Abstract
Tetanus is a highly fatal disease caused by tetanus neurotoxin (TeNT) and remains a major threat to human and animal health, despite preventive strategies. TeNT is composed of heavy and light chain linked by a disulfide bond. The antibody response to TeNT is polyclonal and directed to multiple epitopes within both the light and heavy chains, leading to toxin neutralization. This study was undertaken to localize and compare neutralizing epitopes recognized by human and mouse TeNT-specific antibodies at a clonal level. In the present study, 22 murine hybridoma clones and 50 human lymphoblastoid cell lines secreting monoclonal antibodies (mAb) were generated against TeNT. The specificity of these mAb was determined using different recombinant fragments of tetanus toxin. Moreover, this study investigated the in vitro toxin neutralizing activity of these mAb by a ganglioside GT1b assay. The results showed that tetanus toxoid immunization in humans and BALB/c mice induced a vigorous humoral immune response against different fragments of TeNT, particularly the carboxyl-terminal fragment of the heavy chain (known as fragment C). The fragment C-specific human and mouse mAb could largely neutralize TeNT. However, while all fragment C-specific human mAb reacted with the carboxyl-terminal part of this fragment (HCC), the majority of the mouse mAb failed to recognize this region. These results suggested that fragment C is the major target for the TeNT neutralizing antibodies, although different epitopes seem to be targeted by human and mouse antibodies.
Introduction
Tetanus is a highly fatal disease, with 20–50% mortality, particularly in children. There have been an estimated 61 000 tetanus deaths in less than 5 years around the world and neonatal tetanus has not been effectively controlled, especially in developing countries, despite extensive universal vaccinations (Kitchin, Citation2011; Luo et al., Citation2012). Clostridial neurotoxins (CNT) that are composed of tetanus neurotoxin and seven antigenically-distinct botulinum neurotoxins (BoNT/A-G) are produced by species of clostridias (Lalli et al., Citation2003). CNT block neurotransmission by specific proteolysis of components of the vesicular fusion machinery and are exclusively responsible for neuroparalytic syndromes of tetanus and botulism (Chaddock & Marks, Citation2006). Despite variations within their targets and biological activities, CNT share a common structure composed of a 50 kDa light (LC) and a 100 kDa heavy chain (HC) linked by a single disulfide bond (Schiavo et al., Citation2000). The 100 kDa HC mediates the binding and internalization of TeNT into neurons, while the 50 kDa LC is a zinc metalloprotease containing a catalytic domain of the neurotoxin that inhibits neurotransmission (Herreros et al., Citation2000).
The HC is composed of two distinct functional domains; the N-terminal fragment important for LC translocation and the C-terminal fragment (fragment C) that is responsible for receptor binding (Lalli et al., Citation2003; Montecucco & Schiavo, Citation2006). Structural analysis of HC revealed that the fragment C binding domain is composed of two sub-domains: the proximal HCN sub-domain and the extreme carboxyl sub-domain, HCC. Several lines of evidence suggest that a set of conserved residues within the HCC sub-domain form the ganglioside binding motif and take part in neuron binding activity of TeNT (Herreros et al., Citation2000; Turton et al., Citation2002).
The long lasting humoral immune response induced by tetanus toxoid plays a pivotal role in immunity against tetanus (Mayer et al., Citation2002). Tetanus toxoid is a stable and inactivated form of TeNT treated with formaldehyde and represents the major constituent of tetanus vaccines (di Tommaso et al., Citation1994). The polyclonal antibody response to TeNT has been widely studied in both human and mouse models (Lang et al., Citation1993; Poulsen et al., Citation2007; Volkman et al., Citation1982). Many monoclonal antibodies (mAbs) have also been generated against TeNT, particularly in mice, in order to select toxin neutralizing mAb (Ahnert-Hilger et al., Citation1983; Ichimori et al., Citation1985; Kazemi et al., Citation2011; Kenimer et al., Citation1983; Kozbor et al., Citation1982; Larrick et al., Citation1983; Petrušić et al., Citation2011; Sheppard et al., Citation1984; Simpson et al., Citation1990; Trabaud et al., Citation1989; Volk et al., Citation1984; Yousefi et al., Citation2014). However, the anti-TeNT antibody response in human and mouse models has not been extensively studied and compared at a clonal level.
In the present study, 22 stable murine hybridoma clones and 50 human lymphoblastoid cell lines (LCL) secreting mAb against tetanus toxin were established. The fine specificity and toxin neutralizing activity of these mAb were then investigated and compared.
Materials and methods
Animals
Female BALB/c mice (6–8-weeks-of-age) were obtained from the Pasteur Institute (Tehran, Iran). All mice were housed in pathogen-free facilities maintained at 25 °C with a 50–60% relative humidity and a 12-h light/dark cycle. All mice had ad libitum access to standard rodent chow and filtered water throughout the study period. All protocols using the mice were approved by the Ethics Committee of the Tehran University of Medical Sciences.
Generation of mouse TeNT-specific hybidomas
Mouse immunization and anti-TeNT mAb production was carried out as previously described (Yousefi et al., Citation2014). The mice were given an intraperitoneal injection of tetanus toxoid (40 μg TeNT) (Razi Vaccine and Serum Research Institute, Karaj, Iran) emulsified in complete Freund’s adjuvant (CFA, Sigma, St Louis, MO). Subsequently three booster injections (each 10 μg TeNT dissolved in incomplete Freund’s adjuvant [IFA, Sigma]) were also administered at 3-week intervals. Splenocytes from the hyper-immunized mice were then harvested and fused with the mouse myeloma cell line SP2/0 (National Cell bank of Iran, Pasteur Institute of Iran, Tehran) at a 4:1 ratio using PEG 1500 (Sigma). Fused cells were then grown in hypoxanthine/aminopterin/thymidine (HAT) selective medium (Sigma) and cloned by limiting dilution. Supernatants of the growing cells were tested for a presence of antibody with tetanus toxoid and for toxin specificity via an indirect enzyme linked immunosorbent assay (ELISA). All positive hybridomas were further sub-cloned to generate stable clones.
To generate the mouse monoclonal antibodies (mAb), each clone was intraperitoneally injected into dedicated naive BALB/c mice that had been pre-treated with 0.5 ml Pristane solution (Sigma) to obtain ascites fluid. Subsequently, the ascites from each mouse was harvested and mAb present purified using HiTrap™ SPG columns (GE Healthcare, Hatfield, UK), according to standard protocols. The SPG adsorbed mAb was subsequently eluted using 0.1 M glycine-HCL buffer (pH 2.5) and immediately dialyzed with PBS buffer (0.14 M, pH 7.2).
Establishment of human TeNT specific lymphoblastoid cell lines (LCL)
Human LCL producing TeNT specific antibodies were established using an Epstein-Barr virus (EBV) transformation method as described elsewhere (Younesi et al., Citation2010). In brief, human B-cells were isolated from peripheral blood of normal subjects immunized with tetanus vaccine (Razi Vaccine and Serum Research Institute, Karaj, Iran) using magnetic-activated cell sorting (MACS) negative selection kits (Miltenyi Biotec, Bergisch Gladbach, Germany). All subjects had provided consent for use of their blood products prior to use in these studies.
Isolated B-cells were treated with supernatant from a 3-week-old culture of B95.8 cells (National Cell Bank of Iran, Pasteur Institute of Iran, Tehran) that had been grown in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum [FCS] (both from Gibco, Paisley, UK). The B95.8 cell line itself was derived from EBV-transformed Marmoset B-cells. These B95.8 cells secrete EBV particles and are employed as a source of EBV for human B-cell transformation protocols. After 1.5 h of co-stimulation at 37 °C, treated cells were washed with RPMI 1640 culture medium (Gibco) and re-suspended in complete culture medium (RPMI 1640 supplemented with 10% fetal calf serum [FCS], 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco). EBV-infected cells were seeded in microtiter plates on top of allogenic PBMC (50 000/well) that were inactivated by γ-irradiation (3000 Rad). After 3 weeks of culturing, supernatants of the transformed B-cells (LCL) were analyzed for the presence of human anti-tetanus toxoid by indirect ELISA and for toxin antibody using an ELISA.
The clonality of the anti-TeNT antibodies produced by selected LCL was evaluated based on the Poisson statistical analysis as previously described (Younesi et al., Citation2010, Citation2014). This analysis states that, when a population of cells carrying an undefined number of antigen-specific precursors is randomly and independently distributed by limiting dilution among a number of wells, if more than 37% of wells at a given cell dilution (density) are negative for presence of antigen (i.e. TeNT) specific antibody (negative fraction ≥37%), the antigen-specific antibody produced by growing LCL in the remaining wells are likely derived from a single precursor cell (clonal outgrowth). Based on this assumption, the number of wells that were positive and negative for TeNT-specific antibody in each limiting dilution assay (LDA) plate was determined. Subsequently, supernatants from all positive wells at a cell dilution in which anti-TeNT antibody was not detectable in more than 37% of the wells with growing LCL were considered to contain antibody produced by a single specific precursor (monoclonal).
Determination of antibody specificity by ELISA
To investigate mAb specificity, microtiter polystyrene plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with an appropriate concentration (10 μg/ml) of tetanus toxoid, toxin, fragment C (Sigma) and recombinant light chain and HCC (as previously described in Yousefi et al., Citation2013a) in phosphate-buffered saline (PBS, 0.15 M, pH = 7.2) and incubated overnight at 4 °C. After washing with PBS containing 0.05% Tween 20 (Sigma; PBS-T), plates were blocked using blocking buffer (PBS-T containing 3% non-fat skim milk) at 37 °C for 1.5 h. After blocking and washing, 100 μl of 1 μg/ml purified mouse monoclonal antibodies were added and the plates were incubated for 1.5 h at 37 °C. Appropriate dilutions of horseradish peroxidase (HRP)-conjugated rabbit anti-mouse or rabbit anti human (prepared in our laboratory) were subsequently added and the presence of adherent antibodies were detected using 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. After 15 min, the reaction was stopped by addition of 100 μl of 20% H2SO4 and the optical density (OD) in each well was measured using a Multiscan ELISA reader (OrganonTeknika, Boxtel, Belgium) at 450 nm.
Assessment of toxin neutralizing activity of monoclonal antibodies
The ability of mAb to inhibit binding of TeNT to its receptor GT1b ganglioside was assessed in vitro by modification of a previously described procedure (Yousefi et al., Citation2013b). In brief, a concentration of TeNT that resulted in saturation of ganglioside GT1b binding was chosen (20 μg/ml) and mixed with different concentrations of the purified murine anti-TeNT mAb (1–10 μgr/ml) or 100 μl of undiluted LCL supernatants and the mixture then incubated for 2 h at room temperature (RT). Microtiter ELISA plates were coated with GT1b (Sigma) (10 μg/ml in methanol, 100 μl/well) and plates were left at RT overnight to allow evaporation of methanol. The plates were then blocked with 1% BSA/PBS for 2 h at RT. After four washes with PBS-T, 100 μl of pre-incubated TeNT/antibody mixtures were added to wells and the plate incubated for 2 h at RT. The plates were then washed four times and HRP-conjugated human anti TeNT polyclonal Ab (produced in our lab) was then added to each well and the plate was incubated for 2 h at RT. Following addition of TMB substrate solution, the OD value in each well was measured at 450 nm in the multi-scan ELISA plate reader. The inhibition percentage was calculated based on OD values obtained in the presence of anti-TeNT mAb divided by the OD values obtained in the absence of anti-TeNT mAb.
Statistical analysis
Statistical analyses were conducted using the SPSS statistical package (SPSS Inc., v15, Chicago, IL). Statistical comparisons of data were made using Chi-Square and Fisher-Exact tests, as appropriate. A p value ≤ 0.05 was considered significant.
Results
Production and characterization of murine anti-TeNT mAb
The fusion of splenocytes from hyper-immunized mouse with SP2/0 myeloma cells led to generation of growing hybridomas. All actively proliferating hybridomas were initially screened based on reactivity to tetanus toxoid and toxin by indirect ELISA. Finally, 22 stable hybridomas producing antibodies reacting with both tetanus toxoid and toxin were selected. Representative results are presented in . All selected hybridomas were sub-cloned and stable clones producing TeNT-specific mAb were selected (). Seven of 22 murine anti-TeNT mAb (31.8%) reacted with fragment C and only two reacted with the HCC sub-domain. Seven of the remaining mAb (31.8%) reacted with TeNT light chain, three (13.6%) reacted with both fragment C and the light chain and five (22.8%) reacted with neither fragment C, HCC or the light chain ().
Table 1. Representative screening results of mouse TeNT-specific hybridoma clones. (Results represent optical density (OD) values obtained by ELISA at 450 nm.).
Table 2. Reactivity of human and mouse monoclonal antibodies with different fragments of tetanus toxin.
Establishment and characterization of human TeNT-specific LCL
The frequency of anti TeNT LCLs was determined 3 weeks after EBV infection by limiting dilution assay (based on Poisson statistical analysis as explained in the Materials and methods section). The negative fraction was defined as the fraction of wells containing growing LCL that secreted a quantifiable amount of human immunoglobulin, but did not produce anti-TeNT antibody. These were calculated from the number of wells positive for TeNT-specific antibody determined for each LDA plate.
Finally, 50 TeNT-specific LCL were selected. Culture supernatants from all LCL were positive for both tetanus toxin and toxoid. Thirty-four of 50 human anti-TeNT positive supernatants (68%) reacted with fragment C; of these, 26 mAb recognized epitopes located in the HCC sub-domain. Ten (20%) of the remaining mAb reacted with the TeNT light chain, whereas two (4%) reacted with both fragment C and the light chain and four (8%) reacted with none of the TeNT proteins or fragments ().
Ganglioside GT1b binding inhibition by monoclonal antibodies
To investigate the inhibitory effect of each mAb to block TeNT binding to immobilized ganglioside, a GT1b inhibition assay was performed using an indirect ELISA. Representative results are shown in . Only six of 22 (27%) murine anti-TeNT mAb were able to inhibit TeNT binding to ganglioside GT1b. All of these in vitro neutralizing mAb were directed against fragment C, whereas only one reacted with the HCC sub-domain. Of the human anti-TeNT mAb, only eight (16%) were able to block TeNT binding to immobilized GT1b; of these, six were directed against fragment C, one reacted with both fragment C and light chain and one reacted with no fragments. In contrast to mouse fragment C-specific neutralizing mAb, all human anti-fragment C neutralizing mAb reacted with the HCC sub-domain (p = 0.015) (). Representative raw data from among the results presented in and are given in .
Figure 1. In vitro inhibitory activity of the mouse monoclonal antibodies on TeNT binding to GT1b. Individual clones are listed along the right side of the figure.
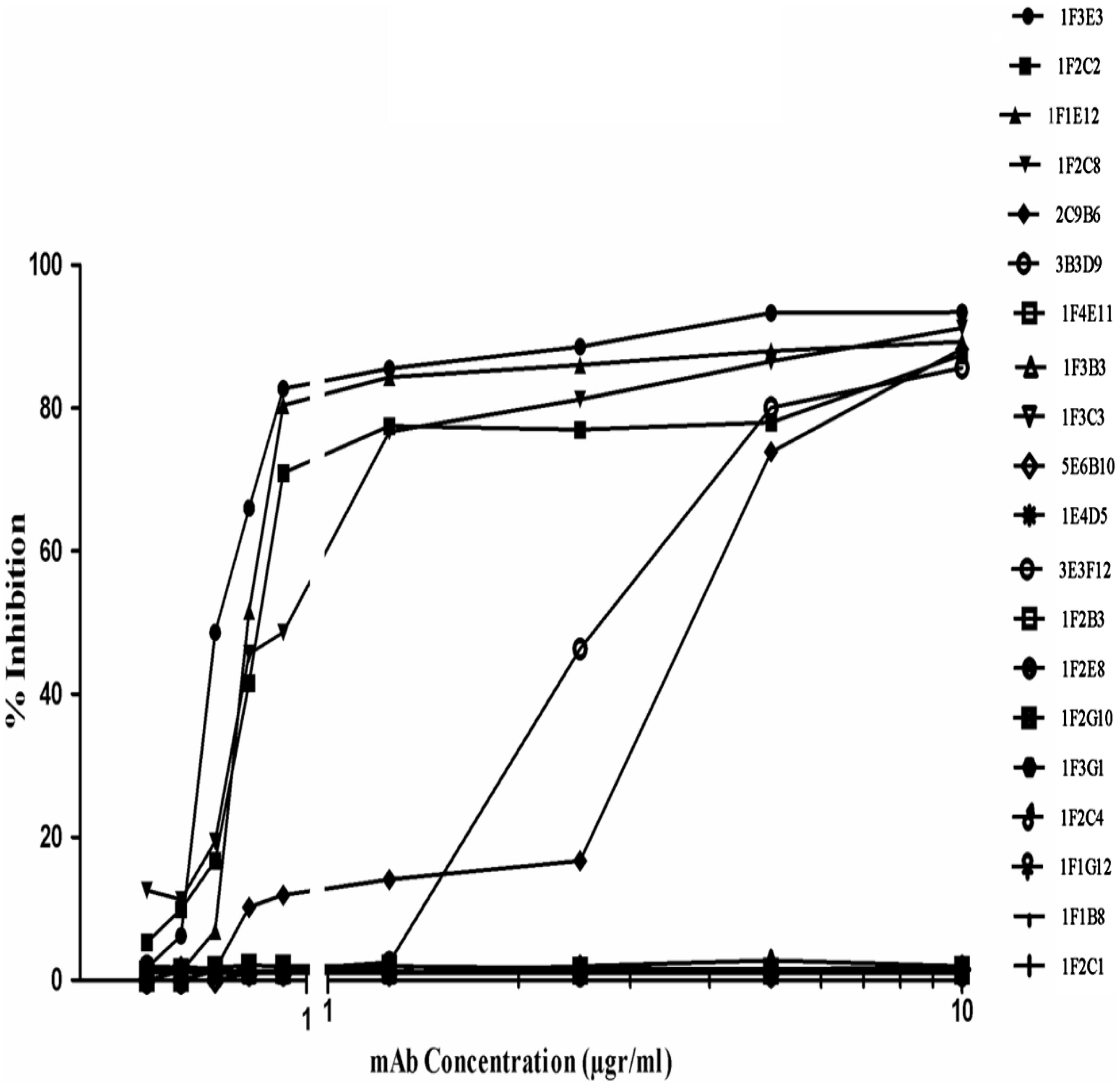
Table 3. Toxin neutralization activity of human and mouse anti-TeNT mAb as determined by GT1b binding inhibition assay.
Table 4. Representative data obtained for a number of murine mAb and human LCLs specific for tetanus toxin. (Results represent optical density (OD) values obtained by ELISA at 450 nm.).
Discussion
We have recently reported the dominance of the fragment C specific antibody response in mice following immunization with tetanus toxoid (Yousefi et al., Citation2013b). In the present study, 22 murine and 50 human anti-TeNT mAb were produced using hybridoma technology and EBV transformation methods, respectively. The results showed that the antibody responses in the human and mouse models were mainly directed against fragment C. Despite this overall similarity between the two systems, there are some key differences. The number of fragment C- and HCC-specific mAb was significantly higher in humans compared to mice (p = 0.009 and p = 0.0004, respectively). Conversely, light chain-specific mAb were more prevalent among mice (31.8%) compared to humans (20%) mAb, although the difference was insignificant (). These data suggested to us there are some inter-species differences between humans and mice regarding the immune response to TeNT. As such, this leads us to believe that results obtained from animal studies might not always be generalizable to humans.
Several studies have shown that certain sequences of TeNT could sensitize CD4+ T-cells of almost all subjects (Diethelm-Okita et al., Citation1997; Ho et al., Citation1990; Reece et al., Citation1993). The molecular mechanism through which these “universal epitopes” sensitize most CD4+ cells is not fully understood. One explanation is processing and presentation of these epitopes by multiple MHC Class II molecules. Alternatively, the T-cells may cross-react with unrelated peptides (presented by various Class II molecules) and enhance the immune response against universal epitopes (Cresswell, Citation1994; Madden, Citation1995; Watts, Citation1997). These epitopes have been localized to fragment C, both the N-terminal region (HCN) and particularly the C-terminal region (HCC) of this fragment (Diethelm-Okita et al., Citation2000). These epitopes have been widely used to enhance vaccine immunogenicity and efficacy in a variety of immunotherapeutic systems, particularly in the field of cancer (Anderson et al., Citation1996; King et al., Citation1998; Robinson et al., Citation2004).
As shown in , three mouse and two human anti-TeNT mAb models reacted with both fragment C and light chain. It seems these mAb might recognize a conformational epitope to which both fragment C and light chain contribute to its expression or cross-react with epitopes located on both LC and fragment C. Another interesting finding of the present study was the neutralization activity of anti-fragment C mAb. The results showed that all (six out of six) mouse and 75% (six out of eight) human neutralizing antibodies were directed against fragment C, whereas none of the human or mouse anti-light chain antibodies could neutralize the toxin in vitro (). Interestingly, only one of these fragment C-specific mouse mAb reacted with HCC, whereas six of the human fragment C positive mAb reacted with HCC (p = 0.015).
As previously mentioned, the fragment C HCC sub-domain contains key amino acids responsible for tetanus toxin binding to gangliosides of the 1b series (as the most important part of the clostridium neurotoxin receptor). Thus, antibodies against fragment C were expected to be neutralizing; these findings would be consistent with results of several other published studies (Arunachalam et al., Citation1992; Fitzsimmons et al., Citation2000; Gustafsson et al., Citation1993; Sheppard et al., Citation1984; Simpson et al., Citation1990). However, contradictory results showing involvement of the other parts of the toxin have also been reported (Ahnert-Hilger et al., Citation1983; Gigliotti & Insel, Citation1982; Kamei et al., Citation1990; Lang et al., Citation1993; Matsuda et al., Citation1992). The neutralization activity of such mAb directed against other parts of the toxin may act through conformational changes in the structure of the toxin and toxin-binding domain.
In conclusion, the results here show that, in the human and mouse models, the neutralizing anti-body response was directed mainly against fragment C – although different epitopes might be involved. These findings suggest that this non-toxic fragment of TeNT could be considered as a suitable candidate for tetanus vaccination. Moreover, the fragment C-specific mAb could be potentially useful for passive immunotherapy of tetanus.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors would like to thank Jalal Khoshnoodi and Azam Brook for their technical assistance.
References
- Ahnert-Hilger, G., Bizzini, B., Goretzki, K., et al. 1983. Monoclonal antibodies against tetanus toxin and toxoid. Med. Microbiol. Immunol. 172:123–135
- Anderson, R., Gao, X. M., Papakonstantinopoulou, A., et al. 1996. Immune response in mice following immunization with DNA encoding fragment C of tetanus toxin. Infect. Immun. 64:3168–3173
- Arunachalam, B., Ghosh, S., Talwar, G., and Raghupathy, R. 1992. A single human monoclonal antibody that confers total protection from tetanus. Hybridoma 11:165–179
- Chaddock, J., and Marks, P. M. 2006. Clostridial neurotoxins: Structure-function led design of new therapeutics. Cell. Mol. Life Sci. 63:540–551
- Cresswell, P. 1994. Assembly, transport, and function of MHC Class II molecules. Ann. Rev. Immunol. 12:259–291
- di Tommaso, A., de Magistris, M. T., Bugnoli, M., et al. 1994. Formaldehyde treatment of proteins can constrain presentation to T-cells by limiting antigen processing. Infect. Immun. 62:1830–1834
- Diethelm-Okita, B. M., Okita, D. K., Banaszak, L., and Conti-Fine, B. M. 2000. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 181:1001–1009
- Diethelm-Okita, B. M., Raju, R., Okita, D. K., and Conti-Fine, B. M. 1997. Epitope repertoire of human CD4+ T-cells on tetanus toxin: Identification of immunodominant sequence segments. J. Infect. Dis. 175:382–391
- Fitzsimmons, S. P., Clark, K. C., Wilkerson, R., and Shapiro, M. A. 2000. Inhibition of tetanus toxin fragment C binding to ganglioside GT1b by monoclonal antibodies recognizing different epitopes. Vaccine 19:114–121
- Gigliotti, F., and Insel, R. 1982. Protective human hybridoma antibody to tetanus toxin. J. Clin. Invest. 70:1306–1309
- Gustafsson, B., Whitmore, E., and Tiru, M. 1993. Neutralization of tetanus toxin by human monoclonal antibodies directed against tetanus toxin fragment C. Hybridoma 12:699–708
- Herreros, J., Lalli, G., Montecucco, C., and Schiavo, G. 2000. Tetanus toxin fragment C binds to a protein present in neuronal cell lines and motoneurons. J. Neurochem. 74:1941–1950
- Ho, P. C., Mutch, D. A., Winkel, K. D., et al. 1990. Identification of two promiscuous T-cell epitopes from tetanus toxin. Eur. J. Immunol. 20:477–483
- Ichimori, Y., Sasano, K., Itoh, H., et al. 1985. Establishment of hybridomas secreting human monoclonal antibodies against tetanus toxin and hepatitis B virus surface antigen. Biochem. Biophys. Res. Commun. 129:26–33
- Kamei, M., Hashizume, S., Sugimoto, N., et al. 1990. Establishment of stable mouse/human-human hybrid cell lines producing large amounts of anti-tetanus human monoclonal antibodies with high neutralizing activity. Eur. J. Epidemiol. 6:386–397
- Kazemi, T., Tahmasebi, F., Bayat, A. A., et al. 2011. Characterization of novel murine monoclonal antibodies directed against extracellular domain of human HER2 tyrosine kinase receptor. Hybridoma 30:347–353
- Kenimer, J. G., Habig, W. H., and Hardegree, M. C. 1983. Monoclonal antibodies as probes of tetanus toxin structure and function. Infecti. Immun. 42:942–948
- King, C. A., Spellerberg, M. B., Zhu, D., et al. 1998. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nature Med. 4:1281–1286
- Kitchin, N. R. 2011. Review of diphtheria, tetanus and pertussis vaccines in clinical development. Expert Rev. Vaccines 10:605–615
- Kozbor, D., Roder, J. C., Chang, T. H., et al. 1982. Human anti-tetanus toxoid monoclonal antibody secreted by EBV-transformed human B-cells fused with murine myeloma. Hybridoma 1:323–328
- Lalli, G., Bohnert, S., Deinhardt, K., et al. 2003. The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol. 11:431–437
- Lang, A., Cryz, S., Schurch, U., et al. 1993. Immunotherapy with human monoclonal antibodies. Fragment A specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J. Immunol. 151:466–472
- Larrick, J. W., Truitt, K. E., Raubitschek, A. A., et al. 1983. Characterization of human hybridomas secreting antibody to tetanus toxoid. Proc. Natl. Acad. Sci. USA 80:6376–6380
- Luo, P., Qin, L., Mao, X., et al. 2012. Identification of a novel linear epitope in tetanus toxin recognized by a protective monoclonal antibody: Implications for vaccine design. Vaccine 30:6449–6455
- Madden, D.R. 1995. The three-dimensional structure of peptide-MHC complexes. Ann. Rev. Immunol. 13:587–622
- Matsuda, M., Kamei, M., Sugimoto, N., et al. 1992. Characteristics of toxin-neutralization by anti-tetanus human monoclonal antibodies directed against the three functional domains [A], [B], and [C] of the tetanus toxin molecule and a reliable method for evaluating the protective effects of monoclonal antibodies. Eur. J. Epidemiol. 8:1–8
- Mayer, S., Laumer, M., Mackensen, A., et al. 2002. Analysis of the immune response against tetanus toxoid: Enumeration of specific T-helper cells by ELISPOT assay. Immunobiology 205:282–289
- Montecucco, C., and Schiavo, G. 2006. Mechanism of action of tetanus and botulinum neurotoxins. Mol. Microbiol. 13:1–8
- Petrušić, V., Živković, I., Stojanović, M., et al. 2011. Production, characterization and applications of a tetanus toxin specific monoclonal antibody T-62. Acta Histochemica 114:480–486
- Poulsen, T. R., Meijer, P. J., Jensen, A., et al. 2007. Kinetic, affinity, and diversity limits of human polyclonal antibody responses against tetanus toxoid. J. Immunol. 179:3841–3850
- Reece, J. C., Geysen, H. M., and Rodda, S. J. 1993. Mapping the major human T-helper epitopes of tetanus toxin. The emerging picture. J. Immunol. 151:6175–6184
- Robinson, K., Chamberlain, L., Lopez, M., et al. 2004. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect. Immun. 72:2753–2761
- Schiavo, G., Matteoli, M., and Montecucco, C. 2000. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80:717–766
- Sheppard, A. J., Cussell, D., and Hughes, M. 1984. Production and characterization of monoclonal antibodies to tetanus toxin. Infect. Immun. 43:710–714
- Simpson, L. L., Lake, P., and Kozaki, S. 1990. Isolation and characterization of a novel human monoclonal antibody that neutralizes tetanus toxin. J. Pharmacol. Exp. Ther. 254:98–103
- Trabaud, M. A., Lery, L., and Desgranges, C. 1989. Human monoclonal antibodies with a protective activity against tetanus toxin. APMIS 97:671–676
- Turton, K., Chaddock, J. A., and Acharya, K. R. 2002. Botulinum and tetanus neurotoxins: Structure, function and therapeutic utility. Trends Biochem. Sci. 27:552–558
- Volk, W., Bizzini, B., Snyder, R., et al. 1984. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect. Immun. 45:604–609
- Volkman, D., Allyn, S., and Fauci, A. 1982. Antigen-induced in vitro antibody production in humans: Tetanus toxoid-specific antibody synthesis. J. Immunol. 129:107–112
- Watts, C. 1997. Capture and processing of exogenous antigens for presentation on MHC molecules. Ann. Rev. Immunol. 15:821–850
- Younesi, V., Nikzamir, H., Yousefi, M., et al. 2010. Epstein Barr virus inhibits the stimulatory effect of TLR7/8 and TLR9 agonists but not CD40 ligand in human B-lymphocytes. Microbiol. Immunol. 54:534–541
- Younesi, V., Shirazi, F. G., Memarian, A., et al. 2014. Assessment of the effect of TLR7/8, TLR9 agonists and CD40 ligand on the transfor–mation efficiency of Epstein-Barr virus in human B-lymphocytes by limiting dilution assay. Cytotechnology 66:95–105
- Yousefi, M., Khosravi-Eghbal, R., Hemmati, A., and Shokri, F. 2013a. Production and characterization of recombinant light chain and carboxyl terminal heavy chain fragments of tetanus toxin. Avicenna J. Med. Biotech. 5:220–226
- Yousefi, M., Khosravi-Eghbal, R., Mahmoudi, A. R., et al. 2013b. Comparative in vitro and in vivo assessment of toxin neutralization by anti-tetanus toxin monoclonal antibodies. Human Vaccines Immunother. 10:16–15
- Yousefi, M., Tahmasebi, F., Younesi, V., et al. 2014. Characterization of neutralizing monoclonal antibodies directed against tetanus toxin fragment C. J. Immunotoxicol. 11:28–34

