Abstract
Background: This is the 28th Annual Report of the American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS). All US poison centers upload case data automatically with a median time interval of 19.0 [11.9, 40.6] (median [25%, 75%]) minutes, creating a near real-time national exposure and information database and surveillance system.
Methodology: We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center cases with medical outcomes of death were evaluated by a team of 33 medical and clinical toxicologist reviewers using an ordinal scale of 1 (Undoubtedly responsible) – 6 (Unknown) to determine Relative Contribution to Fatality (RCF) of the exposure to the death.
Results: In 2010, 3,952,772 closed encounters were logged by NPDS: 2,384,825, human exposures, 94,823 animal exposures, 1,466,253 information calls, 6537 human confirmed nonexposures, and 334 animal confirmed nonexposures. Total encounters showed a 7.7% decline from 2009 while health care facility calls increased by 2.7%. Human exposures with more serious outcomes (minor, moderate, major or death) increased 4.5% while those with less serious outcomes (all other medical outcome categories) decreased 5.9%. All information calls decreased 12.6% and health care facility (HCF) information calls decreased 13.6%, Drug ID calls decreased 10.9%, and human exposures decreased 3.8%. The top 5 substance classes most frequently involved in all human exposures were analgesics (11.5%), cosmetics/personal care products (7.7%), household cleaning substances (7.3%), sedatives/hypnotics/ antipsychotics (6.0%), and foreign bodies/toys/miscellaneous (4.2%). Analgesic exposures as a class increased the most rapidly by 32.8% over the last decade. The top f ve most common exposures in children age 5 years or less were cosmetics/personal care products (13.2%), analgesics (9.4%), household cleaning substances (9.2%), foreign bodies/toys/miscellaneous (7.2%), and topical preparations (6.8%). THC homolog and designer amphetamine (“Bath Salts”) exposures were identified as emerging public health threats. Drug identification requests comprised 64.3% of all information calls. NPDS documented 1730 human exposures resulting in death with 1146 human fatalities judged related with an RCF of 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory.
Conclusions: These data support the continued value of poison center expertise and need for specialized medical toxicology information to manage the more severe exposures, despite a decrease in calls involving less severe exposures. Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls. The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response and situational awareness tracking. NPDS is a model system for the nation and global public health.
WARNING: Comparison of exposure or outcome data from previous AAPCC Annual Reports is problematic. In particular, the identification of fatalities (attribution of a death to the exposure) differed from pre-2006 Annual Reports (see Fatality Case Review — Methods). Poison center death cases are described as all cases resulting in death and those determined to be exposure-related fatalities. Likewise, Table 22 (Exposure Cases by Generic Category) since year 2006 restricts the breakdown including deaths to single-substance cases to improve precision and avoid misinterpretation.
Introduction
This is the 28th Annual Report of the American Association of Poison Control Centers’ (AAPCC; http://www.aapcc.org) National Poison Data System (NPDS).Citation1 On 1 January 2010, sixty regional Poison Centers (PCs) serving the entire population of the 50 United States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands submitted information and exposure case data collected during the course of providing telephonic patient tailored exposure management and poison information. On 17 December 2010, the Western New York Poison Center (Buffalo) serving Western New York ceased operations. The Ruth A. Lawrence Poison Center (Rochester) closed on 30 December 2010. The Long Island Regional Poison Control Center (Mineola) ceased operations on 31 December 2010. New York State is now served by two poison centers based in New York City and Syracuse. During this transition national coverage remained seamless.
NPDS is the data warehouse for the nation's poison centers. Poison Centers (PCs) place emphasis on exposure management, accurate data collection and coding, and the continuing need for poison related public and professional education. The PC's health care professionals are available free of charge to all, 24-hours a day, every day of the year. PCs respond to questions from the public, health care professionals, and public health agencies. The continuous staff dedication at the regional PCs is manifest as the number of exposure and information call encounters exceeds 3.9 million annually. PC encounters either involve an exposed human or animal (EXPOSURE CALL) or a request for information (INFORMATION CALL) with no exposed person or animal.
What's New in NPDS and the Annual Report
Several enhancements were made to the tables and figures for this report. Continuing goals of the writing team have been to remove inconsistencies, improve the reader's ability to clearly understand the data, and provide additional data where appropriate. Two new tables have been added to this year's report: Population-Adjusted Exposures by Age Groups and Substance Categories Most Frequently Involved in Pregnant Exposures (Top 25).
This year, the AAPCC Fatality Review team did not review death (indirect report) cases. Death (indirect report) cases are reports identified through other sources (news feeds, medical examiner data or other) about which no inquiry to the PC was made. In previous years, both death and death (indirect report) cases were reviewed and included in the tables. This year, all the tables related to fatalities contain only death cases with an AAPCC Relative Contribution of Fatality (RCF) of 1, 2,or 3, except , , , , and 21 which also contain death (indirect report) cases—see list below:
Enhancements were added to the NPDS Fatality module to aid the fatality team in performing their review. The assignment of the Annual Report ID for the fatality cases included in Table 21 has now been automated. This will allow the cases in Table 21 to be easily identified when responding to Annual Report questions or comments.
Throughout the year the AAPCC Micromedex Joint Coding Group reviews the Generic Codes and responds to questions and requests for new generic codes. The group consists of AAPCC members and editorial and lexicon staff from Micromedex Poisindex® (Micromedex Healthcare Series [Internet database]. Greenwood Village, CO: Thomson Reuters [Healthcare] Inc.). New Product Codes and AAPCC Generic Codes were added to NPDS to address emerging products. In 2010, new generic codes were added for the following six product classes:
Electronic Cigarettes
Energy Drinks
Hand sanitizers
Opioids
Tetrahydrocannabinol (THC) Pharmaceuticals
Tetrahydrocannabinol (THC) Homologs
At the time of this report, there were 965 active and 12 obsolete generic codes. The active codes are divided into Non-Pharmaceutical (541) and Pharmaceutical (424) groups. These two groups are further divided into Major (67) and Minor (167) categories. New products associated with these classes were also added by Micromedex. Addition of these generic codes provides enhanced report granularity as ref ected in Table 22. Because the new codes were added at different times during the year, the numbers in Table 22 may not accurately reflect all of the cases in these categories, and for completeness certain categories require customized data retrieval until these categories have been in place for a minimum of a full year or more (2011 forward).
The NPDS Application
In 2010, numerous enhancements were introduced in the NPDS web-based application. Many of these focused on enhancing enterprise reports and surveillance functions. One hundred sixty-nine (169) enterprise reports now return multi-year results. The Case Log reports were expanded to support any combination of 24 separate search parameters and nine (9) different result formats. NPDS Case Log reports now support a variety of outputs including case line listing, daily and monthly counts, time series charts, and US maps. Case Log Counts Reports were added that stratify the results based on user defined classifications. To simplify product selection for reports, a new product selection function was added that displays products associated with a specific AAPCC Generic Code. Finally, a new National Case Log report was added that allows Regional Poison Centers to use the power of the Case Log (Generic Code) report to execute a national case listing without geographic or case identifiers.
New surveillance functions were added to support information call and animal call volume surveillance. To aid the AAPCC Surveillance Team anomaly review, a ‘Pending’ Status indicator was added for all anomalies to allow users to identify anomalies that are in the process of being analyzed. In addition, a new Case Classification parameter was added to the Case Based anomalies to allow users to classify the anomaly.
To provide centers with more information on public health events, a Special Projects report was added to the NPDS enterprise reporting system. This report provides geocentric reporting of AAPCC defined products for real time event monitoring. For example, the NPDS report was utilized by regional poison centers to access national cases related to the Gulf of Mexico Oil spill in real-time.
NPDS aggregate and case detail web services operate continuously, allowing external systems or viewers to analyze NPDS data in ways not otherwise possible in the NPDS application. The aggregate web service provides total call volume, human exposure call volume, or clinical effects counts allowing an external system such as RODS (Realtime Outbreak and Disease Surveillance, University of Pittsburgh, Department of Biomedical Informatics) to create time-series or GIS displays. Unique to NPDS, the aggregate case count web service is not only accessible by external computer systems but also directly by system users to create their own time series without the need for external system software. Two state health departments utilize the case detail web service to analyze data from their PCs. Four state health departments access the aggregate count web service for data. The web services allow NPDS data to be provisioned in a federated manner where the data is always current in NPDS and can be readily accessed as needed without the need for costly cloning and warehousing.Citation2
Limitations and Plans
As outlined above, the encounters (exposure reports and information questions) which comprise NPDS are collected from spontaneous, self-reported calls made to US PCs. Exposures in NPDS comprise a portion of the total number of incidents that occurred. These reflect the limitations of this type of passive reporting system (see DISCLAIMER).
Most of the 390,000 proprietary and non-proprietary drugs, chemicals, and biological agents including food poisoning agents in the NPDS products data base are classified by their primary active ingredient into one of 965 AAPCC generic codes. Some multiple ingredient products are coded to multiple product generic codes (e.g., acetaminophen with hydrocodone). Table 22 and other tables reporting information by generic category are organized by this system. Thus our current review and reporting methods do not necessarily distinguish between the individual components of a combination product.
Nonetheless, the scope and immediacy of these data have much to offer. In particular, the 28-years history offers a unique opportunity to assess the long term (secular) trends in exposures and information calls.
There are a number of plans to improve the data system and reporting for 2010 and beyond including:
Enhancements to NPDS real-time geographic information system (GIS) with more data display options for appropriate data analyses;
Enhancements to case-based surveillance systems;
Continued improvements in data quality edits;
Implement security paradigm enhancements to support specific product access for reports and surveillance;
Enterprise report enhancements;
New auto-upload requirements and improved solution;
Lexicon based analysis of the current generic code system to better meet current exposure tracking and surveillance needs;
Review and analysis of NPDS clinical effect coding terminology.
These and other initiatives are under continuous review by the AAPCC Board, NPDS Steering Committee, and CDC.
Methods
Characterization of Participating Poison Centers and Population Served
Sixty participating centers submitted data to AAPCC through 17 December 2010, 59 participating centers submitted data to AAPCC through 30 December 2010, 58 participating centers submitted data to AAPCC through 31 December 2010, with the total center count decreasing to 57 for the remainder of 2010. Fifty-seven centers (95%) were accredited by AAPCC as of 1 July 2010. The entire population of the 50 states, American Samoa, the District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands was served by the US PC network in 2010.Citation3,Citation4
The average number of human exposure cases managed per day by all US PCs was 6,534. Similar to other years, higher volumes were observed in the warmer months, with a mean of 6,950 cases per day in June compared with 6,305 per day in January. On average, US PCs received a call about an actual human exposure every 13.2 sec.
Call Management – Specialized Poison Exposure Emergency Providers
Most PC operations management, clinical education, and instruction are directed by Managing Directors (most are PharmDs and RNs with American Board of Applied Toxicology [ABAT] board certification). Medical direction is provided by Medical Directors who are board-certified physician medical toxicologists. At some PCs, the Managing and Medical Director positions are held by the same person.
Calls received at US PCs are managed by healthcare professionals who have received specialized training in toxicology and managing exposure emergencies. These providers include medical and clinical toxicologists, registered nurses, doctors of pharmacy, pharmacists, chemists, hazardous materials specialists, and epidemiologists. Specialists in Poison Information (SPIs) are primarily registered nurses, PharmDs, and pharmacists. They work under the supervision of a Certified Specialist in Poison Information (CSPI). SPIs must log a minimum of 2,000 calls over a 12-month period to become eligible to take the CSPI examination for certification in poison information. Poison Information Providers (PIPs) are allied healthcare professionals. They manage information-type and low acuity (non-hospital) calls and work under the supervision of a CSPI. Of note is the fact that no nursing or pharmacy school offers a toxicology curriculum designed for PC work and SPIs must be trained in programs offered by their respective PC. Centers are accredited by the AAPCC meeting strict standards and must be reaccredited every 5 years.
NPDS – Near Real-time Data Capture
Launched on 12 April 2006, NPDS is the data repository for all of the US regional PCs. In 2010, all 60 of the 60 US PCs uploaded case data automatically to NPDS through 17 December 2010. The center count decreased to 59 as of 17 December 2010, to 58 as of 30 December 2010 and to 57 as of 31 December 2010. All centers submitted data in near real-time making NPDS one of the few operational systems of its kind. PC staff record calls contemporaneously in 1 of 4 case management systems. Each center uploads case data periodically as it is entered. The time to upload data for all PCs is 19.9 [9.7, 58.7] (median [25%, 75%]) minutes creating a real-time national exposure database and surveillance system.
The web-based NPDS software facilitates detection, analysis, and reporting of NPDS surveillance anomalies. System software offers a myriad of surveillance uses allowing AAPCC, its member centers and public health agencies to utilize NPDS US exposure data. Users are able to access local and regional data for their own areas and view national aggregate data. The application allows for increased “drill-down” capability and mapping via a geographic information system (GIS). Custom surveillance definitions are available along with ad hoc reporting tools. Information in the NPDS database is dynamic. Each year the database is locked prior to extraction of annual report data to prevent inadvertent changes and ensure consistent, reproducible reports. The 2010 database was locked on 9 October 2011 at 0930 hr EDT.
Annual Report Case Inclusion Criteria
The information in this report reflects only those cases that are not duplicates and classified by the regional PC as CLOSED. A case is closed when the PC has determined that no further follow-up/recommendations are required or no further information is available. Exposure cases are followed to obtain the most precise medical outcome possible. Depending on the case specifics, most calls are “closed” within the first hours of the initial call. Some calls regarding complex hospitalized patients or cases resulting in death may remain open for weeks or months while data continues to be collected. Follow-up calls provide a proven mechanism for monitoring the appropriateness of management recommendations, augmenting patient guidelines, and providing poison prevention education, enabling continual updates of case information as well as obtaining final/known medical outcome status to make the data collected as accurate and complete as possible.
Statistical Methods
All tables except and were generated directly by the NPDS web-based application and can thus be reproduced by each center. The figures and statistics in and were created using SAS JMP version 9.0.0 (SAS Institute, Cary, NC) on summary counts generated by the NPDS web-based application.
NPDS Surveillance
As previously noted, all of the active US PCs upload case data automatically to NPDS. This unique near real-time upload is the foundation of the NPDS surveillance system. This makes possible both spatial and temporal case volume and case based surveillance. NPDS software allows creation of volume and case based definitions. Definitions can be applied to national, regional, state, or ZIP code coverage areas. Geocentric definitions can also be created. This functionality is available not only to the AAPCC surveillance team, but to every regional PC. PCs also have the ability to share NPDS real-time surveillance technology with external organizations such as their state and local health departments or other regulatory agencies. Another NPDS feature is the ability to generate system alerts on adverse drug events and other products of public health interest like contaminated food or product recalls. NPDS can thus provide real-time adverse event monitoring and surveillance for resilience response and situational awareness.
Surveillance definitions can be created to monitor a variety of volume parameters, any desired substance or commercial product in the Micromedex Poisindex products database. The database contains over 390,000 entries. Surveillance definitions may be constructed using volume or case based definitions with a variety of mathematical options and historical baseline periods from 1 to 11 years. NPDS surveillance tools include the following:
Volume Alerts Surveillance Definitions
Total Call Volume
Human Exposure Call Volume
Animal Exposure Call Volume
Information Call Volume
Clinical Effects Volume (signs and symptoms, or laboratory abnormalities)
Case Based Surveillance Definitions utilizing various NPDS data fields linked in Boolean expressions
Substance
Clinical Effects
Species
Medical Outcome and others
Incoming data is monitored continuously and anomalous signals generate an automated email alert to the AAPCC's surveillance team or designated regional PC or public health agency. These anomaly alerts are reviewed daily by the AAPCC surveillance team and/or the regional PC that created the surveillance definition. When reports of potential public health significance are detected, additional information is obtained via the NPDS surveillance correspondence system or phone as appropriate from reporting PCs. The regional PC then alerts their respective state or local health departments. Public health issues are brought to the attention of the Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC). This unique near real-time tracking ability is a unique feature offered by NPDS and the regional PCs.
AAPCC Surveillance Team clinical and medical toxicologists review surveillance definitions on a regular basis to fine-tune the queries. CDC, as well as State and local health departments with NPDS access as granted by their respective regional PCs, also have the ability to create surveillance definitions for routine surveillance tasks or to respond to emerging public health events.
Fatality Case Review and Abstract Selection
NPDS fatality cases can be recorded as DEATH or DEATH (INDIRECT REPORT). Medical outcome of death is by direct report. Death (indirect reports) are deaths that the PC acquired from medical examiners or media, but did not manage nor answer any questions related specifically to that death.
Although PCs may report death as an outcome, the death may not be the direct result of the exposure. We define exposure-related fatality as a death judged by the AAPCC Fatality Review Team to be at least contributory to the exposure. The definitions used for the Relative Contribution to Fatality (RCF) classification are defined in Appendix B and the methods to select abstracts for publications is described in Appendix C. For details of the AAPCC fatality review process, see the 2008 annual report.Citation1
Pediatric Fatality Case Review
A focused Pediatric Fatality Review team, comprised of 3 pediatric toxicologists, was assembled this year to evaluate cases in patients under 18 years of age. The panel reviewed the documentation of all such cases, with specific focus on the conditions behind the poisoning exposure and on finding commonality which might inform efforts at prevention. Seventy-one cases were reviewed and found to have a bimodal age distribution. Exposures causing death in children ≤ age 5 years were mostly coded as “Unintentional-General” while those in ages over 12 years were mostly “Intentional”. Often the Reason Code did not capture the complexities of the case. For example, there were few mentions of details such as the involvement of law enforcement or child protective services. While there were some complete and informative reports, in many narratives the circumstances which preceded the exposure thought responsible for the death was unclear or absent. In response to these findings, the pediatric fatality review team will develop Pediatric Narrative Guidelines for the upcoming year, with specific attention to the root cause of these cases. As a result, poison centers will be requested to implement guidelines recommending the most in-depth “causality” investigation possible.
Results
In 2010, the participating PCs logged 3,952,772 total encounters including 2,384,825 closed human exposure cases (), 94,823 animal exposures (), 1,466,253 information calls (), 6,537 human confirmed non-exposures, and 334 animal confirmed non-exposures. An additional 449 calls were still open at the time of database lock. The cumulative AAPCC database now contains nearly 51 million human exposure case records (). A total of 13,357,650 information calls have been logged by NPDS since the year 2001.
shows the human exposures, information calls and animal exposures by day since 2001. Second order (quadratic) least squares regression for 2000–2010 has shown a statistically significant departure from linearity (declining rate of calls since mid-2007) for Human Exposure Calls. Information Calls are declining more rapidly than the quadratic regression this year, and Animal Exposure Calls have likewise been declining since mid-2005.
Fig. 1. Human Exposure Calls, Information Calls and Animal Exposure Calls by Day since 1 January 2000.
Black lines show least-squares second order regression–both linear and second order (quadratic) terms were statistically significant for each of the 3 regressions. (See colour version of this figure online).
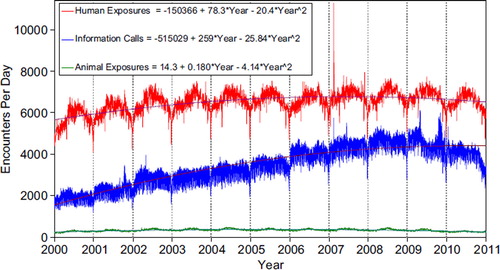
A hallmark of PC case management is the use of follow-up calls to monitor case progress and medical outcome. US PCs made 2,841,477 follow-up calls in 2010. Follow-up calls were done in 46.0% of human exposure cases. One follow-up call was made in 22.4% of human exposure cases, and multiple follow-up calls (range 2–666) were placed in 23.6% of cases.
Information Calls to Poison Centers
Data from 1,466,253 information calls to PCs in 2010 () was transmitted to NPDS, including calls in optional reporting categories such as prevention/safety/education (31,656), administrative (23,546) and caller referral (65,652).
Table 1A. AAPCC Population Served and Reported Exposures (1983–2010)
Table 1B. Non-Human Exposures by Animal Type
Table 1C. Distribution of Information Calls
shows that All Drug ID calls decreased dramatically in mid-2009, and again in late-2010 (no regression was fit to these data). Enforcement Drug ID Calls showed a declining rate of increase. The most frequent information call was for Drug ID, comprising 942,614 calls to PCs during the year. Of these, 566,543 (60.1%) were identified as drugs with known abuse potential; however, these cases were categorized based on the drug's abuse potential without knowledge of whether abuse was actually intended.
Fig. 2. All Drug Identification and Law Enforcement Drug Identification Calls by Day since 1 January 2000.
Black line shows least-squares second order regression–both linear and second order (quadratic) terms were statistically significant for the Law Enforcement Drug ID Calls. (See colour version of this figure online).
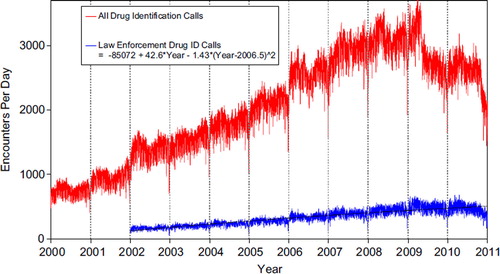
While the number of Drug Information calls decreased 9.4% from 2009 (239,943 calls) to 2010 (217,286), the Drug Information calls as a percentage of all information calls was 14.3% and 14.8%, respectively. Of these, the most common requests were in regards to therapeutic use and indications, followed by drug–drug interactions, questions about dosage and inquiries of adverse effects. Environmental inquiries comprised 1.6% of all information calls. Of these environmental inquiries, questions related to cleanup of mercury (thermometers and other) remained the most common followed by questions involving pesticides.
Of all the information calls, poison information comprised 4.8% of the requests with inquiries involving general toxicity the most common followed by questions involving food preparation practices, plant toxicity, and safe use of household products.
Exposure Calls to Poison Centers
shows a graphic summary and analyses of Health Care Facility (HCF) Exposure and HCF Information calls. HCF Exposure Calls did not depart from linearity (continued to increase at a steady rate) while the rate of HCF Information Calls has been declining since early 2005. This linearly increasing use of the PCs for the more serious exposures (HCF calls) is important in the face of the declining growth of all exposure and information calls. The 2 May 2006, exposure data spike on the figure was the result of 602 children in a Midwest school reporting a noxious odor which caused anxiety, but resolved without sequelae.
Fig. 3. Health Care Facility (HCF) Exposure Calls and HCF Information Calls by Day since 1 January 2000.
Black lines show least-squares first and second order regressions–linear regression for HCF Exposure Calls (second order term was not statistically significant) and second order regression for HCF Information Calls. All terms shown were statistically significant for each of the 2 regressions. (See colour version of this figure online).
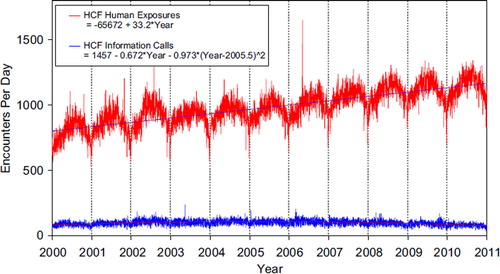
Tables 22A (Nonpharmaceuticals) and 22B (Pharmaceuticals) provide summary demographic data on patient age, reason for exposure, medical outcome, and use of a health care facility for all 2,384,825 human exposure cases, presented by substance categories.
Column 1: Name of the major, minor generic categories and their associated generic codes.
Column 2: No. of Case Mentions (all exposures) in grey shading and displays the number of times the specific generic code was reported in all human exposure cases. If a human exposure case has multiple instances of a specific generic code it is only counted once.
Column 3: No. of Single Exposures–this column was previously named “No. of ‘Single Exposures’” and was renamed in the 2009 report for clarity. This column displays the number of human exposure cases that identified only one substance (one case, one substance).
The succeeding columns (Age, Reason, Treatment Site, and Outcome) show selected detail from these single-substance exposure cases. Death cases include both cases that have the outcome of Death or Death, (indirect report). These death cases are not limited by the relative contribution to fatality.
Tables 22A and 22B restrict the breakdown columns to single-substance cases. Prior to 2007, when multi-substance exposures were included, a relatively innocuous substance could be mentioned in a death column when, for example, the death was attributed to an antidepressant, opioid, or cyanide. This subtlety was not always appreciated by the user of this table. The restriction of the breakdowns to single-substance exposures should increase precision and reduce misrepresentation of the results in this unique by-substance table. Single substance cases reflect the majority (90%) of all exposures yet 41% of fatalities ().
Tables 22A and 22B tabulate 2,759,287 substance-exposures, of which 2,147,248 were single-substance exposures, including 1,125,336 (52.4%) nonpharmaceuticals and 1,021,909 (47.6%) pharmaceuticals. The remaining 4 exposure cases (3 single exposures cases) did not specify if the substance was pharmaceutical or nonpharmaceutical (invalid generic codes).
In 17.6% of single-substance exposures that involved pharmaceutical substances, the reason for exposure was intentional, compared to only 3.5% when the exposure involved a nonpharmaceutical substance. Correspondingly, treatment in a health care facility was provided in a higher percentage of exposures that involved pharmaceutical substances (27.5%) compared with nonpharmaceutical substances (14.7%). Exposures to pharmaceuticals also had more severe outcomes. Of single-substance exposure-related fatal cases, 521 (0.05%) were pharmaceuticals compared with 242 (0.02%) nonpharmaceuticals.
Age and Gender Distributions
The age and gender distribution of human exposures is outlined in . Children younger than 3 years of age were involved in 37.7% of exposures and children younger than 6 years accounted for approximately half of all human exposures (50.5%). A male predominance was found among cases involving children younger than 13 years, but this gender distribution was reversed in teenagers and adults, with females comprising the majority of reported exposures. shows population-adjusted exposures for the same age groups.
Caller Site and Exposure Site
As shown in , of the 2,384,825 human exposures reported, 74.6% of calls originated from a residence (own or other) but 93.7% actually occurred at a residence (own or other). Another 17.5% of calls were made from a health care facility. Beyond residences, exposures occurred in the workplace in 1.6% of cases, schools (1.2%), health care facilities (0.3%), and restaurants or food services (0.2%).
Table 2. Site of Call and Site of Exposure, Human Exposure Cases
Table 3A. Age and Gender Distribution of Human Exposures
Table 3B. Population-Adjusted Exposures by Age Group
Table 4. Distribution of Agea and Gender for Fatalitiesb
Table 5. Number of Substances Involved in Human Exposure Cases
Exposures in Pregnancy
Exposure during pregnancy occurred in 7,849 women (0.33% of all human exposures). Of those with known pregnancy duration (n = 7,193), 31.6% occurred in the first trimester, 37.5% in the second trimester, and 30.9% in the third trimester. Most (72.2%) were unintentional exposures and 20.4% were intentional exposures. Medical outcome was No effect in 16.9%, Minor effect in 20.3%, Moderate effect in 5.76%, and Major Effect in 0.542%. There was one death in a pregnant female in 2010.
Chronicity
Most human exposures, 2,136,572 (89.6%) were acute cases (single, repeated, or continuous exposure occurring over 8 hr or less) compared to 869 acute cases of 1730 fatalities (50.2%). Chronic exposures (continuous or repeated exposures occurring over >8 hr) comprised 2% (47,700) of all human exposures. Acute-on-chronic exposures (single exposure that was preceded by a continuous, repeated, or intermittent exposure occurring over a period greater than 8 hr) numbered 174,777 (7.3%).
Reason for Exposure
The reason category for most human exposures was unintentional (81.4%) with unintentional general (57.3%), therapeutic error (11.3%) and unintentional misuse (5.4%) of all exposures ().
Table 6A. Reason for Human Exposure Cases
Scenarios
Of the total 285,277 therapeutic errors, the most common scenarios for all ages included: inadvertent double-dosing (29.1%), wrong medication taken or give (14.7%), other incorrect dose (12.9%), doses given/taken too close together (9.5%), and inadvertent exposure to someone else's medication (9.0%). The types of therapeutic errors observed are different for each age group and are summarized in .
Table 6B. Scenarios for Therapeutic Errorsa by Ageb
Reason by Age
Intentional exposures accounted for 14.7% of human exposures. Suicidal intent was suspected in 9.2% of cases, intentional misuse in 2.5% and intentional abuse in 2.2%. Unintentional exposures outnumbered intentional exposures in all age groups with the exception of ages 13–19 years (). Intentional exposures were more frequently reported than unintentional exposures in patients aged 13–19 years. In contrast, of the 1,146 reported fatalities with RCF 1–3, the majority reason reported for children <5 years was unintentional while most fatalities in adults (>20 years) were intentional ().
Table 7. Distribution of Reason for Exposure by Age
Table 8. Distribution of Reason for Exposure and Age for Fatalitiesa
Route of Exposure
Ingestion was the route of exposure in 83.5% of cases (), followed in frequency by dermal (7.2%), inhalation/nasal (5.7%), and ocular routes (4.5%). For the 1,146 exposure-related fatalities, ingestion (87.5%), inhalation/nasal (7.7%), and parenteral (3.1%) were the predominant exposure routes. Each exposure case may have more than one route.
Table 9. Route of Exposure for Human Exposure Cases
Clinical Effects
The NPDS database allows for the coding of up to 131 different clinical effects (signs, symptoms, or laboratory abnormalities) for each case. Each clinical effect can be further defined as related, not related, or unknown if related. Clinical effects were coded in 849,516 (35.6%) cases. (17.9% had 1 effect, 9.3% had 2 effects, 4.9% had 3 effects, 2% had 4 effects, 0.8% had 5 effects, and 0.9% had >5 effects coded). Of clinical effects coded, 79.2% were deemed related to the exposure(s), 9.3% were considered not related, and 11.5% were coded as unknown if related.
The duration of effect is required for all cases that report at least one clinical effect and have a medical outcome of minor, moderate, or major effect (n = 514,203; 21.6% of exposures). demonstrates an increasing duration of the clinical effects observed with more severe outcomes.
Case Management Site
The majority of cases reported to PCs were managed in a non-health care facility (71.3%), usually at the site of exposure, primarily the patient's own residence (). 1.8% of cases were referred to a health care facility but refused referral. Treatment in a health care facility was rendered in 25.2% of cases.
Table 10. Management Site of Human Exposures
Of the 601,197 cases managed in a health care facility, 292,289 (48.6%) were treated and released, 97,650 (16.2%) were admitted for critical care (intensive care or monitored unit), and 62,346 (10.4%) were admitted to a noncritical unit.
The percentage of patients treated in a health care facility varied considerably with age. Only 11.1% of children <5 years or younger and only 12.7% of children between 6 and 12 years were managed in a health care facility compared to 48.6% of teenagers (13–19 years) and 40.1% of adults (age > 20 years).
Medical Outcome
displays the medical outcome of human exposure cases distributed by age. A greater number of severe medical outcomes is observed in the older age groups. compares medical outcome and reason for exposure and shows a greater frequency of serious outcomes in intentional exposures.
Table 11. Medical Outcome of Human Exposure Cases by Patient Agea
Table 12. Medical Outcome by Reason for Exposure in Human Exposuresa
Table 13. Duration of Clinical Effects by Medical Outcome
Decontamination Procedures and Specific Antidotes
and outline the use of decontamination procedures, specific antidotes, and measures to enhance elimination in the treatment of patients reported in the NPDS database. These must be interpreted as minimum frequencies because of the limitations of telephone data gathering.
Table 14. Decontamination and Therapeutic Interventions
Table 15. Therapy Provided in Human Exposures by Age.
Ipecac-induced emesis for poisoning continues to decline as shown in and . Ipecac was administered in only 163 (0.01%) pediatric exposures in 2010. The continued decrease in ipecac syrup use over the last two decades was likely a result of ipecac use guidelines issued in 1997 by the American Academy of Clinical Toxicology, European Association of Poisons Centres, and Clinical Toxicologists and updated in 2004.Citation5,Citation6 In a separate report, the American Academy of Pediatrics concluded not only that ipecac should no longer be used routinely as a home treatment strategy, but also recommended disposal of home ipecac stocks.Citation7 A decline was also observed since the early 1990s for reported use of activated charcoal. While not as dramatic as the decline in use of ipecac, reported use of activated charcoal decreased from 3.7% of pediatric cases in 1993 to just 1.4% in 2010.
Table 16A. Decontamination Trends (1985–2009)
Table 16B. Decontamination Trends: Total Human and Pediatric Exposures ≤5 Years (2010)a
Top Substances in Human Exposures
presents the most common 25 substance categories, listed by frequency of human exposure. This ranking provides an indication where prevention efforts might be focused, as well as the types of exposures PCs regularly manage. It is relevant to know whether exposures to these substances are increasing or decreasing.
Table 17A. Substance Categories Most Frequently Involved in Human Exposures (Top 25)
To better understand these relationships, we examined exposures per year over the last 11 years for the change over time for each of the 67 major generic categories via least squares linear regression. Despite an overall decrease in human exposure calls (3.8%) for this year, the calls per year increased for 42 and decreased for 25 of the 67 major categories. The change over time for the 11-yearly values was statistically significant (p < 0.05) for 49 of the 67 categories. shows the 25 categories which were increasing the most rapidly. Statistical significance of the 25 regressions can be verified by noting the 95% conf dence interval on the rate of increase excludes zero. shows the linear regressions for the top 4 increasing categories in . and present exposure results for children and adults, respectively, and show the differences between substance categories involved in pediatric and adult exposures.
Fig. 4. Change in Encounters from 2009 to 2010 with Graphical Breakdown of Exposure Calls.
The figure shows how the decrease of 94,530 in Human Exposure Calls divides among the 10 Medical Outcomes. The More Serious Exposures (Minor, Moderate, Major and Death) all increased and their combined increase was 22,175 calls (23.5% of the 94,530 total decrease). The Less Serious Exposures (the other 6 outcome groups) decreased by 116,705 (−123.5% of the 94,530 total decrease). (See colour version of this figure online).
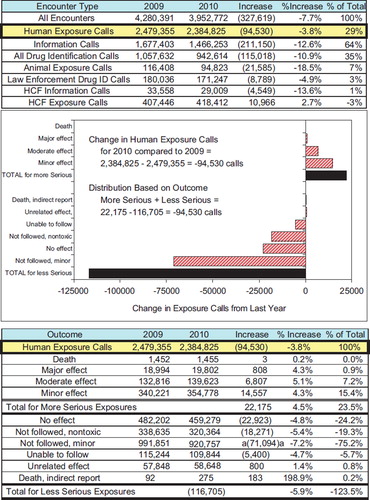
Fig. 5. Gulf Oil Spill Encounters per Day.
Black line for Gulf Oil Spill Encounters (human and animal exposure and information calls) shows a spline smoothing fit (lambda V 0.0000117, rsquare V 0.710). Map shows number of calls per day. (See colour version of this figure online).
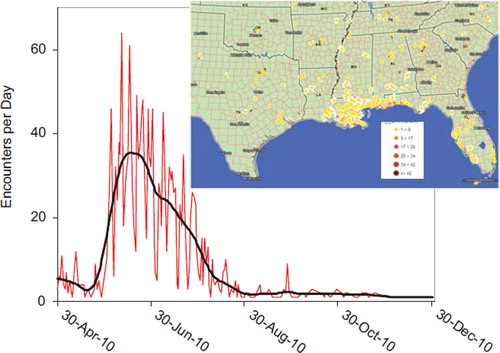
Fig. 6. Emerging Trends: Bath Salts and THC Homologs Exposures.
Black line for Bath Salt Exposures shows a spline smoothing fit (lambda = 0.01, rsquare = 0.829).
Black line for THC Homolog Exposures shows s show least-squares second order regressions: THC Exposures = −26,223 + 13.0*Year −3.83* (Year-2011)ˆ2 (rsquare = 0.708). All 3 terms in this regression were statistically significant (p < 0.05). (See colour version of this figure online).
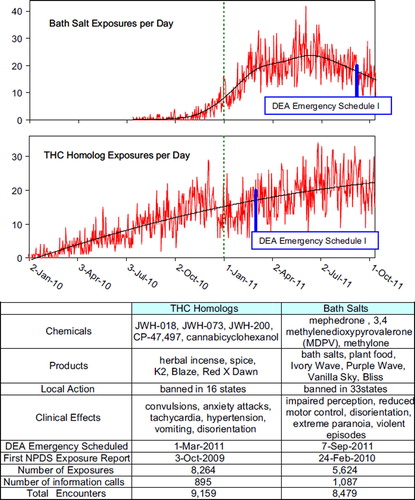
Fig. 7. Human Exposure Calls By Year 2000–2010 – Top 4 Categories.
Solid lines show least-squares linear regressions for the Human Exposure Calls per year for that category (□). Broken lines show 95% confidence interval on the regression. (See colour version of this figure online).
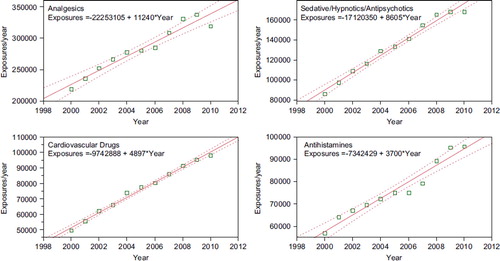
Table 17B. Substance Categories with the Greatest Rate of Exposure Increase (Top 25)
Table 17C. Substance Categories Most Frequently Involved in Pediatric (≤5 years) Exposures (Top 25)a
Table 17D. Substance Categories Most Frequently Involved in Adult (≥ 20 years) Exposures (Top 25)a
reports the 25 categories of substances most frequently involved in pediatric (≤5 years) fatalities in 2010.
Table 17E. Substance Categories Most Frequently Involved in Pediatric (≤5 years) Deathsa
reports the 25 Drug ID categories most frequently identified in 2010. The most often identified drug category is miscellaneous and unknown; this category includes medications which could not be identified. Drug ID information is of value to AAPCC, public health, public safety, and regulatory agencies. Internet based resources do not allow data capture nor do they afford the caller the ability to speak with a specialist in poison information if the inquiry is more than a drug identification question. Proper resources to continue this vital public service are essential, especially since the top 10 substance categories include antibiotics as well as drugs with widespread use and abuse potential such as opioids and benzodiazepines.
Table 17F. Substance Categories Most Frequently Identified in Drug Identification Calls (Top 25)
(new this year) reports the 25 substance categories most frequently reported in exposures involving pregnant patients.
Table 17G. Substance Categories Most Frequently Involved in Pregnant Exposuresa (Top 25)
Table 18. Categories Associated with Largest Number of Fatalities (Top 25)a
Changes from Last Year
shows the year-to-year changes for 2009–2010 for all encounters and for several major categories.
The graphic breaks down the change in exposure calls by outcome category. Although overall exposure calls have decreased by 94,530 calls (−3.8%), there is a consistent increase in the exposures with a more serious outcome (minor, moderate, major or death) and as a group increased by 22,175 encounters (4.5%).
Thus we see a consistent increase in exposure calls from HCFs and for the more severe exposures, despite a decrease in calls involving less severe exposures.
Distribution of Suicides
shows the modest variation in the distribution of suicides and pediatric deaths over the past two decades as reported to the NPDS national database. Within the last decade, the percent of exposures determined to be suspected suicides ranged from 45.0 to 54.3% and the percent of pediatric cases has ranged from 2.2% to 3.2%.
Table 19A. Comparisons of Death Data (1985–2010)a
Table 19B. Comparisons of Direct and Indirect Death Data (2000–2010)a
Plant Exposures
provides the number of times the specific plant was reported to NPDS (N = 53,526). The 25 most commonly involved plant species and categories account for 40% of all plant exposures reported. The top 3 categories in the table are essentially synonymous for unknown plant and comprise 13.2% (7,076/53,526) of all plant exposures. For a variety of reasons it was not possible to make a precise identification in these three groups. The top most frequent plant exposures where a positive plant identification was made were (descending order): Phytolacca americana (L.), Spathiphyllum spp. Not otherwise specified (NOS), Ilex spp. (NOS), Philodendron spp. (NOS), and plants-pokeweed.
Table 20. Frequency of Plant Exposures (Top 25)a
Deaths and Exposure-related Fatalities
A listing of cases (Table 21) and summary of cases (, , , , , and 22) are provided for fatal cases for which there exists reasonable confidence that the death was a result of that exposure (exposure-related fatalities). , , and 19 list all deaths, irrespective of the RCF. Beginning in 2010, cases with outcome of death, indirect were not reviewed and the Relative Contribution to Fatality (RCF) was determined by the individual poison center team.
There were 275 death, indirect and 1,455 deaths. Of these 1,730 cases, 1,366 were judged exposure-related fatalities (RCF = 1, Undoubtedly responsible; 2, Probably responsible; or 3, Contributory). The remaining 364 cases were judged as follows: 73 as RCF = 4, Probably not responsible; 27 as 5, Clearly not responsible; and 264 as RCF = 6, Unknown.
Deaths are sorted in Table 21 according to the category, substance deemed most likely responsible for the death (Cause Rank), and then by patient age. The Cause Rank permits the PC to judge two or more substances as indistinguishable in terms of cause, e.g., two substances which appear equally likely to have caused the death could have Substance Rank of 1, 2 and Cause Rank of 1, 1. Additional agents implicated are listed below the primary agent in the order of their contribution to the fatality.
As shown in Table 5, a single substance was implicated in 90.0% of reported human exposures, and 6.4% of patients were exposed to two or more drugs or products. The exposure-related fatalities involved a single substance in 474 cases (41.4%), 2 substances in 270 cases (23.6%), 3 in 159 cases (13.9%) and 4 or more in the balance of the cases.
In Table 21, the Annual Report ID number [bracketed] indicates that the abstract for that case is included in Appendix C. The letters following the Annual Report ID number indicate: i = Death, Indirect report (occurred in 220, 16.1% of cases), p = prehospital cardiac and/or respiratory arrest (occurred in 666 of 1,366, 44.4% of cases), h = hospital records reviewed (occurred in 53, 3.9% of cases), a = autopsy report reviewed (occurred in 356, 26.1% of cases). The distribution of NPDS RCF was: 1 = Undoubtedly responsible in 559 cases (40.9%), 2 = Probably responsible in 599 cases (43.9%), 3 = Contributory in 208 cases (15.2%). The denominator for these Table 21 percentages is 1,366.
All fatalities – all ages
Table 4 presents the age and gender distribution for these 1,146 exposure-related fatalities (excluding death, indirect). The age distribution of reported fatalities is similar to that in past years with 91 (7.9%) of the fatalities in children (< 20 years old), 1,052 of 1,146 (92.1%) of fatal cases occurring in adults (age ≥ 20 years) and 3 (0.3%) of fatalities occurring in Unknown Age patients. Although children ≤5 years old were involved in the majority of exposures, the 32 fatalities comprised just 2.8% of the exposure-related fatalities. Most (71.4%) of the fatalities occurred in 20-to 59-year-old individuals.
Table 21 lists each of the 1,366 human fatalities (including death, indirect report) along with all of the substances involved. Please note: the substance listed in column 3 of Table 21 (alternate name) was chosen to be the most specific generic name based upon analysis of the Micromedex Poisindex product name and generic code. Alternate names are maintained in the NPDS for each substance involved in a fatality. The cross-references at the end of each major category section in Table 21 list all cases that identify this substance as other than the primary substance. This Alternate name may not agree with the AAPCC generic categories used in the summary tables (including Table 22).
Table 18 lists the top 25 minor generic substance categories associated with reported fatalities and the number of single substance exposure fatalities for that category—miscellaneous sedative/hypnotics/antipsychotics, miscellaneous cardiovascular drugs, opioids, and acetaminophen combination products, lead this list followed by miscellaneous antidepressants, miscellaneous alcohols, acetaminophen alone, miscellaneous anticonvulsants, and miscellaneous stimulants and street drugs. Note that Table 18 is sorted by all substances to which a patient was exposed (i.e., a patient exposed to an opioid may have also been exposed to 1 or more other products) and shows single substance exposures in the right hand column.
The first ranked substance (Table 21) was a pharmaceutical in 1,104 (80.8%) of the 1,366 fatalities. These 1,104 first ranked pharmaceuticals included:
526 analgesics (92 acetaminophen, 89 acetaminophen/ hydrocodone, 67 methadone, 53 oxycodone, 36 salicylate, 27 morphine, 20 acetaminophen/oxycodone, 17 fentanyl, 16 acetaminophen/diphenhydramine, 16 acetaminophen/propoxyphene, 14 tramadol, 12 fentanyl (transdermal), 11 colchicine, 11 opioid, 7 propoxyphene)
128 cardiovascular drugs (24 amlodipine, 17 cardiac glycoside, 13 verapamil, 7 atenolol, 7 beta blocker, 7 diltiazem, 7 diltiazem (extended release), 7 metoprolol)
112 antidepressants (29 amitriptyline, 12 doxepin, 9 citalopram, 9 venlafaxine, 8 trazodone, 7 tricyclic antidepressant, 6 bupropion, 6 bupropion (extended release), 5 venlafaxine (extended release))
106 sedative/hypnotic/antipsychotics (31 quetiapine, 16 alprazolam, 8 clonazepam, 7 diazepam, 6 lorazepam), 86 stimulants/street drugs (28 methamphetamine, 20 heroin, 20 cocaine, 10 methylenedioxymethamphetamine (MDMA))
The exposure was acute in 706 (51.7%), A/C = acute on chronic in 266 (19.5%), C = chronic exposure in 86 (6.3%) and U = unknown in 308 (22.6%).
A total of 1,639 tissue concentrations for 1 or more related analytes were reported in 692 cases. Most of these (1,537) are listed in Table 21, while all tissue concentrations are available to the member centers through the NPDS Enterprise Reports. These 125 analytes included: 231 acetaminophen, 153 ethanol, 76 hydrocodone, 67 oxycodone, 60 alprazolam, 60 methadone, 54 salicylate, 33 diphenhydramine, 28 morphine.
Route of exposure was: Ingestion only in 1057 cases (77.4%), inhalation/nasal only in 89 cases (6.5%), parenteral in 23 cases (1.7%). Most other routes were combination routes or unknown.
The Intentional exposure reason was: Suspected suicide in 673 cases (49.3%), Abuse in 230 cases (16.8%), and Misuse in 49 cases (3.6%). Unintentional exposure reason was: Environmental in 36 cases (2.6%), Therapeutic error in 29 cases (2.1%), and Misuse in 21 cases 1.5%), and Occupational in 15 (1.1%). Adverse drug reaction was the reason in 27 (2.0%).
Pediatric fatalities – age ≤5 years
Although children younger than 6 years were involved in the majority of exposures, they comprised 55 of 1,730 (3.2%) of fatalities. These numbers are similar to those reported since 1985 (). The percentage fatalities in children ≤5 years related to total pediatric exposures was 32/1,207,575 = 0.00265%. By comparison, 1,052/858,982 = 0.122% of all adult exposures involved a fatality. Of these 32 pediatric fatalities, 18 (81.3%) were reported as unintentional and 2 (6.3%) were coded as resulting from malicious intent (Table 8).
The 37 fatalities in children <5 years old in Table 21 (includes death, indirect reports, and RCF 1 - 3) included 16 pharmaceuticals and 21 nonpharmaceuticals. The first ranked substances associated with these fatalities included: carbon monoxide in 3 cases, aluminum phosphide, hydrofluoric acid, lamp oil, smoke, diphenhydramine, flecainide in 2 cases each, and 22 other substances (1 each).
Pediatric fatalities – ages 6–12 years
In the age range 6–12 years, there were 3 reported fatalities, 1 of which was unintentional misuse, 1 was intentional abuse, and 1 unknown reason (Table 8). The 4 fatalities listed in Table 21 (includes death, indirect reports, and RCF 1–3) included: 2 fluorinated hydrocarbons, 1 carbon monoxide, and 1 oxycodone.
Adolescent fatalities – ages 13–19 years
In the age range 13–19 years, there were 56 reported fatalities including 46 intentional and 4 unintentional (Table 8). The 67 fatalities listed in Table 21 (includes death, indirect reports, and RCF 1–3) included 53 pharmaceuticals and 14 nonpharmaceuticals. The first ranked pharmaceuticals associated with these fatalities included: acetaminophen/hydrocodone, methylenedioxymethamphetamine (MDMA), oxycodone, and salicylate (3 cases each); acetaminophen, acetaminophen/diphenhydramine, clonazepam, colchicine, diphenhydramine, methadone, morphine (2 cases each); and the balance 1 substance each. The first ranked nonpharmaceuticals associated with these fatalities included: ethanol in 3 cases, cyanide, and ethylene glycol (2 cases each); and the balance 1 substance each.
Pregnancy and Fatalities
A total of 25 deaths of pregnant women have been reported from the years 2000 through 2010. The majority (21 of 25) were intentional exposures (misuse, abuse, or suspected suicide). There was 1 death in a pregnant women reported to NPDS in 2010. A 25 year-old female at 27 weeks gestation presented to the maternity ward with fetal demise. The patient was acidotic and in fulminate hepatic failure following an intentional ingestion of acetaminophen and aspirin. She expired on the afternoon of hospital day 3. The fatality was judged undoubtedly responsible to the acetaminophen and salicylate.
AAPCC Surveillance Results
A key component of the NPDS surveillance system is the variety of monitoring tools available to the NPDS user community. In addition to AAPCC national surveillance definitions, 38 regional PCs utilize NPDS as part of their surveillance programs. Five state health departments plus CDC run surveillance definitions in NPDS. Since Surveillance Anomaly 1, generated at 2:00 pm EDT on 17 September 2006, over 177,000 anomalies have been detected. More than 600 were confirmed as being of public health significance with regional PCs working collaboratively with their local and state health departments and in some instances CDC on the public health issues identified.
At the time of this report, 375 surveillance definitions run continuously, monitoring case and clinical effects volume and a variety of case based definitions from food poisoning to nerve agents. These definitions represent the surveillance work by many regional PCs, state health departments, the AAPCC, and the Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, Centers for Disease Control, and Prevention (CDC).
In 2010, the NPDS surveillance application module underwent incremental improvements. Information call surveillance tools were enhanced with the activation of animal exposure call surveillance for volume definitions. Analysis process improvements included the addition of anomaly status tracking and case based time series reports.
Automated surveillance continues to remain controversial as a viable methodology to detect the index case of a public health event. Uniform evaluation algorithms are not available to determine the optimal methodologies.Citation8 Less controversial is the benefit to situational awareness that NPDS can provide. Typical NPDS surveillance data detects a response to an event rather than event prediction. This aids in situational awareness and resilience during and after a public health event.
2010 Gulf of Mexico Oil Spill
On Tuesday, 20 April 2010, an explosion aboard the Deep-water Horizon drilling rig working on a well one mile below the surface of the Gulf of Mexico created the largest unintentional oil spill in history. The human, animal, and environmental impact spanned thousands of miles across the Gulf Coast and the coastal states of Alabama, Florida, Louisiana, Texas, and Mississippi. US Poison Centers began receiving calls related to the spill. In order to better track these calls to aid state and CDC public health response, the AAPCC Rapid Coding Team in concert with Micromedex Poisindex staff activated a Gulf Oil Spill surveillance code. Product codes were also initiated for the two dispersants used (Corexit EC 9500A and EC 9527A, Nalco Company, Naperville, IL). As the disaster progressed, another product code was implemented for Contaminated Seafood so seafood questions could be tabulated. This code allowed the tracking of these calls in NPDS. US poison centers received 1932 calls (1222 exposure calls and 710 information calls). shows the distribution of calls received as of 1 July 2010 over space and time.
This data demonstrates how the public utilizes PCs in times of crisis. This unique system can be supported and enhanced to serve as a national public health hotline providing information and management beyond traditional poison exposure calls. PCs represent the only 24/7 system that is always open in the US and requires no membership or preregistration where the public can speak to a health care professional at no charge. This data demonstrates how the public utilizes PCs in times of crisis. This unique system can be supported and enhanced to serve as a national public health hotline providing information and management beyond traditional poison exposure calls.
THC Homologs and Bath Salts
In 2009 US poison centers began receiving an increased number of calls about THC homologs and Bath Salts. Several PCs worked with their state governments to ban sales of these products. On 1 March 2011, the US Drug Enforcement Administration used its emergency scheduling authority to place 5 THC homologs into controlled substances Schedule I.Citation9 Likewise on 7 September 2011, the DEA placed 3 synthetic stimulants into Schedule I.Citation10 This action makes possessing and selling these chemicals or the products that contain them illegal in the US for at least one year while the DEA and the Department of Health and Human Services decide whether these chemicals should be permanently controlled.
shows these emerging trends for each substance along with the time of the DEA action. The continuation of the graphics through October 2011 illustrates the real-time nature of NPDS.
Discussion
The exposure cases and information requests reported by PCs in 2010 do not reflect the full extent of PC efforts which also include poison prevention activities and public and health care professional education programs.
NPDS exposure data may be considered as providing “numerator data”, in the absence of a true denominator, that is, we do not know the number of actual exposures that occur in the population. NPDS data covers only those exposures which are reported to PCs.
NPDS regression analyses indicate that all reported analgesic exposures including opioids and sedatives are increasing year after year. This trend is shown in Table 17B and . NPDS data mirrors CDC data that demonstrates similar findings.Citation11 Thus NPDS provides a real-time view of these public health issues without the need for data source extrapolations.
One of the limitations of NPDS data has been the perceived lack of fatality case volume compared to other reporting sources. Although NPDS fatality volumes are less than those reported by CDC for prior years, the immediacy of NPDS data offers a current window not readily available with other US surveillance systems.
Overall, NPDS encounter volume is larger than similar reporting systems for pharmaceutical exposures, adverse events, or food borne cases. Perceived limitations in NPDS volume are due in part to the fact that electronic surveillance systems seldom follow a federated approach with common output streams. This is particularly apparent with the various electronic medical record systems available. It is important to build a federated approach similar to the one modeled by NPDS to allow data sharing, for example, between hospital emergency departments and other medical record systems including medical examiner offices nationwide. Enhancements to NPDS can promote interoperability between NPDS and electronic medical records systems to better trend poison-related morbidity and mortality in the US and internationally.
Summary
Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls.
Changes in encounters in 2010 compared to 2009 shown in include:
Total encounters (all exposure and information calls) decreased by 7.7%;
All information calls decreased 12.6%, Drug ID calls decreased 10.9%, and human exposures decreased 3.8%;
Health care facility (HCF) information calls decreased 13.6% while HCF exposures increased 2.7%;
Human exposures with less serious outcomes decreased 5.9% while those with more serious outcomes (minor, moderate, major, or death) increased 4.5%;
These data support the continued value of poison center expertise and need for specialized medical toxicology information to manage the more severe exposures, despite a decrease in calls involving less severe exposures.
The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response, and situational awareness tracking. NPDS is a model system for the nation and global public health.
Disclaimer
The American Association of Poison Control Centers (AAPCC; http://www.aapcc.org) maintains the national database of information logged by the country's regional Poison Centers (PCs) serving all 50 United States, Puerto Rico, and the District of Columbia. Case records in this database are from self-reported calls: they reflect only information provided when the public or healthcare professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure, etc.), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s).
www.informahealthcare.com/ctx/10.3109/15563650.2011.635149
Download PDF (765.1 KB)References
- National Poison Data System: Annual reports 1983–2009 [Internet]. Alexandria (VA): American Association of Poison Control Centers; Available from: http://www.aapcc.org/dnn/NPDSPoisonData/AnnualReports/tabid/125/Default.aspx
- Savel TG, Bronstein A, Duck, M, Rhodes MB, Lee, B, Stinn J, Worthen K. Using Secure Web Services to Visualize Poison Center Data for Nationwide Biosurveillance: A Case Study [Internet]. Online Journal of Public Health Informatics. 2010;2:1–9; [cited 2010 Nov 1] http://ojphi.org/htbin/cgiwrap/bin/ojs/index.php/ojphi/article/view/2920/2505
- US Census Bureau. Table 1. Preliminary Annual Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2000 to July 1, 2010 [downloaded 2011 Oct 9] www.census.gov/popest/states/tables/NST-PEST2010-01.xls
- US Census Bureau: International Data Base (IDB) Demographic Indicators for: American Samoa, Federated States of Micronesia, Guam, Virgin Islands, [downloaded 2011 Oct 9]: http://www.census. gov/ipc/www/idb/country.php
- Position statement: ipecac syrup. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:699–709.
- American Academy of Clinical Toxicology European Association of Poisons Centres and Clinical Toxicologists. Position Paper: Ipecac Syrup. J Toxicol Clin Toxicol. 2004;42:133–143.
- American Academy of Pediatrics Policy Statement. Poison treatment in the home. Pediatrics. 2003;112:1182–1185.
- Sosin DM, DeThomasis J. Evaluation challenges for syndromic surveillance–making incremental progress. MMWR Morb Mortal Wkly Rep. 2004;53 Suppl:125–129.
- US Drug Enforcement Administration, Chemicals Used in “Spice” and “K2” Type Products Now Under Federal Control and Regulation. News Release, 1-Mar-2011, [downloaded 2011 Oct 9] http://www.justice.gov/dea/pubs/pressrel/pr030111p.html
- US Drug Enforcement Administration, DEA Moves to Emergency Control Synthetic Stimulants - Agency Will Study Whether To Permanently Control Three Substances. News Release, 7-Sep-2011, [downloaded 2011 Oct 9] http://www.justice.gov/dea/pubs/pressrel/pr090711.html
- Centers for Disease Control and Prevention. QuickStats: Number of Poisoning Deaths * Involving Opioid Analgesics and Other Drugs or Substances–United States, 1999–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1026
- McGraw-Hill's AccessMedicine, Laboratory Values of Clinical Importance (Appendix), Harrison's Principles of Internal Medicine 17e. McGraw-Hill Professional, 2008 [cited 2010 Nov 1]. Available from: http://www.accessmedicine.com/.
- Goldfrank's Toxicologic Emergencies, Ninth Edition. New York, NY, McGraw-Hill Companies, 2010.
- Dart RC, editor. Medical Toxicology, Third Edition. Philadelphia, Lippincott, Williams & Wilkins, 2004.
