Abstract
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a class of synthetic compounds that have widespread use in consumer and industrial applications. PFAS are considered environmental pollutants that have various toxic properties, including effects on the immune system. Recent human studies indicate that prenatal exposure to PFAS leads to suppressed immune responses in early childhood. In this study, data from the Norwegian BraMat cohort was used to investigate transcriptomics profiles in neonatal cord blood and their association with maternal PFAS exposure, anti-rubella antibody levels at 3 years of age and the number of common cold episodes until 3 years. Genes associated with PFAS exposure showed enrichment for immunological and developmental functions. The analyses identified a toxicogenomics profile of 52 PFAS exposure-associated genes that were in common with genes associated with rubella titers and/or common cold episodes. This gene set contains several immunomodulatory genes (CYTL1, IL27) as well as other immune-associated genes (e.g. EMR4P, SHC4, ADORA2A). In addition, this study identified PPARD as a PFAS toxicogenomics marker. These markers can serve as the basis for further mechanistic or epidemiological studies. This study provides a transcriptomics connection between prenatal PFAS exposure and impaired immune function in early childhood and supports current views on PPAR- and NF-κB-mediated modes of action. The findings add to the available evidence that PFAS exposure is immunotoxic in humans and support regulatory policies to phase out these substances.
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are synthetic fluorinated compounds that have been produced since the 1950s. Due to their attractive water and oil repellent properties, PFAS have been produced on a large scale for use in various consumer products and industrial applications. PFAS form a diverse class of compounds and their terminology and classification has been described by Buck et al. (Citation2011). Among PFAS, worldwide interest from a regulatory point of view mainly lies with “long-chain” perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs). Currently, PFAS are considered environmental pollutants and some Western manufacturers halted production or agreed to a voluntary phase out of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) production in the 2000s. However, because of poor biodegradability as well as continued production elsewhere and because of other PFAS compounds (than PFOS and PFOA), PFAS are still commonly detected in wildlife as well as humans (Houde et al., Citation2006; Lau et al., Citation2007). Human PFAS exposure can be mainly attributed to dietary sources, with the highest concentrations found in products like fish, meat and eggs (Haug et al., Citation2010; Vestergren & Cousins, Citation2009). Indoor dust has also been described as a contributor to human PFAS exposure (Fromme et al., Citation2009).
Human exposure to PFAS occurs before birth due to placental transfer (Gutzkow et al., Citation2012). Prenatal toxicant exposure has been recognized as being of particular importance because the immune system develops extensively in utero, which can possibly lead to higher fetal susceptibility to immunotoxicants following exposure (Holsapple et al., Citation2004; West, Citation2002). Besides adverse effects occurring in early childhood, fetal responses to toxicants may lead to persistent changes throughout later life, possibly leading to predisposition to cancer and immune diseases (Gluckman et al., Citation2008).
Immunotoxic effects of PFAS have been found in rodent studies. For example, DeWitt et al. (Citation2008) described how IgM antibodies in mice were suppressed after PFOA exposure. Peden-Adams et al. (Citation2008) reported that PFOS exposure affects antibody production in mice. The combined evidence to date on rodent literature studies indicates that antibody production is a key endpoint sensitive to modulation by PFAS (Corsini et al., Citation2014). More recently, immunotoxic effects of PFAS exposure were also suggested in humans. Grandjean et al. (Citation2012) described an association between reduced humoral immune responses to childhood vaccinations in Faroese children and increased PFAS concentrations in maternal blood. However, several other studies found no evidence for an association between prenatal PFAS exposure and allergic and infectious diseases in children (Fei et al., Citation2010; Okada et al., Citation2012; Wang et al., Citation2011). Granum et al. (Citation2013) reported evidence for immunosuppression in children by prenatal exposure, such as inverse association between maternal serum concentrations of four PFAS at the time of delivery and anti-rubella antibody levels in serum of 3-year old children. Positive associations were also observed between the maternal PFAS concentrations and the number of episodes of common cold and gastroenteritis in the children during the first 3 years of life. In contrast, no effects on allergy- and asthma-related health outcomes were found (Granum et al., Citation2013). Taken together, the literature indicates that prenatal exposure to PFAS, more specifically to long-chain PFCAs and PFSAs, is primarily associated with immunosuppression in early childhood.
This led us to further examine the connection between prenatal PFCA and PFSA exposure and early life immunosuppression by identifying common toxicogenomics profiles, in neonatal samples from the Norwegian BraMat cohort. Whole-genome transcriptomics data from neonatal umbilical cord blood samples, as described by Hochstenbach et al. (Citation2012), were compared to maternal serum concentration of four PFAS, namely PFOS, PFOA, perfluorononanoate (PFNA) and perfluorohexane sulfonate (PFHxS), for the corresponding mother–child pairs. Additionally, the transcriptomics data were compared to data for the two most prominent endpoints for immunosuppression identified by Granum et al. (Citation2013), namely anti-rubella antibody levels and the reported number of episodes of common cold until 3 years. By making these comparisons, we identified genes correlating with multiple PFAS concentrations (hence a common signature for long-chain PFCAs/PFASs) and those correlating with either of the two neonatal immune functionality endpoints. Further analysis of the overlapping genes between these sets provided genes and pathways related to PFAS-related immunosuppression and add to the available evidence that prenatal exposure by PFAS is associated with immunosuppression.
Materials and methods
Cohort
Blood samples, PFAS determinations and childhood health data were obtained from the Norwegian BraMat cohort. The BraMat cohort has been previously described in Stølevik et al. (Citation2011) and is a subcohort nested within the Norwegian Mother and Child Cohort Study (MoBa), a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (Magnus et al., Citation2006). Study protocols were approved by the Regional Committees for Medical and Health Research Ethics and the Data Inspectorate. Informed consent was obtained from all participating mothers.
Microarray data
The umbilical cord blood microarray data used in this study were assembled based on the dataset described by Hochstenbach et al. (Citation2012) and available at GEO (www.ncbi.nlm.nih.gov/geo) under accession number GSE31836. Briefly, blood samples used for microarray gene expression profiling were collected immediately after birth from the cord vein of babies whose mothers participated in the Norwegian BraMat cohort. Samples were collected by trained nurses at the maternity wards of the Oslo University Hospital, Ullevål and Akershus University Hospital, Norway. For preserving RNA, aliquots of heparin-anticoagulated whole blood (0.4 ml) were mixed with 1.2 ml RNAlater (Ambion/Applied Biosystems, Nieuwerkerk aan den IJssel, the Netherlands) as soon as possible after blood collection and subsequently stored at −80 °C until shipped on dry ice to the research laboratory. Methods for RNA isolation and gene expression profiling using Agilent 4x44k human oligonucleotide microarrays (Agilent Technologies, Palo Alto, CA) have previously been described by Hochstenbach et al. (Citation2012).
The default (manufacturer) probe to gene annotation that was used in the studies by Hochstenbach et al. contains multiple probes for some genes. We considered this redundancy somewhat undesirable, as it might cause ambiguity during later analysis steps. Therefore, we applied a re-normalization to the raw data to remove this redundancy. Raw microarray signal data were normalized in R (www.r-project.org), using the four-step approach, as described by Baken et al. (Citation2006); namely, (1) natural log-transformation, (2) quantile normalization of all scans, (3) correcting the sample spot signal for differences in the corresponding reference spot signal between arrays and (4) averaging data from spots annotated with an identical gene symbol. This resulted in a data matrix with (ln-transformed) gene expression levels for 19 595 unique genes.
PFAS
As a measure of prenatal exposure to PFAS, we used concentrations of PFAS in maternal blood samples collected up to 3 days after delivery as part of the BraMat cohort. The data and the methods used were previously described by Granum et al. (Citation2013). In brief, 19 PFAS were determined using liquid chromatography-triple quadrupole mass spectrometry (LC-MS/MS). Due to low PFAS concentrations/detection frequency, further statistical analyses were performed only for PFOS, PFOA, PFNA and PFHxS. The limit of quantification (LOQ) was 0.050 ng/ml for all PFAS; concentrations < LOQ were set to 0.035 ng/ml (the LOQ divided by square root of 2). For quantification of PFOS, the total area of the linear and branched isomers was integrated.
Episodes of common cold
At the age of 1, 2 and 3 years, a questionnaire was sent to the participants. Among other questions on the child's health, the mothers were asked how many episodes of common colds and other upper respiratory tract infections (hereafter called common cold) the child had experienced in the last 12 months. The sum of the number of reported incidences for all 3 years was used in this study. Data for children with missing values were excluded in further analyses.
Rubella titers
Children received rubella vaccinations as part of the Norwegian Childhood Vaccination Program. Antibody titers against rubella at 3-years-of-age were measured as previously described in Stølevik et al. (Citation2013).
Data analysis
The data analysis approach used is analogous to that described by Hochstenbach et al. (Citation2012), namely a separate comparison of microarray data to substance exposure and immune parameters (episodes of common cold and rubella titers), respectively, followed by identification of genes and pathways informative for both aspects studied. Due to differences in sample collection and analysis, the number of samples available differed for the various comparisons, 66 samples were available for microarray to PFAS comparison, 73 for microarray to common cold episodes and 58 for microarray to anti-rubella level comparison.
Dose–response relationships were investigated by Pearson's correlation analyses of individual gene expressions and individual values for PFOS, PFOA, PFNA and PFHxS exposure estimates, rubella antibody levels and episodes of common cold. Significantly correlating transcripts were selected using an unadjusted p value cutoff of < 0.05. For the PFAS exposures, we restricted further analyses to genes that could be considered associated with exposure to long-chain PFCAs/PFSAs as a substance class rather than with exposure to individual compounds. To this end, we identified genes that had significant correlations with at least two compounds and these significant correlations were consistently positive or negative.
Adjustments for potentially confounding factors with regard to statistical analyses on the associations between PFAS exposure and the health outcomes common cold and rubella titers have been performed previously (Granum et al., Citation2013). Since we are including only exposure–health outcomes found to be statistically significant after adjustment for confounding factors in Granum et al., adjustments for confounding factors are not repeated in the statistical analyses in the present study. The criterion to be included in statistical analyses as a confounding factor in Granum et al. was that the variable had to be associated with PFAS exposure as well as at least one health outcome, at p values ≤ 0.25.
Functional over-representation analysis was performed using DAVID (http://david.abcc.ncifcrf.gov). Here, we used functional/pathway information from Gene Ontology (GO), KEGG, BBID, BioCarta, Panther and Reactome; with otherwise default settings. Related pathways were grouped together using the DAVID functional annotation clustering option. Additional information on gene function related to human fetal development was obtained from the DFLAT database (http://dflat.cs.tufts.edu) (Wick et al., Citation2014). Literature associations between genes and pathways were determined by CoPub textmining version 5.0 (http://services.nbic.nl/copub5/) (Fleuren et al., Citation2011). For functional interpretation, information obtained from these databases was combined into a non-redundant list of gene–function relations. These relations were visualized as a network using Cytoscape (www.cytoscape.org).
Results
PFAS
The number of genes significantly (p < 0.05) associated with individual PFAS compounds ranged between 601 and 1307 (, Supplemental Table 1). With the exception of PFHxS, the number of positively associated genes was comparable to the number of negatively associated genes. About half (578) of the associated genes per compound were found positively or negatively associated with two or more substances and were, therefore, considered as substance type-associated markers in further analyses. Ten genes were positively associated with all four compounds (APLF, C8orf31, DLG2, FBLL1, LOC254057, LOC389791, MAGEA8, NCKAP1, SCN1A, TNFSF15), whereas only one gene (GPR22) was negatively associated with all four substances.
Table 1. Number of genes correlated with PFAS levels and immune parameters.
Functional over-representation analysis indicated that a diverse set of pathways were over-represented among the 578 PFAS-associated genes (Supplemental Table 1). By using the DAVID pathway clustering option, these were grouped into several categories. Among the most significantly associated functionalities were those associated with plasma membrane or adhesion, immunological pathways, nucleotide metabolism, translation, signaling and development.
The subset of immunological pathways included terms as “regulation of T-cell activation”, “T-cell receptor signaling pathway” and “thymic T-cell selection”. Among the other pathways, a number of functional terms were found over-represented that relate to WNT-signaling, which is involved in several immunological (T-cell development, regulatory T-cell activation, dendritic cell maturation) as well as other kinds of developmental processes.
Common cold
For the comparison of transcriptomics to the number of common cold episodes, we identified 330 positively and 250 negatively associated genes, respectively (, Supplemental Table 2). These genes showed predominantly over-representation for pathways related to membrane and adhesion, immunology and development. Among the over-represented immunological pathways were “NF-κB binding”, “NF-κB Signaling Pathway”, “Signaling in Immune system”, “somatic diversification of immune receptors” and “regulation of lymphocyte apoptosis”. Developmental pathways enriched among the associated genes included e.g. “anatomical structure morphogenesis” and “cell differentiation”.
The study found 27 genes overlapping between those correlated to the number of common cold episodes and those correlated to PFAS exposure (). Of these, three could be related to immunological and/or hematopoietic functions, namely PPARD, SHC4 and CYTL1. Six genes (ADAMTS20, CYTL1, FAT1, PCDHA11, PPARD, SGPL1) are involved in development and/or morphogenesis.
Table 2. Overlap between PFAS- and common cold-associated genes.
Rubella
This study found 1231 genes associated with rubella titers, of which 522 genes showed positive and 709 showed negative association (, Supplemental Table 3). Functional annotation analysis showed a clear over-representation of cell division pathways, such as “M phase of mitotic cell cycle” and “cell division”, as well as functionally related terms such as “spindle”, “kinetochore” and “DNA replication”. An association between expression of cell division-related genes and immunotoxicology has been described by several literature studies (Baken et al., Citation2008; Frawley et al., Citation2011), indicating that a mechanistic relation between cell division and the immune functionality endpoint (rubella titers) is feasible. However, as no clear over-representation of cell division pathways among either the PFAS-associated genes or those associated with common cold was found, the link between cell division and rubella titers is probably less dependent on PFAS exposures.
Additionally, this study found over-representation of pathways involved in immunology, signaling, development and membrane and adhesion. The immunological pathways that were enriched included among others “cellular defense response”, “CTL mediated immune response against target cells”, “natural killer cell activation” and “positive regulation of isotype switching to IgG isotypes” (Supplemental Table 3). Especially this last pathway can be envisaged to be involved in long-term effects on antibody titers. The analyses also found over-representation of developmental pathways, such as “anatomical structure morphogenesis” and “developmental process”. Although the immunological and developmental pathways did not correspond exactly to those regulated by PFAS, the finding that there is an association between such pathways and PFAS serum levels as well as rubella titers suggests that the immunotoxic effect of PFAS probably proceeds through an effect on the developing immune system, rather than by interfering with more general processes such as cell division.
On the gene expression level, the overlap between PFAS and rubella titers-associated genes consisted of 26 genes (). These include two genes involved in the pathway “regulation of T cell activation”, i.e. IL27 and ADORA2A, as well as one other associated with immunological functionality, i.e. CYTL1. Additionally, seven of the genes that correlated with both PFAS and rubella are involved in development and/or morphogenesis, e.g. ADORA2A, CDC42BPB, CYTL1, DVL1, HOXD8, ODF2, RAB3IP.
Table 3. Overlap between PFAS- and rubella titer-associated genes.
Common signatures
The analyses so far have resulted in a set of 27 genes associated with PFAS and common cold episodes (set27PC) and a set of 26 genes associated with PFAS exposure and rubella titers (set26PR) ( and ). A comparison of these two lists revealed that there is just one gene in common, namely cytokine-like 1 (CYTL1). As both of these obtained gene sets showed enrichment for similar functions (including immunology and development), we performed further in silico functionality analyses to determine if common mechanisms could connect PFAS response to rubella titers and common cold incidence, respectively. For the combined set of 52 genes, gene function information obtained from databases (GO, KEGG, DFLAT) and by literature text mining (CoPub) was combined into a gene-function network ().
Figure 1. Gene–function association network. Blue, genes correlated with PFAS and episodes of common cold; red, genes correlated with PFAS and rubella titers; purple, genes correlated with all three parameters; green: functions/pathways.
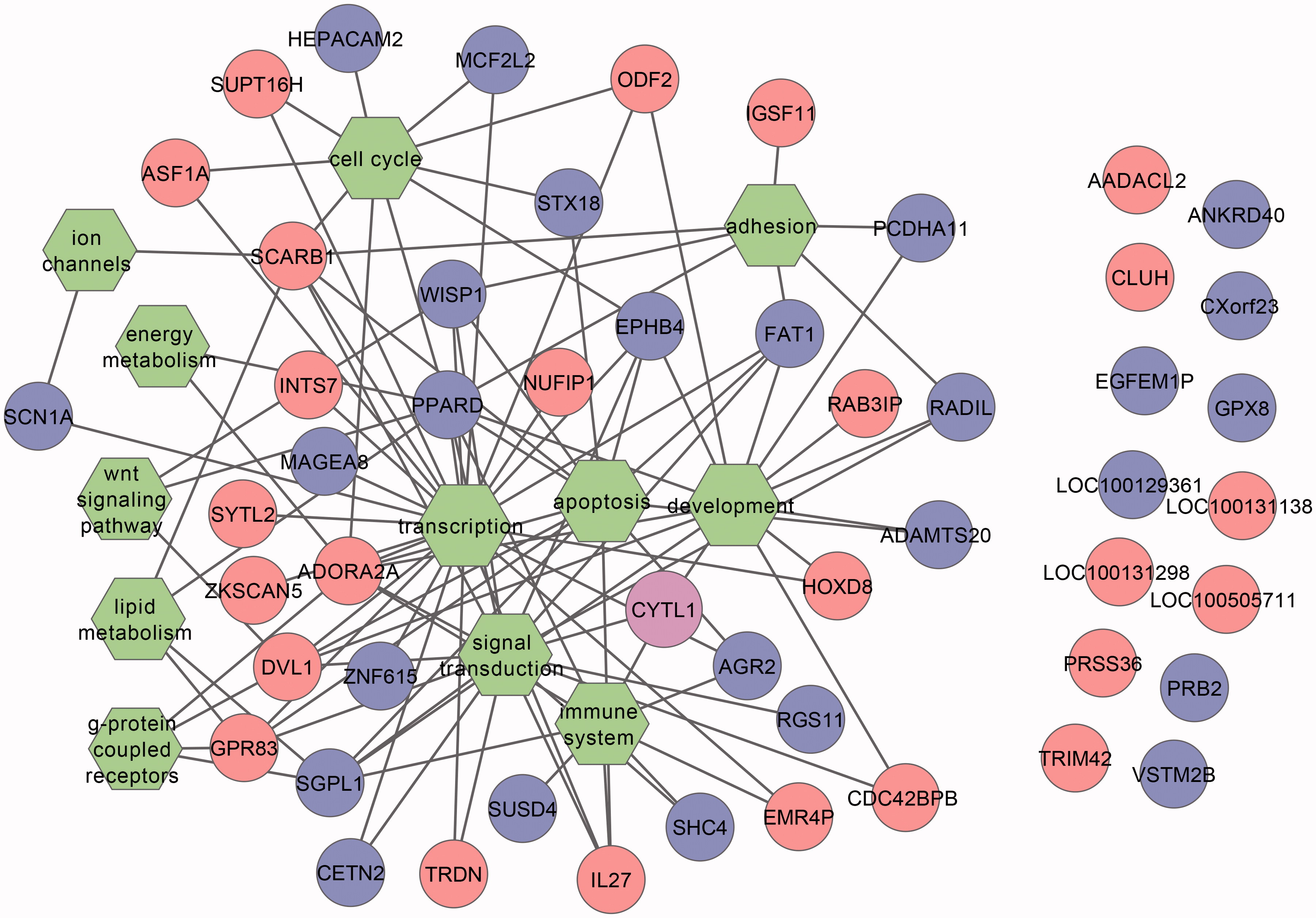
This network connects 38 (of the 52) genes with 12 functional terms. Network analysis indicated that most hub positions are positioned together in the center of the network, such as transcription (25 edges), signal transduction (17 edges), development (14), apoptosis (13) and immune system (9). These hubs are positioned together because they are connected to similar genes, indicating that these functions should not be interpreted on their own, but rather in context. Among the genes, a hub function is taken by PPARD, which is linked to 10 out of the 12 pathways, suggesting that this gene might play a central regulatory role in PFAS-mediated immunotoxicity.
Discussion
Prenatal exposure to PFAS has previously been associated with reduced immune functionality (DeWitt et al., Citation2008; Grandjean et al., Citation2012; Granum et al., Citation2013; Peden-Adams et al., Citation2008). The knowledge on mechanisms underlying the immunomodulatory effects of PFAS in experimental animals and humans is still incomplete. In a recent paper, Corsini et al. (Citation2014) reviewed the literature to date on the immunotoxic effects of PFAS and current insights into possible modes of action. Based on the evidence currently available, they propose that PFAS immunotoxicity may well occur via multiple pathways, some of which are regulated by PPARs (e.g. fatty acid metabolism, glucose uptake) and others (e.g. cytokines, chemokines, adhesion molecules) being regulated by NF-κB (Corsini et al., Citation2014). To improve our understanding of PFAS-associated immunosuppression, this study compared transcriptomics data to maternal PFAS exposures as well as to two immune endpoints in early childhood.
The former part of this analysis was focused on genes that show significant correlation (in the same direction) with two or more PFCA and PFSA exposures. The rationale for this choice is that genes whose expression relates to multiple long-chain PFCA/PFSA substances can be said to be informative for this compound class as a whole rather than for individual substances. Such shared markers are, therefore, more useful for providing insights into mechanisms behind long-chain PFCA/PFSA-related immunotoxic effects, as well as applications as biomarkers for monitoring environmental exposures. This would, ultimately, also provide a more reliable basis for policy making.
For the various PFAS, this study found a different number of genes that were significantly correlated with each individual compound. Interestingly, genes that show a significant correlation with more than one compound almost always show agreement in their direction of correlation. For the dataset as a whole, only three genes show a positive correlation with one PFAS and a negative correlation with another. A weakness of the present study is the relatively small study population where minor differences in gene expression, PFAS exposure and incidence of the health outcomes may considerably impact the statistical analyses. However, as the genes show agreement in their direction of correlation, this is evidence that our results are not due to chance correlations alone. Nevertheless, the present results should not be seen as hypothesis-confirming, but rather as contributing to the available evidence to date on this subject.
Although there have been reports on differences in gene expression response to different perfluorinated compounds, due to different chemical structures as well as chain length, in this data set no clear differences between gene expression associations nor evidence for different responses to long-chain PFCAs (PFOA and PFNA) as opposed to PFSAs (PFAS and PFHxS) was found. This lends further support to our assumption that the response to long-chain PFCAs and PFSAs is predominantly a common response to this type of compound. However, based on our data, it cannot be predicted to what extent this response will also be found for short-chain PFAS.
Among the genes associated with one or more of the respective endpoints, the analyses consistently found over-representation for immunological and developmental pathways, as well as GO-terms related to the plasma membrane. The underlying effects on immunological and developmental pathways support the motivation for this study, namely that long-chain PFCAs and PFSAs exposure affects prenatal development and that the immune system is particularly susceptible to adverse effects by compound exposure (Holsapple et al., Citation2004; West, Citation2002).
The current study identified 52 transcripts associated with long-chain PFCAs/PFSAs and at least one immunological parameter. In a gene–function association network (), it can be seen that these genes are associated around a number of hub processes such as development, apoptosis and the immune system. Similar gene–function associations are found among those correlated with rubella and common cold. Moreover, these genes are scattered evenly across the network, indicating that the gene sets for both immunological endpoints, despite having little overlap at the individual gene level, relate to similar underlying functional mechanisms. Among these genes, several have a biological function that is related to reduced immune functionality or potential underlying toxicity mechanisms.
One gene, CYTL1, is especially relevant as its expression is significantly positively correlated with PFAS exposure, negatively correlated with rubella titers and positively correlated with the number of common cold episodes. CYTL1, also known as C17 or C4orf4, codes for a cytokine-like protein of 136 amino acids including a signal sequence. CYTL1 was first described as specifically expressed in CD34+ (but not CD34−) bone marrow and cord blood mononuclear cells (Liu et al., Citation2000). CYTL1 expression increased in cultured bone marrow-derived CD34+ cells that were exposed to cytokines that favor maintenance of stem cells, whereas CYTL1 expression decreased upon exposure to hematopoietic factors that stimulate cell differentiation. As CD34+ cells are early hematological stem/progenitor cells, a role of CYTL1 in the hematopoiesis and possibly the immunological system was inferred. Later studies also found expression of CYTL1 outside hematopoietic cells. Begley et al. (Citation2008) described that the aging prostate shows up-regulation of CYTL1 as well as several inflammatory mediators, which promotes cellular proliferation of both epithelial and stromal fibroblast cell types. CYTL1 is also expressed in chondrocytes and plays a role in chondrogenesis (Kim et al., Citation2007). However, although Cytl knockout mice are more sensitive to osteoarthritic cartilage destruction, their cartilage and bone development is not affected, which indicates a role in a pathway involved in cartilage homeostasis, such as the immune system, rather than a strictly developmental role (Jeon et al., Citation2011). Indeed, Chao et al. (Citation2011) showed that, in a murine arthritis model, CYTL1 is sufficient for joint protection in a preventive setting, but no therapeutic effects were observed. This, combined with sequence analysis, led to the hypothesis that CYTL has immune-regulatory properties and contributes to immune homeostasis systemically and in a tissue-specific manner. Proteomics analyses on human osteoarthritic patients have lent further support to this hypothesis (Stenberg et al., Citation2013). Although the exact mechanism is not yet clear, protein structure modeling indicated that CYTL1 could be a structurally and functionally related analog of CCL2, capable of signaling through the chemokine receptor CCR2 (Tomczak & Pisabarro, Citation2011).
The present study also found a negative association between gene expression of PPARD (peroxisome proliferator-activated receptor-δ) and PFAS exposure as well as the common cold. PPARD also takes a hub position in the gene–function association network, being connected to 10 out of 12 functional processes. The PPAR family is a group of nuclear receptors that regulate physiological processes such as lipid homeostasis, adipogenesis, reproduction, as well as inflammation. It has been described that PFAS can bind and activate PPAR members, especially PPARα (van den Heuvel et al., Citation2006). For this reason, several studies have looked at the potential role of PPARs in PFAS-mediated immunotoxicity. However, results obtained are not always consistent on whether or not there is a direct effect on immune cells (Corsini et al., Citation2012; DeWitt et al., Citation2012; Midgett et al., Citation2014). Based on the experimental literature available to date and taking into account that PPARs show considerable interspecies differences in their ligand-receptor binding and activation, Corsini et al. (Citation2014) concluded that the role of PPARs differed among PFAS substances as well as species and/or strains. The present study, however, is one of the first to suggest a role of PPARδ in PFAS-mediated immunotoxicity in humans.
In addition to CYTL1 and PPARD, a number of other genes with a mechanistic role in immune functionality showed significant association for multiple substance exposures and a health outcome. Interleukin-27 (IL-27, although also known as IL-30) is another cytokine that is related to IL-12A. IL-27 is an immunoregulatory cytokine, especially for T-cells, with pro- and anti-inflammatory properties (Bosmann & Ward, Citation2013). IL-27 forms a heterodimer with Epstein-Barr virus induced gene 3 (EBI3), which drives rapid clonal expansion of naive but not memory CD4-positive T-cells (Pflanz et al., Citation2002). EMR4P (egf-like module containing, mucin-like, hormone receptor-like 4 pseudo-gene) is a member of the EGF-TM7 receptor gene family, which plays a role in leukocyte adhesion and migration. However, due to sequence divergence since the human–chimpanzee split, this particular member most likely represents a truncated protein that lacks the seven-span transmembrane region and is predicted to be expressed as a soluble secreted protein (Caminschi et al., Citation2006). Finally, SHC4 (Src homology 2 domain containing family, member 4) plays a role in the chemokine signaling pathway as well as natural killer cell mediated cytotoxicity. Both these roles involve an intermediate connecting role between initial membrane receptors and MAPK signaling further downstream.
The data presented in this study support our hypothesis that there is an association at the transcriptomics level between prenatal long-chain PFCAs/PFSAs exposure levels and the number of common cold episodes as well as rubella antibody levels in early childhood. A particular strength of this study is that our transcriptomics profiling on cord blood study strongly indicates that prenatal exposure contributes to these immunological effects. In contrast, previous epidemiological associations between prenatal PFAS exposure and immunosuppression in childhood are limited in that they cannot really exclude that the effects observed are due to postnatal exposure (via milk or food), since these are correlated to maternal exposures. Our findings add to the available evidence that long-chain PFCAs/PFSAs are immunotoxic in humans and support current regulatory policies to phase out these substances. Moreover, our data support and extend the insights to date on the mode of action underlying PFAS-mediated immunotoxicity (Corsini et al., Citation2014) by finding associations for genes involved in both proposed modes of action. The former of these comprises PPAR activation. In the present study, we observed changes in the transcriptional activity of PPARD, as well as several downstream PPAR-regulated genes, such as those involved in lipid metabolism (). The latter toxicity pathway involves interference with NF-κB signaling by inhibiting I-κB degradation, but more importantly by inhibiting the phosphorylation of NF-κB p65 at Ser536, which is required for optimal transcription activity. In the present study, we observed perturbation of several known NF-κB targets (IL 27, ADORA2A) as well as genes with functions regulated downstream of NF-κB signaling, such as cytokines or immune modulating genes (CYTL1, IL27, SHC4) as well as immune cell adhesion (EMR4P) (). Perturbation by long-chain PFCAs/PFSAs of NF-κB signaling and downstream processes will interfere with the functionality of one or more types of immune cells, leading to health effects such as reduced antibody levels as well as an increased incidence of common cold. Taken together, the functionality of the associated genes also strengthens the current views on immunotoxicity of PFAS substances.
In addition to strengthening current knowledge on PFAS-mediated immunosuppression, the data presented in this paper also provide transcriptomics biomarkers for monitoring prenatal PFAS exposure and the related effects on the development of the immune system. Among the markers found in this study, several can be prioritized for such purposes and for further studies it can be envisaged that a small set of markers can be measured by means of a (multiplexed) quantitative PCR rather than a whole-genome microarray. Likely markers to be included in such a panel would be SCN1A (sodium channel, voltage-gated, Type I, α subunit) and MAGEA8 (melanoma antigen family A, 8), which are significantly correlated with all four PFAS as well as common cold episodes and are moderately (albeit non-significantly) correlated with rubella titers, with R values of −0.21 and 0.18, respectively (Supplemental Table 1). Especially CYTL1, however, is interesting as a marker for long-chain PFCAs/PFSAs exposure and both immunological parameters, and a literature link to a proposed mode of action. Depending on practical consideration, such a panel would then also contain one or more other immunological genes such as IL 27, as well as endogenous control (“housekeeping”) genes for normalization.
Exposure to other environmental contaminants can affect the results of PFAS exposure. We previously studied the correlation between exposure to the different PFAS and polychlorinated biphenyls (PCBs), dioxin-like PCBs and dioxins and acrylamide in the BraMat cohort. Only very weak correlations were found (PCBs: 0 – 0.07; dioxin-like PCBs and dioxins: −0.01 – 0.08; acrylamide: −0.11 – 0.07) (results not published). Adjustments for potentially confounding factors with regard to the associations between PFAS exposure and the health outcomes in the present paper were previously performed in Granum et al. (Citation2013). The criterion to be included in the statistical analyses as a confounding factor was that the variable had to be associated with both health outcomes and PFAS exposure at p values of 0.25 or less. None of the abovementioned environmental chemicals fulfilled this criterion and, therefore, none were included in the adjusted statistical analyses. This is in line with the findings by Grandjean et al. (Citation2012) that most of the PFAS correlated only weakly with PCBs in maternal serum and that adjustment for PCB exposure did not appreciably change their results.
In conclusion, this study adds to the available evidence that long-chain PFCAs/PFSAs are immunotoxic and prenatal exposure to this type of compound leads to suppressed immune system functionality in early childhood. The markers identified in this study can serve as the basis for further mechanistic or epidemiological studies.
Supplementary material available online Supplementary Tables 1–3
Pennings_SupplTables.xlsx
Download MS Excel (157.8 KB)Acknowledgments
The authors are grateful to all the participating families in Norway who took part in the BraMat study. The authors thank the technicians Bodil Hasseltvedt, Else-Carin Groeng, Astri Grestad, Berit Arvesen Stensby, Åse Eikeset, Azemira Sabaredzovic, Anne-Cathrine Kristoffersen, Dr Solvor Berntsen Stølevik, Dr Ingeborg S Aaberge and Dr Kirsti Vainio at the Norwegian Institute of Public Health for their assistance in the project. The authors are also grateful to the staff at the maternity wards and laboratories at Oslo and Akershus University Hospitals for their contribution to the recruitment of participants and assistance in blood sampling and processing.
Declaration of interest
The authors report no conflicts of interest. This project was supported by the Research Council of Norway [grant number 228195/H10]. Part of this work was supported by the European Union 6th Framework Programme (Integrated Project NewGeneris [FOOD-CT-2005-016320]) and the Norwegian Institute of Public Health. The authors alone are responsible for the content and writing of the paper.
References
- Baken, K. A., Pennings, J. L., de Vries, A., et al. 2006. Gene expression profiling of bis(tri-n-butyltin)oxide (TBTO)-induced immunotoxicity in mice and rats. J. Immunotoxicol. 3:227–244
- Baken, K. A., Pennings, J. L., Jonker, M. J., et al. 2008. Overlapping gene expression profiles of model compounds provide opportunities for immunotoxicity screening. Toxicol. Appl. Pharmacol. 226:46–59
- Begley, L. A., Kasina, S., MacDonald, J., and Macoska, J. A. 2008. The inflammatory micro-environment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 43:194–199
- Bosmann, M., and Ward, P. A. 2013. Modulation of inflammation by IL-27. J. Leukocyte Biol. 94:1159–1165
- Buck, R. C., Franklin, J., Berger, U., et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 7:513–541
- Caminschi, I., Vandenabeele, S., Sofi, M., et al. 2006. Gene structure and transcript analysis of the human and mouse EGF-TM7 molecule, FIRE. DNA Seq. 17:8–14
- Chao, C., Joyce-Shaikh, B., Grein, J., et al. 2011. C17 prevents inflammatory arthritis and associated joint destruction in mice. PLoS One 6:e22256
- Corsini, E., Luebke, R. W., Germolec, D. R., and DeWitt, J. C. 2014. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol. Lett. 230:263–270
- Corsini, E., Sangiovanni, E., Avogadro, A., et al. 2012. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol. 258:248–255
- DeWitt, J. C., Copeland, C. B., Strynar, M. J., and Luebke, R. W. 2008. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect. 116:644–650
- DeWitt, J. C., Peden-Adams, M. M., Keller, J. M., and Germolec, D. R. 2012. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 40:300–311
- Fei, C., McLaughlin, J. K., Lipworth, L., and Olsen, J. 2010. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ. Res. 110:773–777
- Fleuren, W. W., Verhoeven, S., Frijters, R., et al. 2011. CoPub update: CoPub 5.0 a text mining system to answer biological questions. Nucl. Acids Res. 39:W450–454
- Frawley, R., White, K. Jr, Brown, R., et al. 2011. Gene expression alterations in immune system pathways in the thymus after exposure to immuno-suppressive chemicals. Environ. Health Perspect. 119:371–376
- Fromme, H., Tittlemier, S. A., Volkel, W., et al. 2009. Perfluorinated compounds - exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 212:239–270
- Gluckman, P. D., Hanson, M. A., Cooper, C., and Thornburg, K. L. 2008. Effect of in utero and early-life conditions on adult health and disease. New Engl. J. Med. 359:61–73
- Grandjean, P., Andersen, E. W., Budtz-Jorgensen, E., et al. 2012. Serum vaccine antibody concentrations in children exposed to perfluori–nated compounds. JAMA 307:391–397
- Granum, B., Haug, L. S., Namork, E., et al. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol. 10:373–379
- Gutzkow, K. B., Haug, L. S., Thomsen, C., et al. 2012. Placental transfer of perfluorinated compounds is selective - a Norwegian Mother and Child sub–cohort study. Int. J. Hyg Environ. Health 215:216–219
- Haug, L. S., Salihovic, S., Jogsten, I. E., et al. 2010. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere 80:1137–1143
- Hochstenbach, K., van Leeuwen, D. M., Gmuender, H., et al. 2012. Toxicogenomic profiles in relation to maternal immunotoxic exposure and immune functionality in newborns. Toxicol. Sci. 129:315–324
- Holsapple, M. P., Paustenbach, D. J., Charnley, G., et al. 2004. Symposium summary: Children's health risk - what's so special about the developing immune system? Toxicol. Appl. Pharmacol. 199:61–70
- Houde, M., Martin, J. W., Letcher, R. J., et al. 2006. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 40:3463–3473
- Jeon, J., Oh, H., Lee, G., et al. 2011. Cytokine-like 1 knockout mice (Cytl1–/–) show normal cartilage and bone development but exhibit augmented osteoarthritic cartilage destruction. J. Biol. Chem. 286:27206–27213
- Kim, J. S., Ryoo, Z. Y., and Chun, J. S. 2007. Cytokine-like 1 (Cytl1) regulates the chondro-genesis of mesenchymal cells. J. Biol. Chem. 282:29359–29367
- Lau, C., Anitole, K., Hodes, C., et al. 2007. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 99:366–394
- Liu, X., Rapp, N., Deans, R., and Cheng, L. 2000. Molecular cloning and chromosomal mapping of a candidate cytokine gene selectively expressed in human CD34+ cells. Genomics 65:283–292
- Magnus, P., Irgens, L. M., Haug, K., et al; and the MoBa Study. 2006. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 35:1146–1150
- Midgett, K., Peden-Adams, M. M., Gilkeson, G. S., and Kamen, D. L. 2014. In vitro evaluation of the effects of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on IL-2 production in human T-cells. J. Appl. Toxicol. (in press)
- Okada, E., Sasaki, S., Saijo, Y., et al. 2012. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ. Res. 112:118–125
- Peden-Adams, M. M., Keller, J. M., Eudaly, J. G., et al. 2008. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 104:144–154
- Pflanz, S., Timans, J. C., Cheung, J., et al. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T-cells. Immunity 16:779–790
- Stenberg, J., Ruetschi, U., Skioldebrand, E., et al. 2013. Quantitative proteomics reveals regulatory differences in the chondrocyte secretome from human medial and lateral femoral condyles in osteoarthritic patients. Proteome Sci. 11:43
- Stølevik, S. B., Nygaard, U. C., Namork, E., et al. 2011. Prenatal exposure to polychlorinated biphenyls and dioxins is associated with increased risk of wheeze and infections in infants. Food Chem. Toxicol. 49:1843–1848
- Stølevik, S. B., Nygaard, U. C., Namork, E., et al. 2013. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 51:165–172
- Tomczak, A., and Pisabarro, M. T. 2011. Identification of CCR2-binding features in Cytl1 by a CCL2-like chemokine model. Proteins 79:1277–1292
- van den Heuvel, J. P., Thompson, J. T., Frame, S. R., and Gillies, P. J. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol. Sci. 92:476–489
- Vestergren, R., and Cousins, I. T. 2009. Tracking the pathways of human exposure to perfluoro-carboxylates. Environ. Sci. Technol. 43:5565–5575
- Wang, I. J., Hsieh, W. S., Chen, C. Y., et al. 2011. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ. Res. 111:785–791
- West, L. J. 2002. Defining critical windows in the development of the human immune system. Human Exp. Toxicol. 21:499–505
- Wick, H. C., Drabkin, H., Ngu, H., et al. 2014. DFLAT: Functional annotation for human development. BMC Bioinformatics 15:45

