Abstract
Objectives. To examine whether postpartum maternal prescription of codeine was associated with an increased risk of harm to newborns. Design. Population-based retrospective cohort study. Setting. Ontario, Canada, from April 1, 1998 to March 1, 2008. Participants. A total of 7804 mothers with publically-funded prescription drug coverage. Women who received a prescription for a codeine-containing product within 7 days following hospital discharge and their neonates were matched to 7804 mothers who did not receive codeine following delivery. Main outcome measures. The primary outcome was readmission of the neonate to hospital for any reason within 30 days. Secondary outcomes included arrival to hospital by ambulance, hospitalization for dehydration, for injury, any hospitalization involving resuscitation or assisted ventilation, and all-cause mortality. Results. We studied 7804 infants whose mothers filled a prescription for codeine shortly after delivery and 7804 whose mothers did not. In the primary analysis, infants whose mothers received codeine were no more likely to be readmitted to hospital in the subsequent 30 days than children whose mothers did not (hazard ratio 0.95, 95% confidence interval (CI) 0.81–1.11). Moreover, we found no association between maternal codeine use and the other adverse neonatal outcomes studied. A stratified analysis revealed no differential risk among infants born by Caesarian section (hazard ratio 0.86; 95% CI 0.69–1.08). Conclusions. In this large population-based study, maternal prescription of codeine following delivery was not associated with death or hospitalization in the early neonatal period.
Introduction
Codeine is one of the most widely used analgesics in the world, but is a prodrug and must be converted to morphine for effective analgesia. This biotransformation is catalyzed by cytochrome P450 isoenzyme 2D6 (CYP2D6), a highly polymorphic drug metabolizing enzyme, leading to marked inter-individual variability in the amount of morphine produced.Citation1,Citation2 Approximately 7% of Caucasians display the poor metabolizer (PM) phenotype, lacking functional CYP2D6 activity and deriving minimal analgesia from codeine.Citation1 Individuals with the intermediate metabolizer (IM) and extensive metabolizer (EM) phenotypes have attenuated and normal enzyme levels, respectively, while those displaying the ultrarapid metabolizer (UM) phenotype have increased enzymatic activity. The prevalence of this phenotype varies widely, ranging approximately from 5.5% of Western Europeans to 30% of persons from Saudi Arabia and Ethiopia.Citation3
Like nonsteroidal anti-inflammatory drugs, codeine-containing medications are commonly used for the treatment of pain following childbirth, particularly following Caesarian section or episiotomy. The majority of new mothers also initiate breastfeeding.Citation4 Although the use of codeine during breastfeeding has long been considered safe, a recent case report described a neonate whose death on day 13 was attributed to opioid intoxication after the child's mother was prescribed acetaminophen with codeine for episiotomy-related pain.Citation5 The mother was subsequently found to have a CYP2D6*2 × 2 gene duplication consistent with UM status. The authors concluded that the mother's genotype caused large amounts of morphine to pass into breast milk, causing the child's death.Citation5,Citation6 However, this conclusion has been challenged on the grounds that even very large amounts of breast milk would not be expected to yield the levels of morphine (70 μg/L) or acetaminophen (5.9 mg/L) identified on postmortem analysis of the newborn's blood.Citation7
Although this case report and a small retrospective studyCitation8 raised concern about the safety of postpartum codeine use, particularly among mothers exhibiting the CYP2D6 UM phenotype, other evidence suggests that neonates born to mothers with CYP2D6 EM status (a much larger group in most areas of the world) are also at risk for morphine accumulation.Citation9 In light of the global prevalence of the EM and UM CYP2D6 phenotypes and the widespread use of codeine following delivery, it is likely that many neonates worldwide are exposed to morphine in breast milk each year. However, the potential relationship between maternal use of codeine and adverse neonatal outcomes has to date only been described in case reports and small observational studies.Citation6,Citation8–10 Using 10 years of linked healthcare data in a large population, we examined whether postpartum maternal prescription of codeine was associated with an increased risk of death or hospital admission among newborns.
Methods
Study population
We conducted a population-based retrospective cohort study using propensity score matching, an advanced modeling technique that uses a large amount of healthcare data to identify highly comparable groups of patients.Citation11 The analysis included women living in Ontario, Canada who gave birth in hospital between April 1, 1998 and March 31, 2008 and who, along with their newborn, were discharged within 7 days of delivery. Ontario is Canada's most populous province, with an estimated population of 13 million at the end of the study period. We included only singleton deliveries and excluded women younger than 16 years of age at the time of delivery. We limited the analysis to women with available prescription records by excluding all women who did not receive at least one publically-funded prescription within 180 days following hospital discharge. This project was approved by the ethics review board of Sunnybrook Health Sciences Centre, Toronto.
Data sources
We identified prescription records from the Ontario Public Drug Benefit Program, which contains comprehensive prescription records for all provincial residents younger than 65 years of age receiving social assistance, either by virtue of unemployment or because of high prescription drug costs relative to net household income; this sample represents approximately 5.5% of the total provincial population. We identified deliveries and hospitalizations using the Canadian Institute for Health Information Discharge Abstract Database, which contains detailed diagnostic and procedural information for all hospital admissions. We identified mother–child pairs based upon the mother's hospital chart number and, when necessary, by matching according to healthcare facility, discharge date, and home postal code using an algorithm described in detail elsewhere.Citation12,Citation13 Basic demographic information, including date of death, was identified using the Registered Persons Database, which contains a unique entry for all Ontario residents ever issued a health card. These databases are routinely used to study drug safetyCitation14–16 and were linked in an anonymous fashion using encrypted 10-digit health-card numbers.
Identification of study subjects
We identified two cohorts of women and their neonates for comparison. The exposed group was defined as those women who filled a prescription for a codeine-containing analgesic within 7 days following hospital discharge while the unexposed group did not fill a codeine prescription during the same interval. For each mother who received a prescription for codeine, one untreated mother was identified using propensity score matchingCitation17,Citation18 an advanced modeling technique that incorporated numerous factors that might influence maternal drug exposure or neonatal outcomes (Supplementary Appendix 1 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.681052). Treated and untreated mothers were matched on calendar year of delivery, maternal age (within 3 years), mode of delivery (Caesarian section or vaginal delivery), and propensity score (within 0.2 standard deviations).
We conducted several sensitivity analyses using maternal prescriptions for iron products, nonsteroidal anti-inflammatory drugs and hemorrhoid preparations, with the rationale that, unlike codeine, maternal prescriptions for these products should have no bearing on neonatal health status. Within the treated group, mothers who received prescription for both a codeine-containing analgesic and a nonsteroidal anti-inflammatory drug within 7 days of discharge were excluded from the analysis.
Outcomes
All neonates were followed for up to 30 days from the date of the mother's codeine prescription (the index date) or, in the case of untreated mother–child pairs, a 30-day period that commenced after the same interval between hospital discharge and maternal codeine prescription for the corresponding exposed mother–child pair.
The primary outcome was defined as any readmission of the neonate to hospital within 30 days of the index date. Secondary outcomes (Supplementary Appendix 2 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.681052) included hospital admission for dehydration within 30 days, hospital admission for injury within 30 days, arrival to hospital by ambulance, hospitalization with resuscitation or assisted ventilation, and death from any cause. Only the first occurrence of a study outcome was considered among infants who experienced multiple outcomes over the study period, and children who died during follow-up were censored on the date of death. Finally, we conducted a subgroup analysis according to mode of delivery, with the rationale that women who gave birth by Caesarian section would be more likely to take opioid analgesics following delivery, and likely at higher doses.Citation19
Statistical analysis
We compared baseline characteristics of mothers treated and not treated with codeine, and their respective infants, using standardized differences, which represent the difference between means divided by the pooled standard deviation. Values less than 0.10 suggest negligible differences between groups. The primary analyses used matched Cox proportional hazards regression, with neonates born to women not prescribed codeine as the reference group. The proportional hazards assumption was verified by testing the statistical significance of a covariate that allowed treatment to have a time-varying effect. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina) and used a two-tailed Type 1 error rate of 0.05 as the threshold for statistical significance.
Results
Over the 10-year study period, we identified 7804 mothers who met our criteria and received a prescription for a codeine-containing product within 7 days of hospital discharge. These women were matched to an equal number of mothers who did not receive codeine following delivery, with excellent matching on all measured variables (). Infants of mothers prescribed codeine were observed for an average of 28.4 days (standard deviation 3.7 days) while those whose mothers were not prescribed codeine were observed for an average of 28.3 days (standard deviation 3.8 days).
Table 1. Characteristics of study subjects.
In the primary analysis, 294 (3.8%) children born to mothers who filled a prescription for codeine and 312 (4.0%) children whose mothers did not were hospitalized for any reason within 30 days. We found no difference in the risk of readmission within 30 days among children whose mothers were prescribed codeine relative to those whose mothers were not ( – hazard ratio 0.95; 95% confidence interval (CI) 0.81–1.11; ). Similarly, we found no significant difference in risk of neonatal hospitalization for dehydration, injury, arrival to hospital by ambulance, or hospitalization requiring resuscitation or assisted ventilation (). We could not draw meaningful inferences regarding neonatal mortality given the infrequent occurrence of neonatal death within 30 days, although the majority of deaths occurred in children whose mothers were not prescribed codeine.
Fig. 1. Kaplan–Meier curves for all-cause hospital readmission among neonates, by maternal codeine prescription. Cumulative proportion of neonates readmitted to hospital for any reason within 30 days of the date of issue for the maternal codeine prescription among exposed neonates (solid line) and the corresponding date among unexposed infants (dashed line).
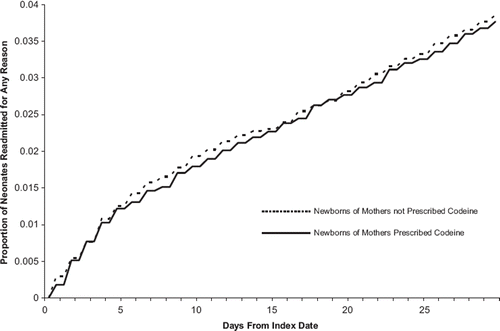
Table 2. Association between maternal medication use and neonatal readmission to hospital for any reason.
Table 3. Association between maternal codeine exposure and secondary neonatal outcomes.
In a subgroup analysis, we found consistent results regardless of whether the child was delivered vaginally (adjusted hazard ratio 1.04; 95% CI 0.83–1.31) or by Caesarian section (adjusted hazard ratio 0.86; 95% CI 0.69–1.08) (). As expected, we found no association between maternal prescriptions for iron, nonsteroidal anti-inflammatory drugs or hemorrhoid products, and neonatal readmission within 30 days (). Finally, we conducted an additional sensitivity analysis in which observation of all neonates began on the day of hospital discharge. In this analysis, we again found no difference in the risk of hospital admission in the two groups of neonates (hazard ratio 0.98; 95% CI 0.84–1.14).
Finally, we conducted an analysis examining any visit to a physician for any reason within 30 days. In this analysis (which was not prespecified), neonates born to women prescribed codeine post-partum were marginally more likely to see a physician for any reason (hazard ratio 1.09; 95% CI 1.04–1.13). However, the same was true of children born to women prescribed hemorrhoid therapies after delivery (hazard ratio 1.35; 95% CI 1.00–1.83). Of note, in both of these analyses, the proportional hazards assumption was not met.
Discussion
Using 10 years of longitudinal healthcare data, we studied nearly 8000 neonates whose mothers were prescribed codeine following delivery, and found that these children were at no greater risk of several adverse health outcomes than children whose mothers were not prescribed codeine.
Importantly, our results should not be construed as an endorsement of codeine therapy, either during breastfeeding or in any other setting. Despite its widespread popularity and familiarity to clinicians, codeine is an inherently irrational analgesic given the unpredictable extent of its conversion to morphine, due to CYP2D6 polymorphisms and interactions with other drugs.Citation2 In most instances, the administration of a known quantity of codeine amounts to the administration of an unknown quantity of morphine.
Some limitations of our study merit emphasis. First, our analyses derive from women and children receiving social assistance. Whether the results can be generalized to more affluent populations is not known. Second, we cannot identify nonprescription drug use, including low-dose codeine (8 mg per tablet), which is available without a prescription in Ontario. By design, this is more likely in our unexposed cohort, and would tend to bias our results toward the null, as would use of higher strengths of codeine obtained without a prescription. Third, receipt of a prescription does not necessarily indicate use of a drug; this would lead to an attenuation of our effect estimates. However, a bias of this nature is unlikely to explain our findings, since all of the point estimates in our analyses fall at or below 1.0. Finally, we have no data regarding several important factors including dose and duration of analgesic treatment, ethnicity, CYP2D6 genotype, or breastfeeding status. However, in clinical practice genotype is generally unknown, and would be expected to be distributed evenly among both groups of women in our cohort. Importantly, it has been suggested that neonatal risk is likely only in women who take codeine repeatedly for at least 4 days while breastfeeding,Citation9 and it is probable that only a minority of women in our study fulfill these criteria.
In summary, despite a recent report of neonatal death associated with maternal use of codeine, we found no evidence that prescription of codeine to women following delivery was associated with several measures of neonatal harm in a large population studied over a 10-year period. While these results do not support the prescribing of codeine generally, they do suggest that serious neonatal harm is highly unlikely when the drug is prescribed to women following delivery.
Acknowledgments
We thank Milton Tenenbein for helpful comments on an earlier draft of this manuscript, and we thank Brogan Inc., Ottawa for the use of their Drug Product and Therapeutic Class Database.
Declaration of interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This study was supported by a grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC) Drug Innovation Fund, and by the Institute for Clinical Evaluative Sciences (ICES), a nonprofit research institute sponsored by the Ontario MOHLTC. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.681052
Download PDF (124.6 KB)References
- Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I, . Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 2007; 7:257–265.
- MacDonald N, Macleod SM. Has the time come to phase out codeine? CMAJ 2010; 182:1825.
- Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 2005; 5:6–13.
- Haiek LN, Kramer MS, Ciampi A, Tirado R. Postpartum weight loss and infant feeding. J Am Board Fam Pract 2001; 14:85–94.
- Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006; 368:704.
- Madadi P, Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder JS, . Safety of codeine during breastfeeding: fatal morphine poisoning in the breastfed neonate of a mother prescribed codeine. Can Fam Physician 2007; 53:33–35.
- Bateman DN, Eddleston M, Sandilands E. Codeine and breastfeeding [Letter to the editor]. Lancet 2008; 372:625.
- Madadi P, Ross CJ, Hayden MR, Carleton BC, Gaedigk A, Leeder JS, . Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin Pharmacol Ther 2009; 85:31–35.
- Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther 2009; 86:634–643.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs 2008; 10:399–404.
- D'Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281.
- Urquia ML, Frank JW, Glazier RH, Moineddin R. Birth outcomes by neighbourhood income and recent immigration in Toronto. Health Rep 2007; 18.
- Urquia ML, Frank JW, Moineddin R, Glazier RH. Immigrants’ duration of residence and adverse birth outcomes: a population-based study. BJOG 2010; 117:591–601.
- Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, . Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543–551.
- Juurlink DN, Gomes T, Lipscombe LL, Austin PC, Hux JE, Mamdani MM. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 2009; 339:b2942.
- Park-Wyllie LY, Juurlink DN, Kopp A, Shah BR, Stukel TA, Stumpo C, . Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 2006; 354:1352–1361.
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127(Pt 2):757–763.
- Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics 1996; 52:249–264.
- East N, Dube J, Perrault E. Postpartum pain relief: a randomized comparison of self-administered medication and standard administration. J Obstet Gynaecol Can 2007; 29:975–981.
