To the Editor:
Flufenoxuron is a newly developed benzoylurea insecticide. The CascadeTM formulation is composed of flufenoxuron (5.3%), polyoxyethylene nonylphenol (8.0%), N-methyl-2-pyrrolidone (20.0%), ethoxylated nonylphenol phosphate (10.0%) and cyclohexanone (56.7%). Its toxicity on human still remains uncertain. Until now, only one case of human poisoning with a flufenoxuron-containing insecticide was reported which was successfully managed.Citation1 We report a fatal case of flufenoxuron-containing insecticide poisoning complicated by lactic acidosis, shock and abdominal compartment syndrome.
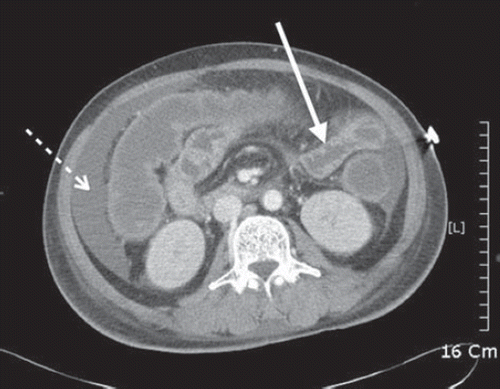
A 66-year-old male presented to emergency department 1 h after self-poisoning with 100 mL of CascadeTM. On arrival, Glasgow coma scale was 5/15 and initial vital signs were: blood pressure 121/50 mmHg, pulse rate 120 beats/min, respiratory rate 24 breaths/min, and temperature 36.5°C. Endotracheal intubation was performed, and he was mechanically ventillated. Blood Laboratory results revealed lactic acidosis (pH 7.11, PaO2 284.9 mmHg, PaCO2 35.8 mmHg, base excess ‐19.8 mmol/L, HCO3‐ 11.0 mmol/L, lactic acid level 9.12 mmol/L and methemoglobin 0.3%. The patient was admitted to the intensive care unit 3 h after ingestion. By 4 h after ingestion, blood pressure was 84/47 mmHg, despite fluid resuscitation. Cardiac index (CI) and systemic vascular resistance index (SVRI) were measured at 2.7 L/min/m2 and 980 dynes-sec/m2/cm5, respectively. Administration of norepinephrine was initiated at the rate of 4 microgram/min and titrated up to 10 microgram/min. Blood pressure returned to normal with this therapy. Lactic acidosis deteriorated (lactic acid 13.56 mmol/L, pH 6.98) despite sodium bicarbonate infusion. Continuous venovenous hemodiafiltration (CVVHDF) was initiated to reverse metabolic acidosis. Metabolic acidosis gradually improved after initiation of CVVHDF and blood pressure stabilized. On hospital day (HD) 7, urine output decreased to less than 10 mL/h and serum creatinine increased to 2.7 mg/dL. A tense and distended abdomen was also observed. Intra-abdominal pressure monitoring (IAP) was initiated with a first reading of 24 mmHg. Subsequently, a non-enhanced abdominal CT scan showed ascites in the abdominal cavity and diffuse bowel wall swelling (). In spite of conservative management, IAP remained more than 20 mmHg with oliguria, hypercapnea and increased peak airway pressure. Decompressive surgery could not be performed because the patient's family refused surgical intervention. His condition deteriorated, and he died of multiple organ failure on HD 19.
Fig. 1. Non-enhanced abdominal CT scan showed ascites in abdominal cavity (dotted arrow) and diffuse bowel wall swelling (solid arrow).
To our best knowledge, this is the first documented case report of a lethal human poisoning with flufenoxuron-containing insecticide. Our case showed three features. First was severe lactic acidosis, which was first reported in flufenoxuron-containing insecticide poisoning by Jeong et al.Citation1 In their case, lactic acidosis returned to a near-normal state with conservative care. Unlike their case, in this patient lactic acidosis could not be reversed by sodium bicarbonate, and CVVHDF was needed to reverse lactic acidosis. The mechanisms of toxicity leading to lactic acidosis is uncertain, but inhibition of the oxygen utilization at the cellular level might contribute. Second was profound shock. Shock might be caused by vasodilation and might also relate in part to cardiogenic failure. While severe metabolic acidosis developed without evidence of hypotension in a previous report,Citation1 profound shock followed the onset of lactic acidosis in our case. As oral ingestion of cyclohexanone, used as solvent in CascadeTM, can cause circulatory shock and metabolic acidosis,Citation2 this may have contributed to the development of circulatory shock and metabolic acidosis in our case. However, the pathophysiology of flufenoxuron-containing insecticide poisoning remains to be elucidated. A third problem was the development of an abdominal compartment syndrome. This might have been secondary to severe acidosis, hypotension, oliguria and large volume fluid resuscitation.Citation3 Severe lactic acidosis and shock induced by flufenoxuron-containing insecticide might have initiated a cycle of capillary leak with fluid sequestration in the abdominal cavity and rising IAP. The clinical features that prompted the suspicion of abdominal compartment syndrome in our case were a tense, distended abdomen on physical examination and sudden decrease in urine output. Earlier monitoring of IAP should be considered in patients with flufenoxuron-containing insecticide poisoning when the patient has risk factors for development of intra-abdominal hypertension.
In conclusion, physicians should be aware that severe lactic acidosis, profound shock and abdominal compartment syndrome can occur in human poisoning with flufenoxuron-containing insecticide. Early and aggressive care for lactic acidosis and shock may be needed, and IAP monitoring may be required in patients.
Achknowledgement
SC Choi and EJ Park equally contributed to this work.
References
- Jeong J, Yeom S, Ryu J, Han SK, Cho SJ, Kim J. A case of human poisoning with a flufenoxuron-containing insecticide. Clin Toxicol 2010; 48(1):87–89.
- Zukerman GB, Lam SC, Santos SM. Rhabdomyolysis following oral ingestion of the hydrocarbon cyclohexanone in an adolescent. J Environ Pathol Toxicol Oncol 1998; 17(1):11–15.
- Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, . Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med 2007; 33(6):951–962.
