To the Editor:
Recently, G. Isbister commented on our study using pharmacokinetic/phamacodynamic (PK/PD) modeling to evaluate the role of whole blood chloroquine concentrations in predicting severity of chloroquine poisoningCitation1 and recommended information more helpful to clinical practice.Citation2 Indeed, chloroquine concentrations are not routinely available in the majority of hospitals. As the epinephrine infusion rate was acknowledged to be a good surrogate marker of poisoning severity for chloroquine,Citation1 and based on the suggestion that the reported ingested dose can be reliably obtained,Citation2 we attempted to develop a dose-based prediction model of cardiovascular toxicity for chloroquine poisoning, for more clinical applicability. Based on our PK/PD analysis,Citation1 we estimated the probability of reaching an epinephrine infusion rate of >3 mg/h in 41 000 theoretically chloroquine-poisoned patients (1000 simulations of our 41 included poisoned adults) as a function of time for different ingested doses (2, 4, 6, 8, and 10 g) of chloroquine, as shown in . We chose 3 mg/h epinephrine infusion rate as the threshold for extreme poisoning severity based on our previous definition of refractoriness of cardiovascular failure in sodium-channel blocker poisonings requiring extracorporeal life support.Citation3 Based on this model, cardiotoxicity appears to occur rapidly after ingestion, with the maximum risk in the first 48 h, a relationship evident with the dose, and a gradual decrease with time.
Fig. 1. Simulated probability over time for having an epinephrine infusion rate > 3 mg/h. Five different chloroquine dose levels are shown including 2, 4, 6, 8, and 10 g.
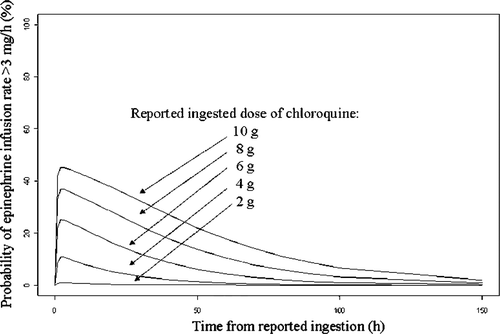
If accurate, the advantages of such a dose-based prediction model of cardiovascular toxicity would be: (1) the ease of obtaining the ingested dose based on history, as patients are awake in the majority of chloroquine poisonings, unless psychotropic drugs have been ingested or cardiac arrest has occurred in the field and (2) the availability of risk assessment at the bedside for anticipating the intensity of resuscitation which may be required. Furthermore, the fact that we did not identify any phenotypic characteristics which influenced pharmacokinetic parameters including clearanceCitation1 supports the reliability in using the reported ingested dose to represent drug exposure. These theoretical arguments are consistent with the findings that an ingested chloroquine dose >5 g is a good predictor of fatality risk with a specificity of 0.98.Citation4 To date, the reported ingested dose of chloroquine, along with other readily available indices including systolic blood pressure and QRS duration, is used to indicate the need for emergent mechanical ventilation, epinephrine, and diazepam, even in the field.
However, several concerns regarding the use of the reported ingested dose may limit prediction of toxicity based on dose-effect modeling. The absence of a relationship between the dose and the likelihood of mortality in a larger series of 167 acute chloroquine poisonings raised doubts regarding the accuracy of patient histories.Citation5 Additionally, in our study, we did not find any meaningful correlation between reported ingested amounts of chloroquine and measured concentrations,Citation1 possibly due to alterations in drug bioavailability (e.g. vomiting, activated charcoal administration, and gastric motility-delaying co-ingestions) or tissue distribution. Both of these observations suggest that blood chloroquine concentrations, a better marker of toxicant body burden, remain more accurate for predicting poisoning severity, as our study demonstrates.Citation1
In conclusion, although the ingested dose cannot be taken as a single reliable predictor of outcome in chloroquine poisonings, dose-effect modeling provides preliminary information for clinicians to estimate poisoning severity, expected prognosis, and required intensity of supportive management including the rate of epinephrine infusion.
References
- Mégarbane B, Bloch V, Hirt D, Debray M, Résiére D, Deye N, Baud FJ. Blood concentrations are better predictors of chioroquine poisoning severity than plasma concentrations: a prospective study with modeling of the concentration/effect relationships. Clin Toxicol (Phila) 2010;48:904–915.
- Isbister GK. Pharmacokinetic-pharmacodynamic modeling in overdose patients – is it worth the trouble? Clin Toxicol (Phila) 2010;48:896–897.
- Baud FJ, Megarbane B, Deye N, Leprince P. Clinical review: aggressive management and extracorporeal support for drug-induced cardiotoxicity. Crit Care 2007;11:207.
- Riou B, Barriot P, Rimailho A, Baud FJ. Treatment of severe chloroquine poisoning. N Engl J Med 1988;318:1–6.
- Clemessy JL, Taboulet P, Hoffman JR, Hantson P, Barriot P, Bismuth C, Baud FJ. Treatment of acute chloroquine poisoning: a 5 years experience. Crit Care Med 1996;24:1189–1195.
