Abstract
Leishmaniasis is one of the most common infectious diseases transmitted by an obligate intracellular genus Leishmania. As there is no efficient vaccination strategy for leishmaniasis, new immunostimulatory components may enhance protective immune responses against this parasite. Lipophosphoglycan 3 (LPG3) is an essential protein required for LPG assembling. In this study, the ability of recombinant LPG3 (rLPG) and its fragments to activate isolated healthy human T-cells and cytokine secretion was evaluated in vitro. The results showed that rLPG3 and its N-terminal fragment (rNT-LPG3) enhanced expression of CD69 on the surface of T-cells and promoted differentiation of CD4+ T-lymphocytes toward a T-helper 1 (TH1) phenotype, in part, through up-regulation of interferon (IFN)-γ expression in a TLR2-independent manner. These results indicated the protective effects of LPG3 (particularly NT-LPG3 fragment) as a potent immunostimulatory component of leishmania in vaccination against leishmaniasis. Further investigations in in vivo assays are clearly warranted.
Introduction
Leishmaniasis, caused by organisms in the genus Leishmania, is considered as the second most common fatal parasitic disease in the world after malaria. This protozoan disease can be induced by many different species of leishmania, with a wide spectrum of manifestations evolving from self-healing cutaneous leishmaniasis (CL) to a life-threating visceral leishmaniasis (VL) that induces severe damage in host organs (Murray et al., Citation2005). In general, Leishmania starts its lifecycle in the digestive tract of an insect vector, where it propagates in its flagellated pro-mastigote stage. Subsequently, virulent meta-cyclic promastigotes infect a host through the bite of an infected sand fly; then organisms take up residence in phagocytes where they can differentiate into aflagellated amastigote forms and escape immune system recognition (McMahon-Pratt & Alexander, Citation2004; Murray et al., Citation2005; Racoosin & Beverley, Citation1997; Tuon et al., Citation2008). Based on World Health Organization reports (WHO, Citation2015), leishmaniasis is endemic in several regions, including the Eastern Mediterranean, Southern and Southeast Asia, and South America. In Iran, it is also estimated that L. major is responsible for 70% of all documented cases (Alvar et al., Citation2012).
Cutaneous leishmania (CL) is the most common form of leishmaniasis, with ≈0.7–1.3 million new cases being recognized annually worldwide. CL is associated with skin sores/nodules that are usually self-limited; however, in some cases, the disease may develop into a progressive chronic form (Murray et al., Citation2005). Generally, leishmaniasis is controlled by chemotherapeutic drugs or by limiting vector transitions. Although CL is self-limiting, patients usually suffer from many problems including long lasting sores, cosmetic problems, expensive drugs, adverse drug side-effects and drug resistance. It is supposed that the effective vaccine immunotherapy may alleviate these problems (Croft et al., Citation2006; Kishore et al., Citation2006; Seyed et al., Citation2011). Consistently, many vaccination strategies have been developed against leishmaniasis, including the use of killed or live attenuated leishman parasites, salivary antigen-based vaccines, recombinant proteins from different stages of Leishmania life cycle and DNA vaccines. AG-702 and Leish-111f leishmaniasis vaccines have recently been assessed in advanced stages of clinical trials; however, none of them was protective (Kedzierski et al., Citation2006).
Lipophosphoglycan (LPG) is one of the most important molecules in survival and virulence of Leishmania. LPG is an abundant surface glycolipid of Leishmania promastigotes, with increased expression occurring during Leishmania transformation into metacyclic promastigotes (Yao et al., Citation2003). Upon a host being bitten by an infected vector, promastigotes invade the host macrophages through LPG molecules and subsequently disrupt different anti-microbial functions of the macrophages (including apoptotic and signaling pathways), phagolysosome maturation and nitric oxide (NO) production, each alone or together can lead to establishment of an infection (McMahon-Pratt & Alexander, Citation2004). Lipophosphoglycan-3 (LPG3) is one of the Class II LPG genes from the HSP90 family that plays a critical role in LPG production (Descoteaux et al., Citation2002). Because LPG3 stimulates host immune responses (Ni & Lee, Citation2007), it can be considered as a potential therapeutic [vaccine] candidate against leishmaniasis (Larreta et al., Citation2000, Citation2002).
Cell-mediated immune response is the main protective response against leishmaniasis; which is, in part, through activation of macrophages by interferon (IFN)-γ, leading to the destruction of phagocytosed parasites in hydrogen peroxide (H2O2) or NO-dependent manners (Pirdel et al., Citation2014). While T-helper (TH)-1 cell type-mediated responses can help to control disease burden, TH2 type responses enhance disease establishment and progression. Therefore, the main objective in the development of effective Leishmania vaccines is the induction of TH1-mediated immune responses (Croft et al., Citation2006).
Toll-like receptors (TLR) are important members of pattern recognition receptors that contribute to inflammatory responses during leishmaniasis (Glushkova et al., Citation2013; Kropf et al., Citation2004). TLRs are expressed on the cell surface and/or intracellular endosomes of various immune cells, including T-cells. Among them, TLR2 can recognize a wide variety of ligands, such as peptidoglycans, bacterial lipoproteins, peptides, triacyl lipopeptides and diacyl lipopeptides (Asgarian-Omran et al., Citation2014; Kropf et al., Citation2004). It has been shown that purified L. major LPG directly binds to TLR2 on the surface of macrophages and natural killer (NK) cells and stimulates their secretion of inflammatory cytokines such as tumor necrosis factor (TNF)-α and IFNγ (Becker et al., Citation2003; de Veer et al., Citation2003; Faria et al., Citation2012).
In the study reported here, the stimulatory effects of recombinant Leishmania LPG3 and its fragments on T-cell activation and cytokine secretion – in the presence and absence of anti-TLR2 inhibitory antibodies – were investigated. Our results showed that LPG3 protein, particularly its NT fragment, had effective immunostimulatory properties for T cells and seemed to cause a shift toward TH1-type responses.
Materials and methods
Blood sampling and PBMC isolation
The fresh heparinized blood samples from 10 normal lab volunteers without history of leishmaniasis were collected in order for isolation of peripheral blood mononuclear cells (PBMC) by density gradient centrifugation over Histopaque (Sigma, St. Louis, MO). Written consent was received from each volunteer. The Ethics Committee of Tabriz University of Medical Sciences approved this study.
T-cell isolation using magnetic activated cell sorting (MACS) technique
CD3+ T-cells were isolated from PBMC by negative selection using magnetically-labeled antibodies against non-T-cell markers (including HLA-DR, CD14, CD15, CD16, CD19, CD25, CD36, CD56, CD57, CD45RO, CD123, CD244, and CD235a) as part of a Naive Pan T-Cell Isolation Kit (Miltenyi Biotech, Bergisch-Gladbach, Germany). In brief, following two washes of the PMBC with MACS buffer (Miltenyi Biotech), the final cell pellet was re-suspended in 30 μl buffer (per 107 total cells). All non-target cells were then labeled by addition of a cocktail of biotin-conjugated monoclonal antibodies (mAb; 10 μl, 107 cells) and incubation for 30 min at 2–8 °C. Thereafter, 30 μl buffer and 20 μl Naive Pan T-Cell MicroBead Cocktail was added (for 107 total cells) and the mixture incubated for a further 10 min at 2–8 °C. After this, all magnetically-labeled non-target cells were depleted from the mixture by passage through a MACS® Column (according to manufacturer instructions). All non-labeled cells (including CD3+ T-cells) were washed out from the column and collected in the eluate.
T-cell purity assessment using flow cytometry
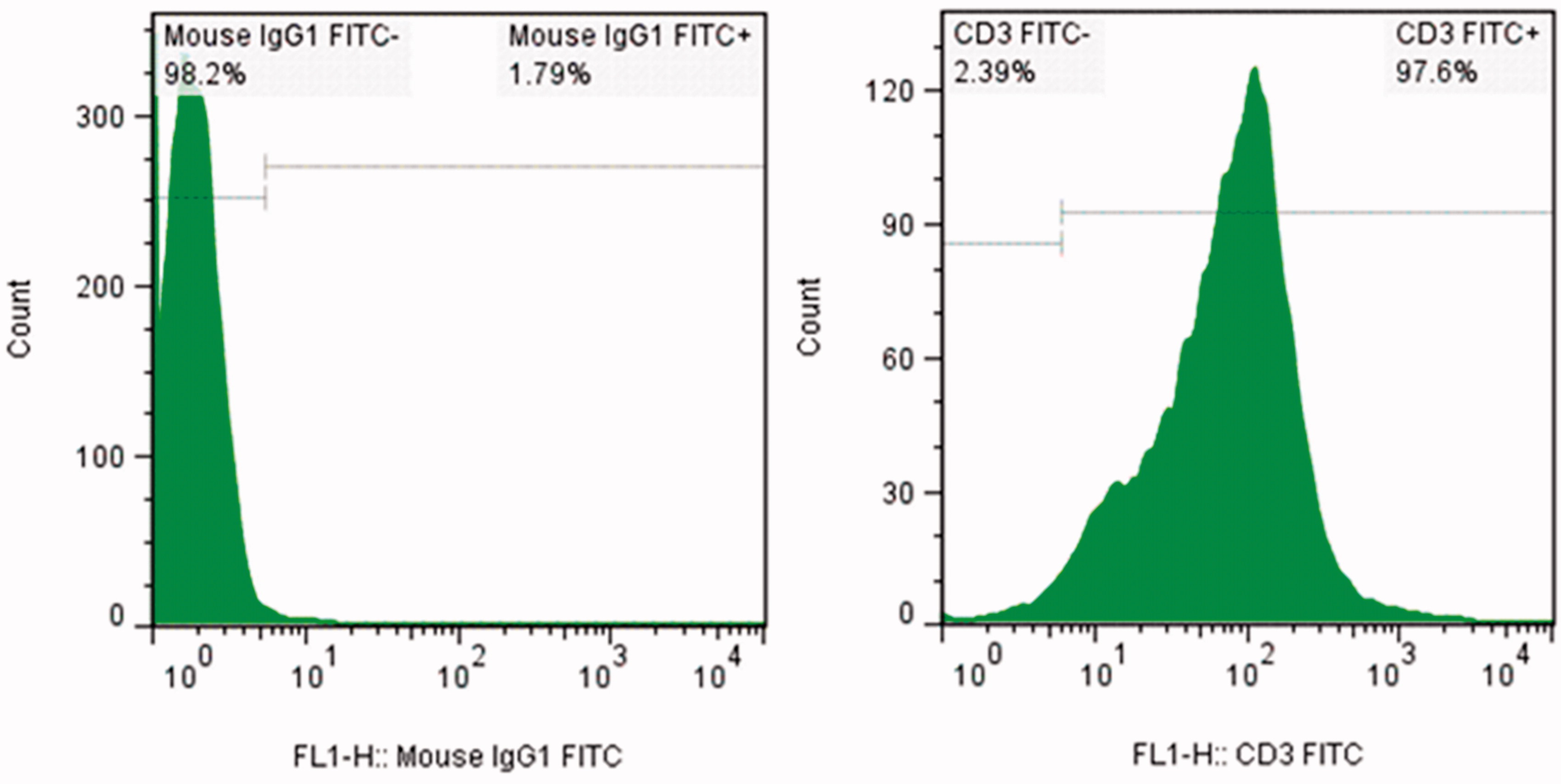
The purity of the isolated T-cells was verified by flow cytometry using fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD3 mAb (eBiosciences, San Diego, CA). Here, T-cells were washed twice with washing buffer (PBS containing 0.5% [w/v] BSA, 0.1% [w/v] NaN3), re-suspended in washing buffer to 104 cells/μl and then stained with the manufacturer-recommended amount of the FITC-mAb. After 40 min incubation at 4 °C in the dark, the purity of the CD3+ cells was determined using a FACSCalibur flow cytometer (BD Biosciences, Heidelberg, Germany). All data were analyzed using FlowJo software (v.7.2.5; Tree Star Inc., Ashland, OR). A minimum of 10 000 events/sample was acquired. The purity of the separated CD3+ T-cells was routinely >90% ().
T-cell treatment with recombinant LPG3, NT-LPG3 and CT-LPG3 fragments in the presence and absence of anti-TLR2 antibody
Recombinant LPG3 (rLPG) N-terminal (NT) and C-terminal (CT) fragments were prepared as described in Abdian et al. (Citation2011) using a highly-pure endotoxin-free plasmid extraction kit (Qiagen, Valencia, CA) to recover the materials expressed in an Escherichia coli system maintained at the Pasteur Institute of Iran (Tehran). For the actual cell treatments, isolated T-cells in complete medium, i.e. RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Paisley, UK), 100 U penicillin/ml and 100 mg streptomycin/ml (Gibco), were placed at 106 cells/well in 24-well plates. The T-cells were then treated with 10 μg/ml (final concentration in well) of rLPG3, NT-LPG3 or CT-LPG3 in the presence and absence of optimized amounts of anti-human TLR2 antibody (10 μg/ml) (Invivogen, San Diego, CA) and then incubated for 48 h at 37 °C in a 5% CO2/95% air chamber.
Flow cytometry
Expression of CD69 on the cells was analyzed by flow cytometry to evaluate the activation state of the T-cells treated with rLPG3, NT-LPG3 or CT-LPG3 in the presence and absence of anti-TLR2 antibody. In brief, after the culture period outlined above, aliquots of each culture containing 106 cells were washed with PBS and then stained with FITC-anti-CD3 and phycoery-thrin-anti-CD69 mAb (eBiosciences). After 45-min incubation at 4 °C in the dark, the cells were washed and analyzed by flow cytometry as above.
Real time PCR
The mRNA levels of TH1 and TH2 lineage specific transcription factors including T-bet and GATA3 (Matsumoto et al., Citation2010) were assessed through real time PCR. Briefly, the total RNA was extracted from T-cells treated with different concentrations of rLPG3 using Trizol (Sigma). Next, cDNA synthesis was achieved by using 1 μg total RNA and a cDNA synthesis kit (Roche, Gipf-Oberfrick, Switzerland), according to manufacturer instructions. Quantification by real-time PCR was used to detect the level of T-bet and GATA3; this was done using a One-Step Quantitech SyberGreen Real Time PCR kit (Qiagen), according to manufacturer instructions. Designated primers for human T-bet were 5′-GATGTTTGTGGACGTGGTCTTG-3′ and 5′-CTTTCCACACTGCACCCACTT-3′, for human GATA3 the primers were 5′-CTCCTCTCTG-CTCTTCGCTACC-3′ and 5′-GACTCTGCAATTCTGCGAGCC-3′. 18 S rRNA was employed as the housekeeping control (Applied Biosystems, Foster City, CA) to analyze mRNA expression. Changes in T-bet and GATA3 expression after treatment were evaluated using the equation ΔΔCt(target) =ΔCt(target, stimulated) − ΔCt(target, resting).
Cytokine assay
The effects of rLPG3, NT-LPG3 or CT-LPG3 on IFNγ and interleukin (IL)-4 secretion were evaluated using commercial ELISA kits (R&D, Minneapolis, MN). The wells of microtiter ELISA plate (Maxisorp, Nunc, Roskilde, Denmark) were coated overnight at 4 °C with anti-IFNγ or anti-IL-4 capture Ab (in 100 mM Na2HPO4 buffer, pH 9.0) and then blocked using phosphate buffered-saline (PBS, pH 7.4) containing 10% FBS and 0.05% Tween-20. Cells culture supernatants (collected at the end of the 48-h treatments) were then added (to triplicate wells/treatment condition) and the plate incubated for 2 h at room temperature. After this incubation, the wells were rinsed with wash buffer (PBS + 0.05% [v/v] Tween-20) and then biotinylated mouse anti-hIFNγ mAb or anti-hIL-4 mAb (each 1:10 dilution in 1% BSA/PBS) was added to specified wells and the plate was incubated at 25 °C for 1 h. After a gentle rinsing with wash buffer to remove all non-adherent antibodies, the presence of anti-cytokine antibody was detected by addition of a streptavidin-alkaline phosphatase-conjugated anti-mouse IgG Ab (Sigma) (diluted 1:300 in Tris-Buffered Saline (TBS, pH 7.6) and incubation of the plate for 20 min at 25 °C. Upon completion of the incubation and a gentle rinsing with wash buffer, p-nitrophenyl phosphate (4 mg/ml) was added to each well as substrate. After incubation at 25 °C for 30 min, the absorbance in each well was assessed at 405 nm in an ELISA plate reader (LabSystems, Helsinki, Finland). The range of the limit concentration of detection was 15–1000 pg/ml. Wells that had received varying amounts of each cytokine (as kit-provided standards) were run as well. This allowed the level of each cytokine in a given supernatant sample to be extrapolated from this standard curve that was generated in parallel.
Statistical analysis
All data are reported as means ± SD. Inter-group comparisons were done using a Kruskal-Wallis non-parametric analysis of variance to determine statistical significance. All analyses were done using Prism v.5.04 software (GraphPad, La Jolla, CA).
Results
Effects of recombinant LPG3, NT fragment and CT fragment on T-cell CD69 expression
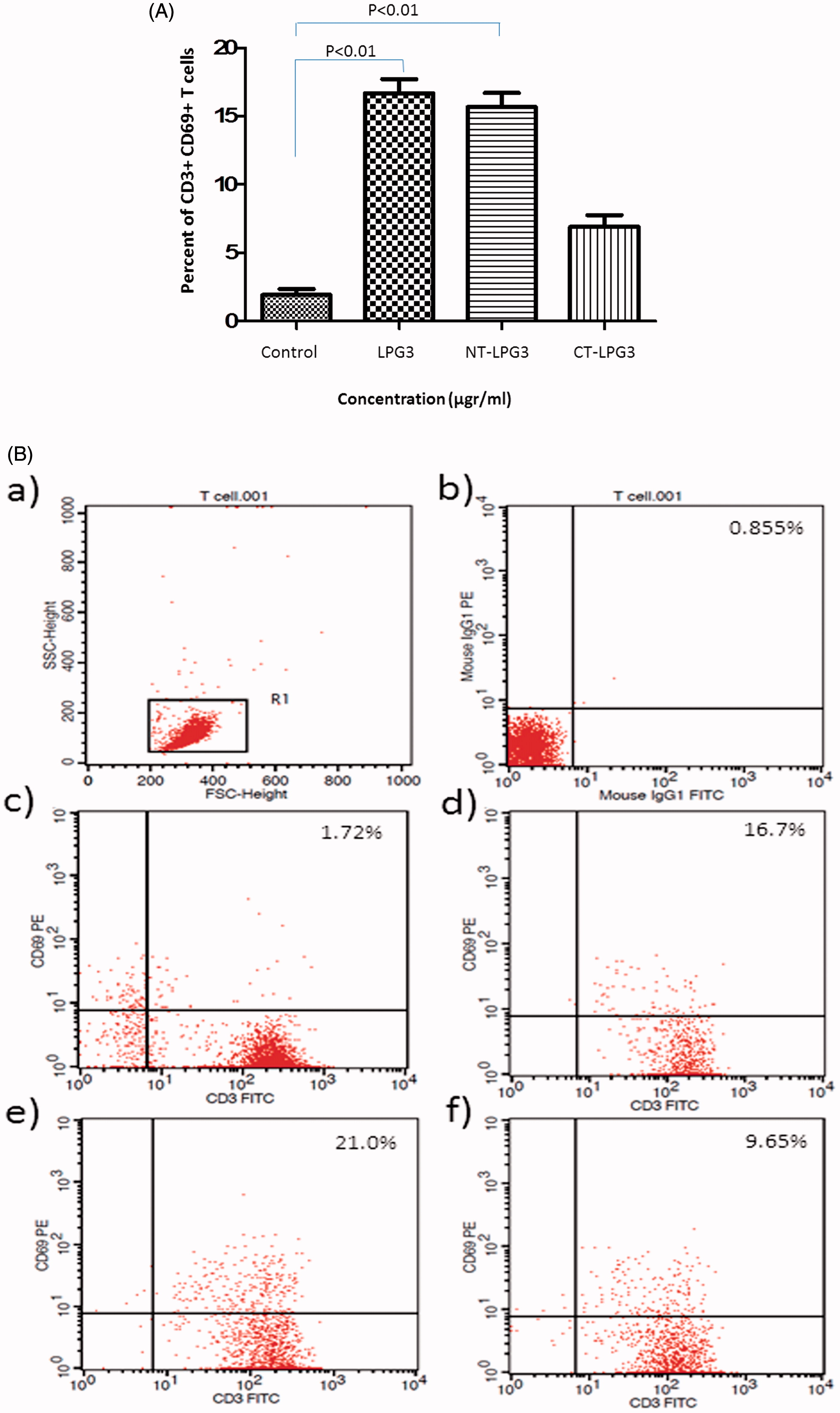
Surface expression levels of CD3 and CD69 on T-cells treated with rLPG3, NT-LPG3 or CT-LPG3 in the presence/absence of anti-TLR2 inhibitory antibodies was investigated. As seen in (conversion of dot-plot representative data shown in ), CD69 expression on cells treated with LPG3 or the NT fragment was significantly increased compared to that on untreated cells (p < 0.01). Average expression increased from 1.7 (± 0.2)% to now 16.7 (± 2.0) and 21.0 (± 2.1)% for, respectively, the LPG3- and the NT fragment-treated cells. However, the CT fragment imparted an increase in expression as well (to 9.6 [± 1.8]%), but the change was not significant. No significant differences in CD69 induction among the varying treatments were seen when the cells were treated with the test agents in the presence of anti-TLR2 blocking antibodies (data not shown).
Figure 2. Flow cytometry analysis of T cells treated with rLPG3, NT-LPG3 or CT-LPG3. (A) Purified T cells were incubated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h and then their level of activation examined by flow cytometry. Results shown are mean (± SD) %CD3+CD69+ T cells present. Values significantly different from control are indicated (p < 0.01). (B) Flow cytometric analysis of the T cells is presented in representative dot-blot forms: (a) Whole population of lymphocytes; (b) isotype control; (c) untreated T cells; (d) T cells treated with rLPG3; (e) T cells treated with NT-rLPG3; and (f) T cells treated with CT-rLPG3.
Effects on T-bet and GATA-3 mRNA levels in LPG3-treated T-cells
The results shown in indicated that rLPG3 could significantly (p < 0.05) increase (when compared with control cell values) the expression of T-bet mRNA in the T-cells treated with moderate (10 μg/ml) and high (20 μg/ml) doses of rLPG3. The fold-induction of T-bet mRNA increased from 1.00 (± 0.28) to now 8.36 (± 0.73) and 10.25 (± 0.51) for, respectively, the moderate and high dose rLPG3-treated cells. In contrast, the LPG3 imparted no significant effects had no effect on mRNA levels of GATA-3 in the cells, albeit there appeared to initially be a trend toward an increase in expression (that, in turn, disappeared as dose doubled from 10 to 20 μg LPG3/ml).
Effects of LPG3, NT fragment and CT fragment on T-cell formation of cytokines associated with TH1 and TH2 response
The levels of IFNγ secreted by T-cells treated with rLPG3, NT and CT fragments in the presence or absence of anti TLR2 antibody were determined (). The results indicated that rLPG3 and its NT fragment had a significant (p < 0.01) effect on IFNγ production compared to that by untreated T-cells. Levels of IFNγ formation increased from 21.70 (± 2.10) pg/ml to now 82.11 (± 3.61) and 88.12 (± 4.35) pg/ml for, respectively, the LPG3 and the NT fragment-treated cells. These effects appeared to occur in a TLR2-independent manner. In contrast, the impact of IFNγ induction in CT fragment-treated cells was not significant.
Figure 4. Cytokine secretion by T cells treated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h. Results shown are mean [± SD] amounts of cytokine secreted by cells (in pg/ml) that were treated in the absence or presence of anti-TLR2 antibody; 10 samples/treatment. (A) Secretion of IFNγ by T cells treated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h (p < 0.01). (B) Secretion of IL-4 by T cells treated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h. (C) IFNγ:IL-4 ratio in co-cultured T cells with LPG3, NT-LPG3 or CT-LPG3 following 48 h incubation (p < 0.05). Values significantly different from control are indicated.
![Figure 4. Cytokine secretion by T cells treated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h. Results shown are mean [± SD] amounts of cytokine secreted by cells (in pg/ml) that were treated in the absence or presence of anti-TLR2 antibody; 10 samples/treatment. (A) Secretion of IFNγ by T cells treated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h (p < 0.01). (B) Secretion of IL-4 by T cells treated with rLPG3, NT-LPG3 or CT-LPG3 for 48 h. (C) IFNγ:IL-4 ratio in co-cultured T cells with LPG3, NT-LPG3 or CT-LPG3 following 48 h incubation (p < 0.05). Values significantly different from control are indicated.](/cms/asset/1843026e-d6fa-45a4-a1d9-d72db2a2e869/iimt_a_1066906_f0004_c.jpg)
With respect to IL-4, there were no significant effects on IL-4 secretion among the T-cells treated with rLPG3, NT-rLPG3 or CT-LPG3. Accordingly, when the data from both analyses were viewed in terms of IFNγ:IL-4 ratios, the results revealed a significant shift in the ratio in T-cells that had been treated with LPG3 or NT-LPG3. Ratio values increased from the baseline of 1.08 (± 0.89) to 3.62 (± 1.13) and 4.60 (± 1.44) for, respectively, the LPG3- and the NT fragment-treated cells.
Discussion
In the present study, the effects of recombinant Leishmania LPG3, NT-LPG3 or CT-LPG3 on T-cell activation and cytokine production were investigated. Using isolated purified cells from healthy volunteer PBMC, isolated T-cells were incubated with rLPG3, or its NT or CT fragments in the presence and absence of anti-TLR2 inhibitory antibodies. To evaluate T-cell activation, treated cells were harvested and expression of CD3 and CD69 was analyzed; culture supernatants were examined for secreted IFNγ and IL-4. Moreover, the activated T-cells were evaluated (using real-time PCR) to assess TH1 and TH2 lineage-specific transcription factors.
CD69 is an early activation antigen expressed on T-cells and is an antagonist of sphingosine 1-phosphate receptor-1 (S1P1), a receptor whose presence/activation is necessary for T-cell migration from lymph nodes. CD69 is induced on the surface of mature T-cells upon stimulation of their antigen receptors in order to inhibit the function of S1P1 (Bankovich et al., Citation2010; Yamashita et al., Citation1993). The present study found that rLPG3 and its NT fragment – but not the CT fragment – had strong inducing effects in CD69 expression on T-cell surfaces and that rLPG3 stimulated differentiation of TH1 cells through up-regulation of T-bet. T-bet is a member of the T-box transcription factors family that regulates lineage commitment in CD4 T helper cells via promoting the TH1 cytokine marker (IFNγ) (Szabo et al., Citation2002). On the other hand, GATA3 is expressed predominantly in TH2 cells and is important for TH2 lineage development (Fields et al., Citation2002). Although T-bet is expressed in natural killer (NK) cells, CD4+ T-cells and CD8+ cytotoxic T-cells, it is critical for control of IFNγ production in only CD4+ and NK cells (Szabo et al., Citation2002).
IFNγ is an important pro-inflammatory cytokine secreted by activated T-cells and plays a critical role in parasite elimination via it ability to activate macrophages (Valencia-Pacheco et al., Citation2014). Here, rLPG3 and its NT fragment caused significant increases in IFNγ production by the T-cells, but had no effect on IL-4 secretion. These results suggest that LPG3 and the NT fragments were unable to likely affect TH2-based immune responses, but could potentially preferentially induce TH1 responses. From these outcomes (i.e. including that the CT fragment had no impact on IFNγ secretion), it seems that LPG3 – particularly the NT-LPG3 fragment – enhanced expression of CD69 on the surface of T-cells and promoted differentiation of CD4+ T-cells toward a T-helper 1 (TH1) phenotype; in part, through up-regulation of IFNγ expression.
Becker et al. (Citation2003) showed that purified LPG enhanced TLR2 expression and stimulated both IFNγ and TNFα secretion by human NK cells in a TLR2-dependent manner. Hence, it was expected that activation of TLR2 could contribute to host resistance against leishmaniasis (Franco et al., Citation2012). We had hypothesized that LPG3 may also stimulate T-cells in a similar manner; thus, IFNγ production by the treated T-cells was examined in the absence/presence of anti-TLR2 monoclonal antibody to clarify the mechanism by which LPG3 could be exerting any stimulatory effects. The results here clearly showed that LPG3 and the NT fragment induced IFNγ production in the presence or absence of the blocking antibody. From this it can be concluded that the stimulation of IFNγ production induced in the T-cells by LPG3 and the NT fragment was via a TLR2-independent pathway(s). Such an outcome would appear to conflict with recent studies that demonstrated that TLR2 and TLR4 were involved in the recognition of L. major (Tuon et al., Citation2012). Moreover, de Veer et al. (Citation2003) showed that TLR2-deficient mice infected with L. major had an increased numbers of lesions. Thus, it would appear that TLR2 must have an important role in host resistance to the parasite.
A role for TLR2 mediating effects of LPG seems clear, based on the literature; however, little is known about the mechanism in which LPG3 stimulate an activate immune system. A previous study showed that L. major LPG stimulated IL-12 and TNFα secretion from macrophages in a myeloid differentiation factor 88 (MyD88)-dependent manner (de Veer et al., Citation2003). That same study showed in cells (in this case, human embryonic kidney epithelial 293T cells transfected with various TLR constructs) that LPG-induced activation of TLR2 in turn resulted in activation of NF-κB transcription factor. Because MyD88 is a universal adapter protein – as it is used by almost all TLRs (except TLR3) to activate NF-κB – it was all the more surprising here that, during the activation of human T-cells by LPG3, unlike with LPG, there was an upregulation of activation marker expression and of some key inflammatory cytokines, but in a TLR2-independent manner. As the present study was the first to assess the immunogenicity of LPG3 in freshly isolated non-transformed human T-cells, it is plausible that other signaling mechanisms (apart from MyD88-based ones described for the macrophages, epithelial cells) may also have been activated by the LPG3. Ongoing studies are investigating this latter hypothesis to define such pathways that could be unique to normal human T-cells and, thus, more relevant to clinical situations.
There are other potential explanations for why our outcomes vary from the previous/expected ones. There is evidence that suggested that isolation of T-cells using the MACS method might introduce confounders, such as contamination by other immune cell subsets like monocytes. Lancioni et al. (Citation2009) argued that isolation of CD4+ T-cells using the MACS technique could result in populations contaminated with CD14+ monocytes and other cell types, including NK cells, plasmacytoid and myeloid dendritic cells, CD8+ T cells and B cells, in conjunction with the collected CD4+ T cells. The presence of contaminating antigen-presenting cells (APC)/other accessory cells could have impacted on the measured outcomes here by processing and then presenting the LPG3 to the co-harvested T cells. Thus, our measures would then be assessing both direct and indirect effects of the LPG3 on the human T cells. As such, pending further analyses, it remains a remote possibility that some of the observed stimulation of the isolated T cells could have been a result, in part, of such APC contamination.
Even with the inability to state if the effects on the T cells by LPG3 were direct or indirect (or a combination of the two), immunostimulatory effects of LPG3 and its NT fragment have previously been reported (Abdian et al., Citation2011). Those investigators showed that LPG3 and its NT fragment could significantly stimulate secretion of IgG1 and IgG2a antibodies in mouse models. Further, in line with what was seen here in vitro, higher amounts of IFNγ were noted in ex vivo cultures of splenocytes from mice that had been immunized with vaccines containing LPG3 (Abdian et al., Citation2011). Another study reported there was a now mixed TH1/TH2 response in mice after immunization with DNA encoding L. infantum LPG3 (Pirdel et al., Citation2014).
Conclusions
Due to the comparable immunostimulatory effects of LPG3 and its NT fragment, it was concluded here that the NT fragment was responsible for these effects on T cells. However, further in vivo investigations are required to clarify the full mechanism and to assess the overall potential of LPG3 for use as a protective immunostimulatory component of leishmania against leishmaniasis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This study was supported in part by a grant from Tabriz University of Medical Sciences (Grant #N92-912).
References
- Abdian, N., Gholami, E., Zahedifard, F., et al. 2011. Evaluation of DNA/DNA and prime-boost vaccination using LPG3 against Leishmania major infection in susceptible BALB/c mice and its antigenic properties in human leishmaniasis. Exp. Parasitol. 127:627–636
- Alvar, J., Velez, I. D., Bern, C., et al. 2012. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 7:e35671
- Asgarian-Omran, H., Amirzargar, A. A., Zeerleder, S., et al. 2014. Interaction of Bordetella pertussis filamentous hemagglutinin with human TLR2: Identification of the TLR2-binding domain. APMIS 2:156–162
- Bankovich, A. J., Shiow, L. R., and Cyster, J. G. 2010. CD69 suppresses sphingosine-1-phosphate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 285:22328–22337
- Becker, I., Salaiza, N., Aguirre, M., et al. 2003. Leishmania lipophosphoglycan (LPG) activates NK cells through TLR2. Mol. Biochem. Parasitol. 130:65–74
- Croft, S. L., Sundar, S., and Fairlamb, A. H. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111–126
- de Veer, M. J., Curtis, J. M., Baldwin, T. M., et al. 2003. MyD88 is essential for clearance of Leishmania major: Possible role for lipophosphoglycan and TLR2 signaling. Eur. J. Immunol. 33:2822–2831
- Descoteaux, A., Avila, H. A., Zhang, K., et al. 2002. Leishmania LPG3 encodes a GRP94 homolog required for phosphoglycan synthesis implicated in parasite virulence but not viability. EMBO J. 21:4458–4469
- Faria, M. S., Reis, F. C., and Lima, A. P. 2012. Toll-like receptors in Leishmania infections: Guardians or promoters? J. Parasitol. Res. 12:930257–930269
- Fields, P. E., Kim, S. T., and Flavell, R. A. 2002. Cutting edge: Changes in histone acetylation at the IL-4 and IFNγ loci accompany TH1/TH2 differentiation. J. Immunol. 169:647–650
- Franco, L. H., Beverley, S. M., and Zamboni, D. S. 2012. Innate immune activation and subversion of mammalian functions by Leishmania lipophosphoglycan. J. Parasitol. Res. 16:165126–165137
- Glushkova, O. V., Parfenyuk, S. B., Khrenov, M. O., et al. 2013. Inhibitors of TLR4, NF-κB, and SAPK/JNK signaling reduce the toxic effect of lipopolysaccharide on RAW 264.7 cells. J. Immunotoxicol. 10:133–140
- Kedzierski, L., Zhu, Y., and Handman, E. 2006. Leishmania vaccines: Progress and problems. Parasitology 133:S87–S112
- Kishore, K., Kumar, V., Kesari, S., et al. 2006. Vector control in leishmaniasis. Indian J. Med. Res. 123:467–475
- Kropf, P., Freudenberg, M. A., Modolell, M., et al. 2004. TLR4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 72:1920–1928
- Lancioni, C. L., Thomas, J. J., and Rojas, R. E. 2009. Activation requirements and responses to TLR ligands in human CD4+ T-cells: Comparison of two T-cell isolation techniques. J. Immunol. Meth. 344:15–25
- Larreta, R., Guzman, F., Patarroyo, M. E., et al. 2002. Antigenic proper-ties of Leishmania infantum GRP94 and mapping of linear B-cell epitopes. Immunol. Lett. 80:199–205
- Larreta, R., Soto, M., Alonso, C., and Requena, J. M. 2000. Leishmania infantum: Gene cloning of the GRP94 homologue, its expression as recombinant protein, and analysis of antigenicity. Exp. Parasitol. 96:108–115
- Matsumoto, Y., Horiike, S., Ohshiro, M., et al. 2010. Expression of master regulators of helper T-cell differentiation in peripheral T-cell lymphoma, not otherwise specified, by immuno-histochemical analysis. Am. J. Clin. Pathol. 133:281–290
- McMahon-Pratt, D., and Alexander, J. 2004. Does the Leishmania major paradigm of pathogene-sis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 201:206–224
- Murray H. W., Berman, J. D., Davies, C. R., and Saravia, N. G. 2005. Advances in leishmaniasis. Lancet 366:1561–1577
- Ni, M., and Lee, A. S. 2007. ER chaperones in mammalian development and human diseases. FEBS Lett. 581:3641–3651
- Pirdel, L., Zavaran Hosseini, A., and Rasouli, M. 2014. Immune response in susceptible BALB/c mice immunized with DNA encoding lipophosphoglycan 3 of Leishmania infantum. Parasite Immunol. 36:700–707
- Racoosin, E. L., and Beverley, S. M. 1997. Leishmania major: Promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp. Parasitol. 85:283–295
- Seyed, N., Zahedifard, F., Safaiyan, S., et al. 2011. In silico analysis of six known Leishmania major antigens and in vitro evaluation of specific epitopes eliciting HLA-A2 restricted CD8 T-cell response. PLoS Neglected Trop. Dis. 5:e1295
- Szabo, S. J., Sullivan, B. M., Stemmann, C., et al. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFNγ production in CD4 and CD8 T-cells. Science 295:338–342
- Tuon, F. F., Amato, V. S., Bacha, H. A., et al. 2008. Toll-like receptors and leishmaniasis. Infect. Immun. 76:866–872
- Tuon, F. F., Fernandes, E. R., Duarte, M. I., and Amato, V. S. 2012. Expression of TLR2 and TLR4 in lesions of patients with tegumentary American leishmaniasis. Revista Inst. Med. Trop. São Paulo. 54:159–164
- Valencia-Pacheco, G., Loría-Cervera, E. N., and Sosa-Bibiano, E. L. 2014. In situ cytokines (IL-4, IL-10, IL-12, IFNγ) and chemokines (MCP-1, MIP-1α) gene expression in human Leishmania mexicana infection. Cytokine 69:56–61
- Yamashita, I., Nagata, T., Tada, T., and Nakayama, T. 1993. CD69 cell surface expression iden-tifies developing thymocytes which audition for T-cell antigen receptor-mediated positive selection. Intl. Immunol. 5:1139–1150
- World Health Organization (WHO). 2015. World Health Statistics - 2015. Geneva: WHO Press
- Yao, C., Donelson, J. E., and Wilson, M. E. 2003. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 132:1–16


![Figure 3. GATA-3 and T-bet mRNA levels. Fold-change in T-bet and GATA-3 mRNA expression in T cells that were stimulated with rLPG3 (2, 10 or 20 μg/ml) for 48 h. Results shown are means [± SD] from 10 samples/treatment. Values significantly different from control are indicated (p < 0.05).](/cms/asset/8a5a2f7c-0aff-4dc7-a546-4243b2129830/iimt_a_1066906_f0003_c.jpg)