Abstract
Inflammation is a crucial factor in the pathogenesis of numerous diseases. This study sought to evaluate the effects of thymol and carvacrol, the main components of Thymus vulgaris (thyme) essential oil, on transcription factors regulating inflammation. Lipopolysaccharide (LPS)-stimulated J774.1 mouse macrophages were examined by real time-PCR for interleukin (IL)-1β and tumor necrosis factor (TNF)-α gene expression in the presence of these compounds. Levels of inducible phospho-nuclear factor-κB (pNF-κB) p65, activator protein-1 [AP-1(c-Fos/c-Jun)], and nuclear factors of activated T-cells (NFATs) were also measured using Western blots. Levels of phosphorylation of stress-activated protein kinases (SAPKs)/c-Jun N-terminal kinase (SAPK/JNK), signal transducer, and activator of transcription (STAT-3), p38, IκBα, and NF-κB p65, as well as total levels of IL-1β and TNFα were determined. The results indicated carvacrol significantly reduced both IL-1β and TNFα at the protein and mRNA levels; thymol also significantly reduced IL-1β expression. Western blot analyses of nuclear cell extracts revealed both agents caused significantly decreased expression of c-Fos, NFAT-1, and NFAT-2; decreased expression of c-Jun was only caused by carvacrol. Neither agent inhibited p-NF-κB p65 expression. At the protein level, carvacrol and thymol each caused decreases in inducible phospho-SAPK/JNK and phospho-STAT3 levels, whereas only carvacrol resulted in increased p-p38 levels in the total cell extract. Despite the reduction of phospho-IκBα caused by both agents, p-NF-κB p65 still increased in the presence of carvacrol. Based on these findings, it is concluded that carvacrol and thymol could contribute to reduction of inflammatory responses through modulation of the expression of JNK, STAT-3, AP-1, and NFATs.
Introduction
Chronic inflammation can cause considerable damage to body tissues, leading to numerous problems, depending on location (Murakami & Ohigashi, Citation2007). The nuclear factor (NF)-κB family of transcription factors plays a central role in inflammatory processes and induces the expression of numerous pro-inflammatory genes (Stein et al., Citation1993). Normally, NF-κB transcription factor complex is retained as an inactive form in the cytoplasm due to binding to the IκB inhibitor. Upon activation, IκB is ubiquitinated and degenerated, allowing phosphorylation of the NF-κB complex, and promoting its transporting into the nucleus where it can bind to promoter sites to regulate transcription of target genes (Hoffmann & Baltimore, Citation2006). Similar to NF-κB, activator protein-1 (AP-1), made from a heterodimer of c-Fos and c-Jun oncoproteins, regulates numerous inflammatory genes from which a number require the simultaneous, cooperative activation of NF-κB (Stein et al., Citation1993).
Nuclear factor of activated T-cells (NFAT) is another transcription factor activated through dephosphorylation via calcineurin. Upon activation, this molecule translocates to the nucleus, where it interacts with AP-1 to induce gene transcription (Adcock & Caramori, Citation2001). NFAT signaling after lipopolysaccharide (LPS) stimulation plays a key role in innate immune cell (e.g., macrophages and dendritic cells) activation (Fric et al., Citation2012; Hawiger, Citation2001; Liu et al., Citation2011). Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathways regulate the cell types involved in the beginning, dissemination, and resolution of inflammation by transmitting information from the chemical signals outside the cell into gene promoters on DNA (Kim et al., Citation2003; O'Shea & Murray, Citation2008). STAT-3 mediates activity of cytokines generally associated with inflammatory responses, and its signaling plays a central role in the pathogenesis of inflammatory diseases such as rheumatoid arthritis (Park et al., Citation2014).
The family of mitogen-activated protein kinases (MAPK) regulates cell differentiation, proliferation and gene expression in response to various stimuli (e.g. pro-inflammatory cytokines). The MAPK family is composed of different members, including extracellular signal-regulated kinases (ERK), stress-activated protein kinases (SAPK), c-Jun N-terminal kinases (JNK) and p38 kinases (Ferrer et al., Citation2002). JNK regulates both maturation and activity of T-cells and synthesis of pro-inflammatory cytokines such as interleukin (IL)-2, IL-6 and tumor necrosis factor (TNF)-α. Several recent studies have demonstrated the importance of the JNK pathway in chronic inflammatory disorders that involve expressions of specific proteases and cytokines (Roy et al., Citation2008). The p38 MAPK signaling pathway plays an important role in inflammation and cellular responses to external stress signals and other physiological processes (Ferrer et al., Citation2002). It has been shown that p38 MAPK can also modulate NF-κB activity (Adcock & Caramori, Citation2001). Inhibitors of the p38 family have anti-inflammatory effects in animal models of various diseases (Kumar et al., Citation2003; Saklatvala, Citation2004; Surh et al., Citation2001).
Herbal remedies have become increasingly popular because of their safety and cost-effectiveness (Purushoth et al., Citation2012). A large number of plant species contain a range of bioactive compounds that possess beneficial health properties (Juhas et al., Citation2008). In traditional medicine, various aromatic plants – such as some Thymus species – are used for treatment of infections, arthritis and asthma (Al-Khalaf, Citation2013; Mohamed et al., Citation2013). Thymus vulgaris (thyme) has received extensive attention because of its imparting of antimicrobial, anti-oxidant and anti-inflammatory effects (Ocaña & Reglero, Citation2012). Thyme essential oil is a mixture of monoterpenes that are natural products belonging to the chemical group of terpenes. Two monoterpenes, thymol and carvacrol, are major constituents of thyme oil and are currently used in cosmetic and pharmaceutical preparations, as well as in the food industry (Fachini-Queiroz et al., Citation2012). Both of these agents have been shown to impart anti-inflammatory activities. In in vivo studies, they have significantly inhibited inflammatory edema and leukocyte migration (Fachini-Queiroz et al., Citation2012). There are also a number of reports of thymol or carvacrol regarding their effects on key mediators of inflammation such as cyclooxygenase (COX)-2 (Landa et al., Citation2009), inducible nitric oxide synthase (iNOS), as well as inflammatory cytokines like TNFα and IL-1β (Liang et al., Citation2014). Despite this information, various aspects of the molecular mechanisms of action of thymol and carvacrol remain unclear. Thus, the objective of the present study was to evaluate effects of these components on various signaling molecules that act as key transcription factors in the regulation of inflammation.
Materials and methods
Materials
Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS) and non-essential amino acids were purchased from Gibco (Ashland, KY). Trypan blue, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), cell culture grade dimethyl sulfoxide (DMSO), lipopolysaccharide (LPS; type 0111:B4 from Escherichia coli), thymol, carvacrol, phosphatase inhibitor cocktail 3, phenylmethylsulfonyl fluoride (PMSF), luminol, and coumaric acid were purchased from Sigma (St. Louis, MO). Sodium dodecyl sulfate (SDS) and polyacrylamide gel were obtained from BioRad (Carlsbad, CA). Nuclear and cytoplasmic extraction reagents were purchased from Thermo Scientific (San Jose, CA). A PathScan inflammation multi-target sandwich enzyme-linked immunosorbent assay (ELISA) kit, anti-rabbit IgG horseradish peroxidase (HRP)-linked antibody, as well as rabbit monoclonal antibodies (mAb) against histone H3, phospho-NF-κB p65 (Ser536), c-Fos, c-Jun, NFAT-1 and NFAT-2 were obtained from Cell Signaling Technology (Beverly, MA). IL-1β and TNFα ELISA kits were purchased from eBioscience (San Diego, CA). RNX-Plus Solution for total RNA isolation and cyclosporine A (CSA) were obtained from, respectively, Sinaclon (Tehran, Iran) and Zahravipharma (Tabriz, Iran). High-capacity cDNA reverse transcription kits and SYBR Premix Ex Taq II were purchased from ABI and TAKARA (Foster City, CA), respectively.
Cell culture and compound preparation
The murine macrophage cell line J774.1 (obtained from cell bank of Pasteur Institute, Tehran, Iran) was cultured at a density of 106 cells/ml in DMEM culture medium supplemented with 10% heat-inactivated FBS and 1% non-essential amino acids. The cells were allowed to grow at 37 °C in a humidified 5% CO2 incubator until confluent and then used for further experiments. Cell viability was determined by trypan blue exclusion. After every 10 passages, cells were discarded and replaced with newly-thawed stocks.
Thymol and carvacrol – at stock solutions of 20 mg/ml – were prepared in DMSO. In this paper, DMSO [as vehicle] at the highest concentration used in the tests (e.g. 1% for MTT assay and 0.1% for other experiments) was added to all control cultures that received none of the test components.
Toxicity assessment
To establish the non-toxic concentrations of thymol and carvacrol, the J774.1 cells were cultured in the presence of various concentrations of the compounds and then viability was determined using an MTT reduction assay as described in Amirghofran et al. (Citation2011). In brief, cells were grown in 96-well plates (Nunclone, Thermo Scientific, CA) to a density of 7.5 × 103 cells/well. After overnight incubation, the cells were exposed to different concentrations of either agent (0.1, 1, 10, 20, 30, 50 or 100 µg/ml) in a final volume of 100 µl. Negative control contained only cells; a positive control received cisplatin (50 µg/ml). After 24 h, 10 µl MTT solution (5 mg/ml in phosphate-buffered saline [PBS, pH 7.4]) was added to each well and the plates were incubated for 4 h at 37 °C. Then medium was then removed from the wells and 150 µl DMSO was added to dissolve the formazan produced in the cells. The optical density (OD) in the wells was then measured with a microplate reader (BioTek, Winooski, VT) at 570 nm with a background subtraction at 630 nm. The OD of solubilized formazan in negative control cells was accepted as 100% viability.
Real time-PCR
For this assay, 5 × 105 cells were placed into each well of 24-well plates (Nunclone) in DMEM medium and then received LPS (1 µg/ml) and different concentrations of thymol (10 and 20 µg/ml) or carvacrol (20 and 30 µg/ml). Negative control contained only cells; cells that received LPS and medium served as positive controls. After 24 h of culturing at 37 °C, well supernatants were collected and stored at −80 °C for cytokines evaluation; the cells were used for real time-PCR analysis. In brief, total RNA was prepared using RNX-Plus solution according to the manufacturer protocols. After establishing the sample purity using a NanoDrop 2000c Spectrophotometer (Thermo Scientific, Wilmington, DE), sample target RNA (10 µl) was reverse-transcribed using a high-capacity cDNA reverse transcription kit at 37 °C for 120 min in the presence of dNTP mix, reverse transcriptase and random hexamers. For analysis of IL-1β and TNFα gene expression levels, real time-PCR was performed in a final volume of 20 µl containing 2 µl cDNA, 10 µl SYBR Premix Ex Taq II, 0.4 µl ROX reference dye-2, 0.8 µl PCR forward primer (10 pM), 0.8 µl PCR reverse primer (10 pM) and 6 µl doubly-distilled water. The primers used were: GAPDH [forward], 5′-CGGTGTGAACGGATTTGGC-3′; GAPDH [reverse], 5′-GTGAGTGGAGTCATACTGGAAC-3′; TNFα [forward], 5′-GTC-TCAGCC-TCTTCTCATTC-3′; TNFα [reverse], 5′-GGAACTTCTCATCCCTTTGG-3′; IL-1β forward, 5′-GAAGAAGAGCCCATCCTC-3′; and, IL-1β [reverse], 5′- GTTCATCTCGGAGC-CTGTAG-3′. Real-time PCR was performed in an Applied Biosystems StepOne system (Foster City, CA). The PCR conditions were: one cycle at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 53 °C (TNFα and IL-1β) or 56.3 °C (GAPDH) for 18 s and 72 °C for 30 s. GAPDH served as an endogenous control. Results of target mRNA levels were normalized against GAPDH mRNA in each sample.
Cytokine measurements
The supernatant of the cell cultures were assessed for IL-1β and TNFα levels to quantify inducible production by the J774.1 cells. The level of each cytokine was determined using eBioscience ELISA kits, according to manufacturer instructions. Kit sensitivity was 8 pg/ml for each cytokine.
Transcription factor assessments
Cytoplasmic levels of phospho(p)-SAPK/JNK (Thr183/Tyr185), p-STAT3 (Tyr705), p-p38 (Thr180/Tyr182), p-IκBα (Ser32), p-NF-κB p65 (S536) and NF-κB p65 were measured in J774.1 cells using a PathScan inflammation multi-target sandwich ELISA kit. Briefly, 5 × 105 cells/well were cultured in 24-well culture plates (Nunclone) in DMEM media. The media was then aspirated and the cells treated (in triplicate) with fresh media containing test agent/regulator [thymol (10 and 20 µg/ml), carvacrol (20 and 30 µg/ml) or CSA (2 µg/ml)]. Cells treated just with LPS and those cultured in the absence of LPS + components were used as positive and negative controls, respectively. After an overnight incubation at 37 °C, LPS (1 µg/ml) was added to designated wells and the incubation continued for 15 min (for detection of p-SAPK/JNK, p-STAT3, p-p38 and p-IκBα) or 30 min (for detection of p-NF-κB p65 and NF-κB p65).
After the specified incubation period, the medium was removed and cells in each well collected in ice-cold PBS. Thereafter, 0.5 ml ice-cold cell lysis buffer + 1 mM PMSF was added and the cells incubated on ice for 5 min followed by a brief sonication. The tubes were then centrifuged for 10 min (14 000 rpm) at 4 °C; the supernatant was collected and stored at −80 °C until analyzed. For the ELISA, 100 µl of each cell lysate was added to appropriate wells and the plate incubated for 2 h at 37 °C. After washing with ELISA wash buffer, 100 µl kit-provided detection antibody was added to each well and the plate incubated at 37 °C for 1 h. After washing with wash buffer, 100 µl kit-provided HRP-linked secondary antibody was added to each well and the plate incubated for 30 min at 37 °C. After a final washing with wash buffer, 100 µl tetramethyl-benzidine (TMB) substrate was added to each well and the plate was incubated for 10 min at 37 °C before 100 µl stop solution was added to each well. The absorbance in each well was then measured at 450 nm using the BioTek microplate reader.
Cytoplasmic and nuclear protein extraction
J774.1 cells (2 × 106 cells/well; 2 ml/well) cultured in 6-well culture plates (Nunclone) in DMEM medium were treated with thymol (10 or 20 µg/ml), carvacrol (20 or 30 µg/ml) or CSA (2 µg/ml). Cells treated just with LPS and those cultured in the absence of LPS + components were considered positive and negative controls, respectively. After 24 h, LPS (1 µg/ml) was added and the incubation continued for 30 min before the cells were washed with cold PBS .All cells were collected and a total of 107 cells/treatment were transferred to a 1.5-ml microtube. The cells were pelleted by centrifugation at 500 × g for 3 min and the resultant supernatant then carefully removed. To each cell pellet, 200 µl ice-cold cytoplasmic extraction reagent-1 (CER I) + 1 mM PMSF and phosphatase inhibitor cocktail-3 was added. After vortexing, the pellet was re-suspended in 11 µl ice-cold CER II and then the suspension was centrifuged for 5 min at 16 000 × g. The resulting supernatant (cytoplasmic extract) was transferred to a clean pre-chilled tube. The remaining insoluble fraction (pellet) was suspended in 100 µl ice-cold nuclear extraction reagent (NER) + 1 mM PMSF and phosphatase inhibitor cocktail-3. After vortexing on ice, the tube was centrifuged for 10 min at 16 000 × g. The resulting supernatant (nuclear extract) was immediately transferred to a clean pre-chilled tube and stored at −80 °C until use in Western blot analyses.
Western blot analyses
From each sample, 25 µg of nuclear proteins was separated over 10% sodium dodecyl sulfate-polyacrylamide gels and then electrotransferred to nitrocellulose membranes before being blocked in 2% BSA/Tris-buffered saline containing 0.1% Tween 20 (TBST buffer) overnight. The membranes were then incubated with rabbit mAb against histone H3 (1:1200), c-Fos (1:1000), c-Jun (1:1000), NFAT-1 (1:1000), NFAT-2 (1:1000) or phospho-NF-κB p65 (1:600) at 4 °C for 12 h with gentle agitation. After washing away unbound primary antibody with TBST buffer, anti-rabbit IgG HRP-linked secondary antibody (1:2000) was added and the membrane incubated at 25 °C for a further 1 h with gentle agitation. After gently washing with TBST buffer, all immunoreactive bands were then visualized using a chemiluminescent reagent (5 ml Tris buffer [100 mM, pH 8.5] containing 25 µl luminol, 11 µl coumaric acid and 4 µl H2O2). The visible bands were then quantified using ImageJ software (NIH, Bethesda, MD).
Statistics
All experiments were performed in triplicate and repeated at least three times. All data are reported as mean ± SD. Significant differences between groups were evaluated using Graphpad software (San Diego, CA) and appropriate statistical tests, e.g. one-way analysis of variance (ANOVA) and a Student's t-test. A p value < 0.05 was accepted as significant.
Results
Effects of thymol and carvacrol on cell viability
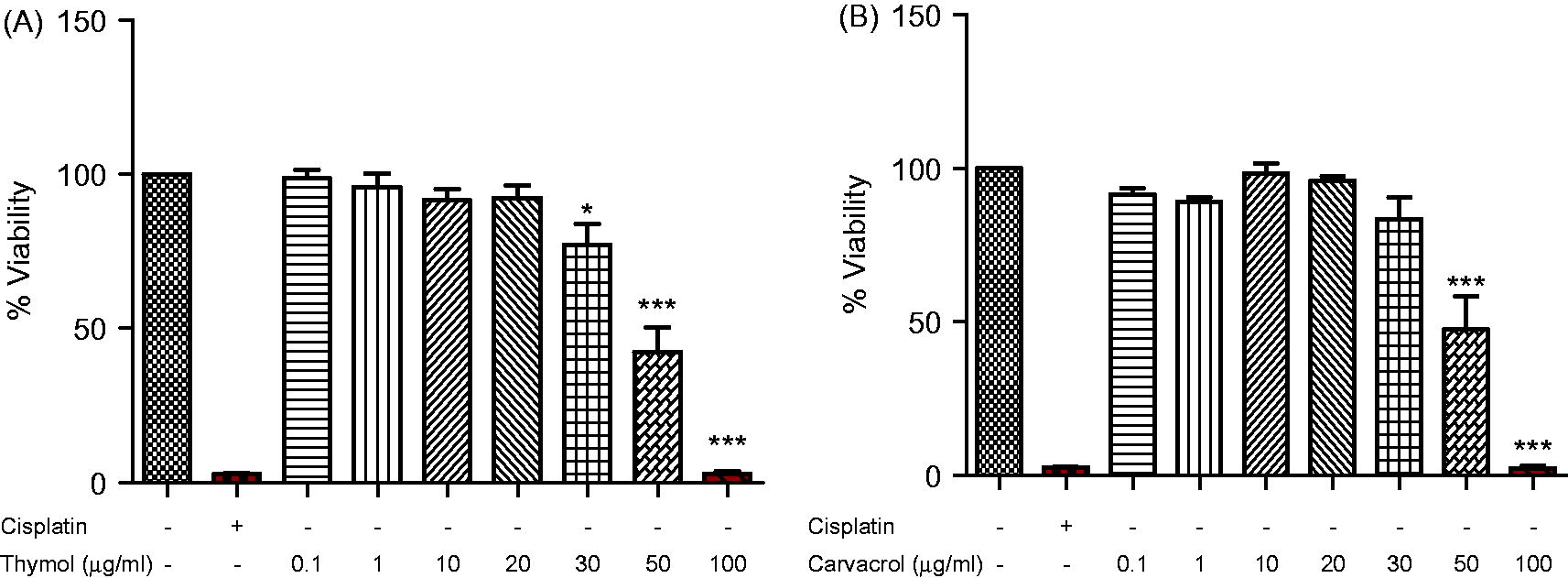
The effects of different concentrations of thymol and carvacrol on the viability of J774.1 cells were determined using an MTT colorimetric assay. As shown in , cell viability at concentrations up to 20 µg thymol/ml () and 30 µg carvacrol/ml () remained unchanged compared to the control. Accordingly, thymol levels of 10 and 20 µg/ml and carvacrol levels of 20 and 30 µg/ml were used for further experiments on this cell line. While each agent clearly was examined at two doses to see if dose–trend effects could be detected for each given agent, at the shared dose of 20 µg/ml direct comparisons of the effects of thymol versus carvacrol could be made.
Figure 1. Effects of thymol and carvacrol on J774.1 cell line viability. The cells were treated with different concentrations of (A) thymol or (B) carvacrol for 24 h. Viability was determined by MTT colorimetic assay. Negative control values were obtained in the absence of components and positive control in the presence of cisplatin (50 µg/ml). Values shown are mean ± SD of three independent experiments in triplicate. *p < 0.05, ***p < 0.001 versus untreated cells.
Effects of agents on inducible IL-1β and TNFα gene expression
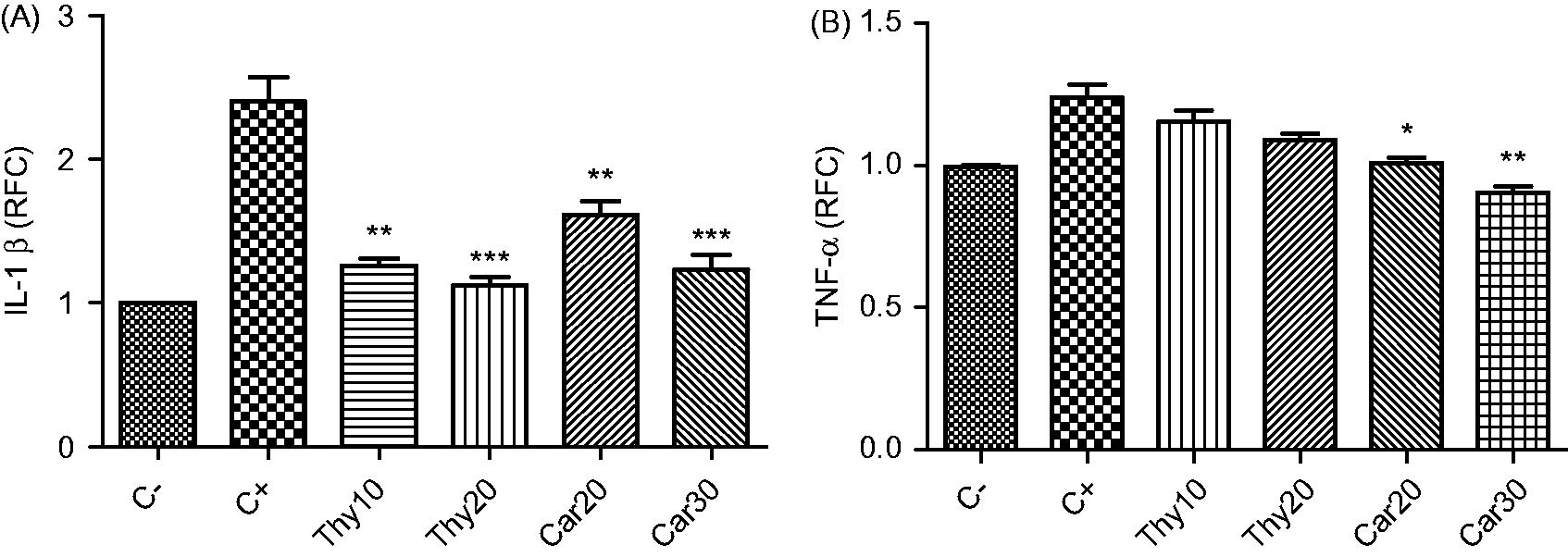
The influence of thymol and carvacrol on expression of genes of two major proinflammatory cytokines, IL-1β and TNFα, was examined in LPS-stimulated cells. The cells were incubated with different concentrations of thymol and carvacrol in the presence of LPS for 24 h, after which they were harvested. As shown in , carvacrol decreased the LPS-inducible expression of both IL-1β and TNFα. IL-1β expression decreased from 2.4 ( ± 0.24)-fold (above untreated cell levels) in cells treated with LPS alone to 1.61 ( ± 0.13)-fold (p < 0.01); TNFα expression decreased from 1.24 ( ± 0.07)-fold to 1.01 ( ± 0.03)-fold (p < 0.05) at the 20 µg carvacrol/ml level. At this same concentration, thymol caused decreases in inducible IL-1β expression from 2.40 ( ± 0.24)-fold to 1.12 ( ± 0.08)-fold (p < 0.001). However, this level of thymol had no effect on inducible TNFα expression.
Figure 2. Effects of thymol and carvacrol on LPS-induced inflammatory gene expression. Cells were treated with thymol (Thy: 10 or 20 µg/ml) or carvacrol (Car: 20 or 30 µg/ml) in the presence of 1 µg LPS/ml for 24 h. Expression of IL-1β (A) and TNFα (B) was evaluated using real time-PCR. Negative control (C−) values were obtained in absence of LPS and compounds; positive controls (C+) were cells treated just with LPS. Values shown are mean ± SD of three independent experiments in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 versus positive control. RFC, relative fold-change.
Effects of treatments on cytokine formation
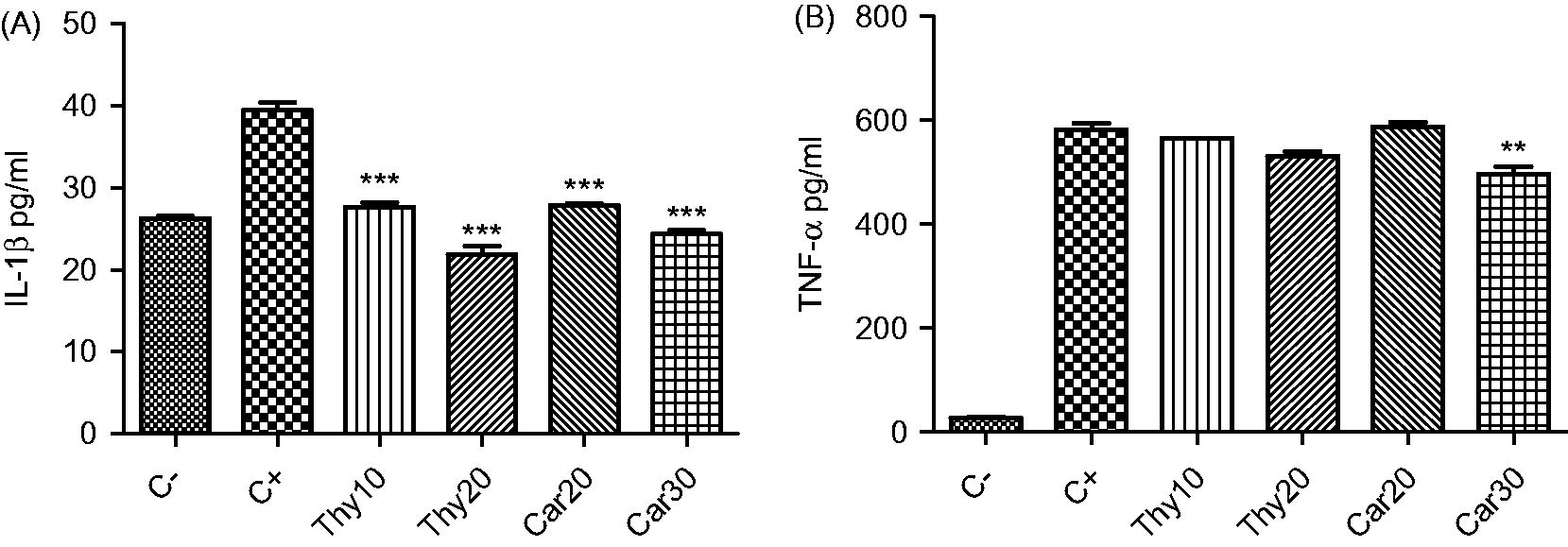
As shown in , the levels of IL-1β [26.3 ( ± 0.4) to 39.5 ( ± 1.4) pg/ml] and TNFα [27.7 ( ± 2) to 582.4 ( ± 17.9) pg/ml] production by the J774.1 cells was significantly increased after LPS challenge (p < 0.001 versus untreated cells). Treatment of cells with either 10 or 20 µg thymol/ml or with 20 or 30 µg carvacrol/ml significantly reduced the inducible levels of IL-1β, Production values were reduced to 27.6 ( ± 0.8) pg/ml and 21.9 ( ± 1.6) pg/ml (10 or 20 µg thymol/ml, respectively; each p < 0.001). For carvacrol, production was reduced to 27.8 ( ± 0.3) and 24.4 ( ± 0.6) pg/ml (20 or 30 µg carvacrol/ml, respectively; each p < 0.001). In contrast, only carvacrol at 30 µg/ml significantly (p < 0.01) reduced inducible TNFα formation from 582.4 ( ± 17.9) to 496.8 ( ± 20.8) pg/ml. Thus, in the context of effects on LPS-inducible IL-1β, thymol appeared to be more inhibitory than carvacrol.
Figure 3. Effects of thymol and carvacrol on LPS-induced inflammatory cytokine levels. Cells were treated with thymol (Thy: 10 or 20 µg/ml) or carvacrol (Car: 20 or 30 µg/ml) in the presence of 1 µg LPS/ml for 24 h. IL-1β (A) and TNFα (B) protein levels were detected using ELISA. Negative control (C−) values were obtained in the absence of LPS and components; positive control (C+) was cells treated only with LPS. Values shown are mean ± SD of three independent experiments in triplicate. **p < 0.01, ***p < 0.001 versus positive control.
Effects of transcription factor inducibility (ELISA)
To determine effects of thymol and carvacrol on regulatory proteins involved in signaling pathways that control inflammatory responses, J774.1 cells were treated overnight with these agents and then activated with LPS. Incubation of cultured cells with LPS caused significant increases in p-SAPK/JNK, p-STAT3, p-IκBα and p-NF-κB p65 levels in the total cell extract (relative to in untreated cells). As shown in , pre-treatment of cells with either 10 or 20 µg thymol/ml significantly inhibited the extent of LPS-inducible phosphorylation of SAPK/JNK and STAT3 (). However, the higher concentration of thymol (20 µg/ml) had the greater inhibitory effect on the phosphorylation of SAPK/JNK [71.1 ( ± 6.1)% versus levels in LPS alone-treated cells; p < 0.01] and of STAT3 [61.0 ( ± 4.4)% versus levels in LPS alone-treated cells; p < 0.01] within 15 min of LPS stimulation. In comparison, use of 20 µg carvacrol/ml resulted in a roughly equivalent significant inhibition of LPS-inducible phosphorylation of SAPK/JNK [68.55 ( ± 3.5)%; p < 0.001] and STAT3 [58.82 ( ± 2.2)%; p < 0.01] versus levels in LPS alone-treated cells. Increasing the dose of carvacrol to 30 µg/ml had little further impact on the inhibitory effect (i.e. SAPK/JNK [62.8 ( ± 4.3)%; p < 0.001], STAT3 [56.0 ( ± 7.2)%; p < 0.01] of levels in LPS-only controls).
Figure 4. ELISA assessment of effects of thymol and carvacrol on LPS-stimulated inflammatory transcription factor levels. Cells were treated with thymol or carvacrol overnight and subsequently stimulated with 1 µg LPS/ml for 15 min [for detection of p-SAPK/JNK (A), p-STAT-3 (B), p-p38 (C), and p-IκBα (D)] or 30 min [for detection of p-NF-κB p65 (E) and NF-κB p65 (F)]. After total cell lysate preparation, transcription factor levels were measured using a PathScan inflammation multi-target sandwich ELISA. Negative control values were obtained in the absence of LPS and compounds. Positive control was cells treated with LPS only. Values shown are mean ± SD of three independent experiments performed in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 versus positive control.
![Figure 4. ELISA assessment of effects of thymol and carvacrol on LPS-stimulated inflammatory transcription factor levels. Cells were treated with thymol or carvacrol overnight and subsequently stimulated with 1 µg LPS/ml for 15 min [for detection of p-SAPK/JNK (A), p-STAT-3 (B), p-p38 (C), and p-IκBα (D)] or 30 min [for detection of p-NF-κB p65 (E) and NF-κB p65 (F)]. After total cell lysate preparation, transcription factor levels were measured using a PathScan inflammation multi-target sandwich ELISA. Negative control values were obtained in the absence of LPS and compounds. Positive control was cells treated with LPS only. Values shown are mean ± SD of three independent experiments performed in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 versus positive control.](/cms/asset/d0040298-def3-4662-828f-056b3b3b4222/iimt_a_1029145_f0004_b.jpg)
While thymol and carvacrol showed seemingly equivalent effects on the phosphorylation of SAPK/JNK and STAT3, it seemed that only carvacrol had any significant impact on LPS-inducible phosphorylation of p38. Specifically, 20 µg cavacrol/ml stimulated phosphorylation of p38 [to 118 ( ± 6.2)% of the level seen in LPS only-treated cells; p < 0.05]. At the higher test concentration (30 µg/ml), the effect was greater, i.e. 123.7 ( ± 4.2)% of level in LPS only-treated cells; p < 0.05]. However, it should be noted that the level of LPS-inducible p38 phosphorylation was increased with 20 µg thymol/ml (to 116.8 ( ± 5.9) versus LPS-only cells), but this change was not significant (p = 0.057).
Despite the fact both thymol and carvacrol at 20 µg/ml significantly reduced p-IκBα levels to ≈41% of the control values (), effects on p-NF-κB p65 expression differed. Twenty micrograms of carvacrol/ml significantly increased p-NF-κB p65 levels to 125.9 ( ± 1.2)% (p < 0.01) and this value increased to 137.4 ( ± 3.4)% (p < 0.001) above those in LPS-only-treated cells after the dose was increased to 30 µg/ml; thymol at either 10 or 20 µg/ml did not significantly impact these levels (). With a significant elevation in p-NF-κB p65 levels induced by each of the carvacrol doses, levels of total NF-κB p65, as expected, significantly decreased accordingly (). Similarly, thymol at either 10 or 20 µg/ml did not significantly impact these levels. Taken together, these results show that, at an equal dose level, thymol and carvacrol appear to have relatively equivalent abilities to impact on LPS-inducible changes in phosphorylation of several key signaling molecules outside of the NF-κB cascade.
Effects of transcription factor inducibility (Western blot)
To evaluate the influence of thymol and carvacrol on p-NF-κB p65, c-Fos, c-Jun, NFAT-1 and NFAT-2 expression, cells were treated with thymol and carvacrol overnight and then activated with LPS for 30 min, after which nuclear cell extracts were prepared and Western blot analyses performed. As seen in , only those cells treated with 30 µg carvacrol/ml displayed any significant (p < 0.05) increase in NF-κB p65 phosphorylation at Ser536 [to 123.6 ( ± 13.4)% of levels seen in cells treated with only LPS]. In a manner similar to what was seen in the ELISA analyses, thymol caused no significant effects on p-NF-κB p65 levels.
Figure 5. Western blot analysis of thymol and carvacrol effects on LPS-stimulated transcription factors. Cells were treated with 10 or 20 µg/ml of thymol (Thy) or 20 or 30 µg/ml of carvacrol (Car) overnight and then activated with LPS to induce (A) p-NF-κB p65 (P-p65), (B) c-Fos, (C) c-Jun, (D) NFAT-1 and (E) NFAT-2. Expression of each factor in cell nuclear extract was measured using Western blots. Transcription factors levels were normalized to Histone H3 expression using ImageJ software. Negative control (C−) values were obtained in the absence of LPS and compounds. Positive control was cells treated just with LPS. Cyclosporine A (CSA) was used as an NFAT suppressor. Values shown are mean ± SD of three independent experiments performed in triplicate.
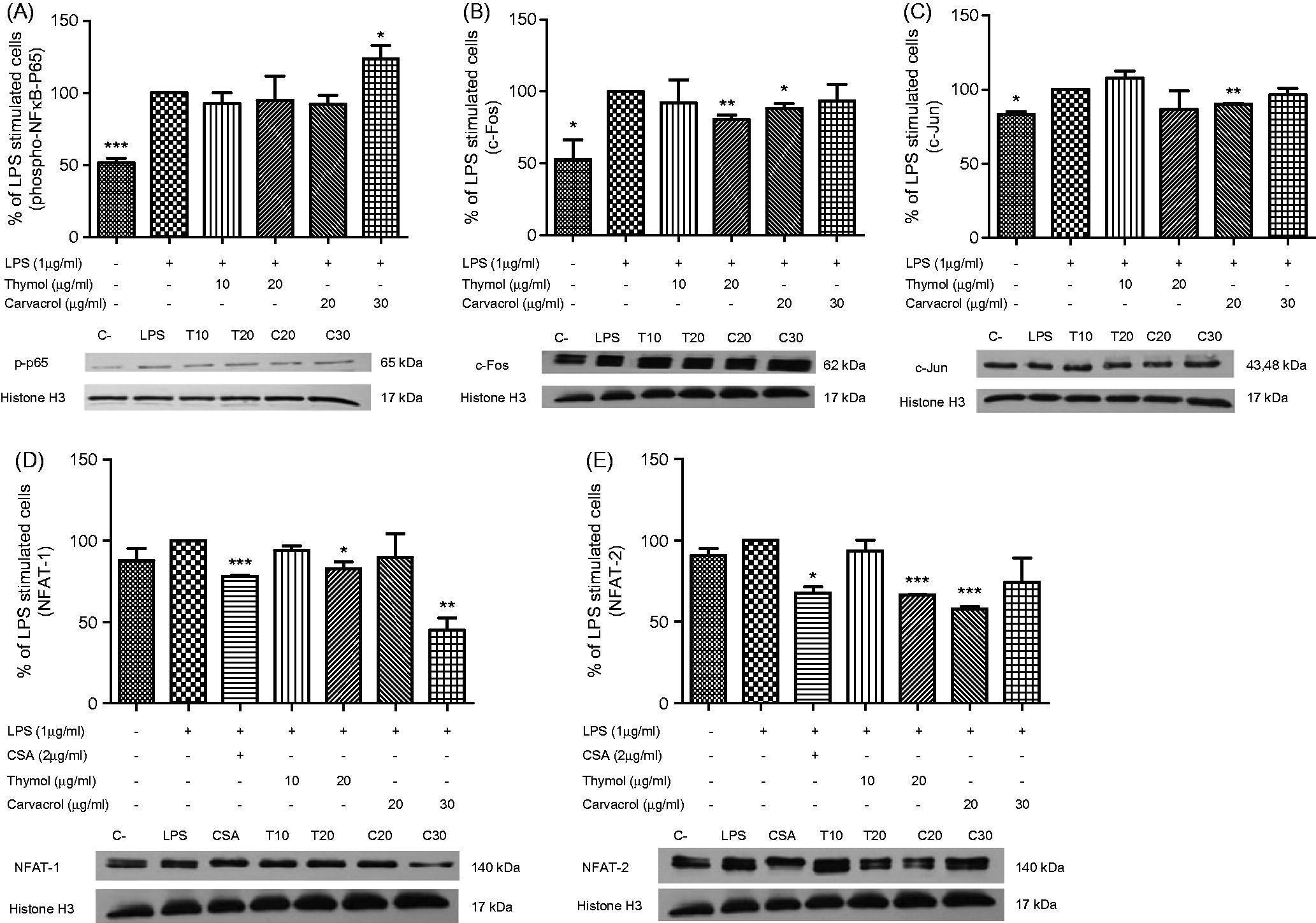
Pre-treatment of cells with 20 µg/ml of thymol or carvacrol significantly reduced nuclear c-Fos levels to 80.5 ( ± 5.5)% (thymol; p < 0.01) and to 88.2 [ ± 6.0])% (cavacrol; p < 0.05) of levels seen in cells treated with only LPS (). Oddly, while treatment with the lower 10 µg thymol/ml could have understandably resulted in lesser inhibition of the LPS effect, treatment with the higher 30 µg cavacrol/ml regimens also did. Similar patterns of effect were also seen with regard to changes in LPS-inducible c-Jun levels in the cells. However, while only treatment with 20 µg carvacrol/ml caused significant (p < 0.05) decreases in inducible nuclear c-Jun (to 90.0 [ ± 0.5])% of levels seen in cells treated with only LPS (), 20 µg thymol/ml caused a reduction as well (to 91.6 ( ± 17.3)% versus LPS-only cells), but this change was not significant (p = 0.67). Again, treatment with the lower 10 µg thymol/ml or higher 30 µg cavacrol/ml regimens resulted in lesser inhibition of the LPS-inducible effect.
In the analyses of effects on LPS-inducible expression of NFAT-1 and NFAT-2 (), the effects on each by the test agents differed. The effects on NFAT-2 were much like those on p-NF-κB p65, c-Fos and c-Jun in that, at 20 µg/ml, both thymol and carvacrol caused significant inhibition of inducible NFAT-2 expression (to 66.7 [ ± 0.1]% {thymol; p < 0.001} and to 58.1 ( ± 1.7)% {carvacrol; p < 0.001}), while the lower (10 µg thymol/ml) or higher (30 µg cavacrol/ml) regimens resulted in lesser inhibition of the LPS-inducible effect (). The NFAT inhibitor CSA (at 2 µg/ml) caused significant decreases in inducible NFAT-2 to 67.9 ( ± 5.3)% [p < 0.05] relative to levels seen in LPS-only-treated cells.
With respect to effects on LPS-inducible expression of NFAT-1, at 20 µg/ml, thymol appeared to have the slightly stronger inhibitory effect. Thymol at this concentration significantly decreased inducible NFAT-1 levels to 82.8 ( ± 6.1)% (p < 0.05) of those found in the LPS-only-treated cells (). At this dose, carvacrol only caused inducible levels to be decreased to 94.7 ( ± 19.1)% (p = 0.80). However, when the dose of carvacrol used was increased to 30 µg/ml, the inducible levels of NFAT-1 were strongly reduced to 45.1 ( ± 10.3)% (p < 0.01) of levels seen in cells treated with only LPS. The NFAT inhibitor CSA (at 2 µg/ml) caused significant decreases in inducible NFAT-1 to 78.2 ( ± 0.7)% [p < 0.001] relative to levels seen in LPS-only-treated cells.
Taken together, these results show that, at an equal dose level (i.e. 20 µg/ml), thymol and carvacrol appear to have relatively equivalent abilities to impact on LPS-inducible changes in transcription factor levels in cells. However, as there is currently no data for 10 µg carvacrol/ml or 30 µg thymol/ml exposures, it is not possible at this point for us to: (a) verify if 20 µg/ml of each agent is the optimal dose for use here/future studies; or (b) establish if each agent becomes “less toxic” (i.e. less inhibitory) as doses increase above 20 µg/ml. Further studies employing more matching test doses (within range initially established here) for both test agents are clearly needed to better establish if there are any/type of dose–response relationships between thymol and carvacrol and each of the endpoints measured here.
Discussion
The aim of the present study was to evaluate the effects of thymol and carvacrol on the main transcription factors involved in regulation of inflammatory processes. These studies first examined the effect of these components on J774.1 macrophage cell viability in an attempt to exclude any probable cytotoxic effects. Next, two non-cytotoxic high concentrations of each agent were investigated for anti-inflammatory effects through measures of the release of pro-inflammatory cytokines TNFα and IL-1β. These cytokines are key mediators of inflammatory responses and their overexpression can lead to severe proinflammatory reactions. Previous studies have reported on the inhibitory effects of thymol or carvacrol on these cytokines (Guimaraes et al., Citation2012; Lima et al., Citation2013; Samara et al., Citation2014). Results of this study showed that pre-treatment of the macrophages with carvacrol significantly inhibited IL-1β and TNFα protein and gene expression and thymol also significantly decreased the expression of IL-1β.
To establish molecular mechanisms underlying the anti-inflammatory effects of these two agents, the effects of thymol and carvacrol on signaling molecules and transcription factors of inflammatory processes (NF-κB, STAT-3, p38, SAPK/JNK, AP-1 and NFATs) were assessed. NF-κB is one of the most ubiquitous important transcription factors that regulate gene expression, including that of iNOS, COX-2, cytokines TNFα and IL-1β, as well as chemokines and adhesion molecules involved in regulation of inflammatory responses (Chen et al., Citation1999; Liang et al., Citation2014). We hypothesized that thymol and carvacrol could inactivate this transcription factor.
There are separate NF-κB-activating pathways; all of these pathways reveal sequentially activated kinases. In a classical pathway, activation of IκB kinase (IKK) complex leads to phosphorylation of IκBα, followed by ubiquitination and degradation of this NF-κB inhibitory molecule via the proteasome pathway. The atypical pathway, which is triggered by DNA damage and several external stimuli, leads to sequential p38 and casein kinase-2 (CK2) activation and involves IκBα phosphorylation and degradation via an IKK-independent pathway (Viatour et al., Citation2005). As seen in the ELISA results here, both thymol and carvacrol significantly decreased the levels of inducible p-IκBα; however, levels of inducible p-NF-κB p65 were significantly increased only with carvacrol and remained unchanged by thymol. In the Western blot analyses, thymol again imparted no significant effects on p-NF-κB p65 levels. However, carvacrol – in parallel with the ELISA results – caused significant increases in the inducible levels of this molecule.
Multiple lines of evidence have indicated that p38 MAPK is one main element in the intracellular signaling cascades responsible for NF-κB activation in response to a wide range of external stimuli (Surh et al., Citation2001). Chan et al. (Citation2005) showed that carvacrol stimulated the active phosphorylation of p38. In the current study, carvacrol was shown to increase the levels of inducible p-p38 molecule. It was possible the increased levels of p-NF-κB p65 seen with carvacrol-treated cells was a result of increased p-p38 that, in turn, could also result in IκBα Ser293 phosphorylation, IκBα degradation and, ultimately, p-NF-κB p65 formation. Numerous studies have reported the ability of various kinases to phosphorylate NF-κB p65 in the cell cytoplasm or nucleus. The mechanism by which phosphorylation regulates the ability of such proteins to repress or induce distinct target genes remains unresolved (Viatour et al., Citation2005).
Because thymol and carvacrol did not inhibit formation of p-NF-κBp65 and p-p38 transcription factors, it could be assumed that their anti-inflammatory effects might be due to inhibition of other pro-inflammatory transcription factors, such as SAPK/JNK, STAT3, AP-1 and various NFAT. In this regard, the current study showed that levels of inducible c-Fos, NFAT-1 and NFAT-2 were significantly decreased by pre-treatments with either thymol or carvacrol; the levels of c-Jun were significantly decreased only by carvacrol. Both thymol and carvacrol significantly decreased STAT-3 and SAPK/JNK phosphorylation in the stimulated macrophage cells (total cell extract); this suggested to us the probable ability of these agents to diminish STAT-3- and JNK-mediated activity of cytokines associated with inflammatory responses (Kortylewski et al., Citation2009). It should be mentioned that, in a previous study on total cell extract of LPS-stimulated mouse mammary epithelial cells, thymol decreased the levels of phosphorylation of NF-κB p65, IκBα, JNK, ERK and p38 MAPK after 1 h (Liang et al., Citation2014). In the current study, according to results of the ELISA assays, carvacrol and thymol also inhibited phosphorylation of IκBα, JNK and STAT-3. However, carvacrol promoted phosphorylation of p38 MAPK. Only carvacrol significantly elevated phosphorylation of NF-κB p65. This discrepancy between the results of the current and the Liang et al. studies could be attributed to differences in incubation time, techniques and particularly the studied cell type.
A number of pharmacological agents have been shown to selectively target key signaling molecules involved in inflammation, including NF-κB. Salicylates and glucocorticoids are two commonly used anti-inflammatory drugs that have inhibitory effects on NF-κB activation (de Bosscher et al., Citation2014; Park et al., Citation2013). Other non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and flurbiprofen act similarly (D'Acquisto et al., Citation2002). Acetaminophen also inhibits binding of NF-κB to DNA in LPS-treated macrophages (Ryu et al., Citation2000). However, not all anti-inflammatory drugs act through inhibition of NF-κB activity. For example, naproxen [a COX inhibitor] and zileuton [a specific inhibitor of lipoxygenase] do not affect NF-κB activity (Kazmi et al., Citation1995). Thus, the development of pharmaceuticals that interfere with transcription factors and signaling molecules (apart from than NF-κB) while still imparting anti-inflammatory effects remains an attractive target in ongoing studies in many laboratories around the world. It is increasingly evident that many natural products, including carvacrol and thymol, may eventually be seen as fulfilling both of these requirements.
In conclusion, reductions in the gene/protein expressions of TNFα and IL-1β due to thymol and carvacrol are suggested to be a result of inhibition of STAT-3, JNK, AP-1 and NFAT and possibly other signaling pathways rather than effects on expression of NF-κB and p38. The present data on the effectiveness of thymol and carvacrol to modulate macrophage function via intracellular signaling pathways supports results from other studies that suggested these compounds might eventually be useful as potential therapeutic agents against inflammatory disorders.
Acknowledgments
This study was extracted from the thesis written by one of the authors, N. Gholijani, and was supported by Iranian National Science Foundation (grant no 93013147) and Shiraz University of Medical Sciences (grant no. 6297).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adcock, I. M., and Caramori, G. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell. Biol. 79:376–384
- Al-Khalaf, M. I. 2013. Thyme and thymol effects on induced bronchial asthma in mice. Life Sci. J. 10:693–699
- Amirghofran, Z., Hashemzadeh, R., Javidnia, K., et al. 2011. In vitro immunomodulatory effects of extracts from three plants of the Labiatae family and isolation of the active compound(s). J. Immunotoxicol. 8:265–273
- Chan, A. S., Pang, H., Yip, E. C., et al. 2005. Carvacrol and eugenol differentially stimulate intracellular Ca2+ mobilization and mitogen-activated protein kinases in Jurkat T-cells and monocytic THP-1 cells. Planta. Med. 71:634–639
- Chen, F., Castranova, V., Shi, X., and Demers, L. M. 1999. New insights into the role of NF-κB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 45:7–17
- D'Acquisto, F., May, M. J., and Ghosh S. 2002. Inhibition of NF-κB: An emerging theme in anti-inflammatory therapies. Mol. Interv. 2:22–35
- de Bosscher, K., Beck, I. M., Dejager, L., et al. 2014. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1. Cell Mol. Life Sci. 71:143–163
- Fachini-Queiroz, F. C., Kummer, R., Estevão-Silva, C.F., et al. 2012. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012:657026
- Ferrer, I., Blanco, R., Carmona, M., et al. 2002. Active, phosphorylation-dependent MAP kinases, MAPK/ERK, SAPK/JNK and p38, and specific transcription factor substrates are differentially expressed following systemic administration of kainic acid to the adult rat. Acta Neuropathol. 103:391–407
- Fric, J., Zelante, T., Wong, A. Y., et al. 2012. NFAT control of innate immunity. Blood 120:1380–1389
- Guimarães, A. G., Xavier, M. A., de Santana, M. T., et al. 2012. Carvacrol attenuates mechanical hyper-nociception and inflammatory response. Naunyn Schmied. Arch. Pharmacol. 385:253–263
- Hawiger, J. 2001. Innate immunity and inflammation: A transcriptional paradigm. Immunol. Res. 23:99–109
- Hoffmann, A., and Baltimore, D. 2006. Circuitry of NF-κB signaling. Immunol. Rev. 210:171–186
- Juhás, Š. D., Bujňáková, P., Rehák, Š., et al. 2008. Anti-inflammatory effects of thyme essential oil in mice. Acta Vet. Brno. 77:327–334
- Kazmi, S. M., Plante, R. K., Visconti, V., et al. 1995. Suppression of NF-κB activation and NF κB-dependent gene expression by tepoxalin, a dual inhibitor of cyclooxygenase and 5-lipoxygenase. J. Cell. Biochem. 57:299–310
- Kim, H. Y., Park, E. J., Joe, E. H., and Jou, I. 2003. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 171:6072–6079
- Kortylewski, M., Xin, H., Kujawski, M., et al. 2009. Regulation of the IL-23 and IL-12 balance by STAT-3 signaling in the tumor microenvironment. Cancer Cell. 15:114–123
- Kumar, S., Boehm, J., and Lee, J. C. 2003. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2:717–726
- Landa, P., Kokoska, L., Pribylova, M., et al. 2009. In vitro anti-inflammatory activity of carvacrol: Inhibitory effect on COX-2 catalyzed PGE2 biosynthesis. Arch. Pharm. Res. 32:75–78
- Liang, D., Li, F., Fu, Y., et al. 2014. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 37:214–222
- Lima, M. D., Quintans-Júnior, L. J., de Santana, W. A., et al. 2013. Anti-inflammatory effects of carvacrol: Evidence for a key role of IL-10. Eur. J. Pharmacol. 699:112–117
- Liu, Z., Lee, J., Krummey, S., et al. 2011. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol. 12:1063–1070
- Mohamed, D. A., Mahmoud, E. A., Abdel-Moniem, S., and Hassan, M. 2013. Anti-inflammatory and anti-arthritic activity of some spices extracts on adjuvant induced arthritis in rats. J. Appl. Sci. Res. 9:5303–5312
- Murakami, A., and Ohigashi, H. 2007. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 121:2357–2363
- Ocaña, A., and Reglero, G. 2012. Effects of thyme extract oils (from Thymus vulgaris, T. zygis, and T. hyemalis) on cytokine production and gene expression of oxLDL-stimulated THP-1-macrophages. J. Obesity 2012:104706
- O'Shea, J. J., and Murray, P. J. 2008. Cytokine signaling modules in inflammatory responses. Immunity 28:477–487
- Park, J. S., Lee, J., Lim, M. A., et al. 2014. JAK2-STAT3 blockade by AG490 suppresses autoimmune arthritis in mice via reciprocal regulation of regulatory T-cells and TH17 cells. J. Immunol. 192:4417–4424
- Park, S., Sung, B., Jang, E. J., et al. 2013. Inhibitory action of salicylideneamino-2-thiophenol on NF-κB signaling cascade and cyclooxygenase-2 in HNE-treated endothelial cells. Arch. Pharm. Res. 36:880–889
- Purushoth, T., Panneerselvam, P., Vijaykumar, R., et al. 2012. Anti-inflammatory, anti-arthritis, and analgesic effect of ethanolic extract of whole plant of Merremia Emarginata Burm. F. Cent. Eur. J. Exp. Biol. 1:94–99
- Roy, P. K., Rashid, F., Bragg, J., and Ibdah, J. A. 2008. Role of JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 14:200–202
- Ryu, Y. S., Lee, J. H., Seok, J. H., et al. 2000. Acetaminophen inhibits iNOS gene expression in RAW 264.7 macrophages: Differential regulation of NF-κB by acetaminophen and salicylates. Biochem. Biophys. Res. Commun. 16:758–764
- Saklatvala, J. 2004. p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 4:372–377
- Samara, R. B., Damasceno, F. R., Nathalia, S., et al. 2014. Carvacryl acetate, a derivative of carvacrol, reduces nociceptive and inflammatory response in mice. Life. Sci. 14:58–66
- Stein, B., Baldwin, A. S., Ballard, D. W., et al. 1993. Cross-coupling of the NF-κB p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879–3891
- Surh, Y. J., Chun, K. S., Cha, H. H., et al. 2001. Molecular mechanisms underlying chemo-preventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. 480:243–268
- Viatour, P., Merville, M. P., Bours, V., and Chariot, A. 2005. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 30:43–52

