophores on rat basophilic (2H3) cells are dependent on cellular ATP and hydrolysis of inositol phospholipids. A comparison with antigen stimulation. J. Biol. Chem. 262:4141–4145
- Mizota, K., and Ueda, H. 2006. Endocrine disrupting chemical atrazine causes degranulation through Gq/11 protein-coupled neurosteroid receptor in mast cells. Toxicol. Sci. 90:362–368
- Narita, S., Goldblum, R. M., Watson, C. S., et al. 2007. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ. Health Perspect. 115:48–52
- Passante, E., Ehrhardt, C., Sheridan, H., and Frankish, N. 2009. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm. Res. 58:611–618
- Pribluda, V. S., Pribluda, C., and Metzger, H. 1994. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc. Natl. Acad. Sci. USA 91:11246–11250
- Rivera, J., and Gilfillan, A. M. 2006. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117:1214–1225
- Sobel, E. S., Gianini, J., Butfiloski, E. J., et al. 2005. Acceleration of autoimmunity by organochlorine pesticides in (NZB × NZW) F1 mice. Environ. Health Perspect. 113:323–328
- Stuchal, L. D., Kleinow, K. M., Stegemen, J. J., and James, M. O. 2006. Demethylation of the pesticide methoxychlor in liver and intestine from untreated, methoxychlor-treated, and 3-methylcholanthrene-treated channel catfish (Ictalurus punctatus): Evidence for roles of CYP1 and CYP3A family isozymes. Drug Metab. Dispos. 34:932–938
- Vaithinathan, S., Saradha, B., and Mathur, P. P. 2010. Methoxychlor induces apoptosis via mitochondria- and FasL-mediated pathways in adult rat testis. Chem. Biol. Interact. 185:110–118
- Versonnen, B. J., Roose, P., Monteyne, E. M., and Janssen, C. R. 2004. Estrogenic and toxic effects of methoxychlor on zebrafish (Danio rerio). Environ. Toxicol. Chem. 23:2194–2201
- Wozniak, A. L., Bulayeva, N. N., and Watson, C. S. 2005. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ. Health Perspect. 113:431–439
- Yamaki, K., Taneda, S., Yanagisawa, R., et al. 2007. Enhancement of allergic responses in vivo and in vitro by butylated hydroxytoluene. Toxicol. Appl. Pharmacol. 223:164–172
- Young, R. M., Holowka, D., and Baird, B. 2003. A lipid raft environment enhances Lyn kinase activity by protecting active site tyrosine from dephosphorylation. J. Biol. Chem 278:20746–20752
- Zaitsu, M., Narita, S., Lambert, K. C., et al. 2007. Estradiol activates mast cells via a non-genomic estrogen receptor-α and calcium influx. Mol. Immunol. 44:1977–1985

