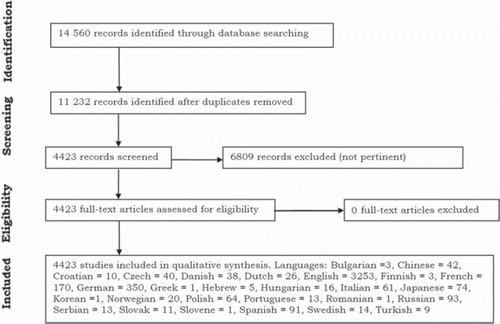
Abstract
Extracorporeal treatments (ECTRs), such as hemodialysis and hemoperfusion, are used in poisoning despite a lack of controlled human trials demonstrating efficacy. To provide uniform recommendations, the EXTRIP group was formed as an international collaboration among recognized experts from nephrology, clinical toxicology, critical care, or pharmacology and supported by over 30 professional societies. For every poison, the clinical benefit of ECTR is weighed against associated complications, alternative therapies, and costs. Rigorous methodology, using the AGREE instrument, was developed and ratified. Methods rely on evidence appraisal and, in the absence of robust studies, on a thorough and transparent process of consensus statements. Twenty-four poisons were chosen according to their frequency, available evidence, and relevance. A systematic literature search was performed in order to retrieve all original publications regardless of language. Data were extracted on a standardized instrument. Quality of the evidence was assessed by GRADE as: High = A, Moderate = B, Low = C, Very Low = D. For every poison, dialyzability was assessed and clinical effect of ECTR summarized. All pertinent documents were submitted to the workgroup with a list of statements for vote (general statement, indications, timing, ECTR choice). A modified Delphi method with two voting rounds was used, between which deliberation was required. Each statement was voted on a Likert scale (1–9) to establish the strength of recommendation. This approach will permit the production of the first important practice guidelines on this topic.
Introduction
The utility of extracorporeal treatments (ECTR) in poisonings is founded upon the premise that the toxicity of a poison correlates with its body burden, and that reducing that burden attenuates toxicity.
The first instance of ECTR use was reported by Abel et al. in 1913, when salicylates were removed from dogs.Citation1 Kyle and coworkers were the first to successfully employ hemodialysis (HD) for the treatment of barbiturate poisoning.Citation2 By 1958, George Schreiner published the first comprehensive review of the use of HD in acute poisoning.Citation3
Currently, there is significant confusion regarding the indications for ECTR in poisoned patients. While a number of reviews are published on the topic, no comprehensive guidelines have been published in recent times to orient patient management in this context.
Background
The EXTRIP workgroup is an international collaborative initiative comprising recognized experts from various clinical specialties (medical toxicology, emergency medicine, nephrology, critical care, pediatrics, and pharmacology) who routinely deal with poisoned patients.Citation4
The guidelines are intended to rely on evidence whenever possible. However, co-chairs acknowledged at the start that the available evidence was likely to be of low quality in most instances. Indeed, few interventions that pre-dated the acceptance of randomized controlled trials in the 1970s (ECTR for poisoning, dialysis for kidney failure, hip replacement for hip fracture) have undergone the scrutiny of a trial.
Furthermore, extracorporeal therapies have been recognized as the gold-standard treatment of several specific poisonings for over 50 years. Physicians have experience and confidence, for example, in the life-saving potential of ECTR in severe salicylate poisoning as illustrated in a recent survey.Citation4 This suggests that, at least in selected cases, ECTR is an element of standard care in the management of poisonings and clinicians support its use overwhelmingly.Citation4,Citation9 In such instances a randomized trial would not be feasible or ethical. Alternatively for poisons for which ECTR is frequently used, studies have questioned the benefit of ECTR on outcome.Citation5
To add to these difficulties, even when consistent published results are available, new technical advances in ECTR (e.g., filters, dialysate, anticoagulation, catheters) have likely rendered older data obsolete; for example, a poison that was not considered dialyzable in the 1970s may be dialyzable today with new technology. Furthermore, new supportive care and antidotes may influence the role of ECTR for some poisons.
These factors illustrate the inherent difficulties of the EXTRIP initiative. Rather than await the arrival of new data, the EXTRIP workgroup has elected to proceed with the systematic identification, review, and critique of all available evidence using the GRADE system.Citation6 Because case reports of ECTR in poisoning may reveal important information about toxicokinetics, and because there is an assumption of a physiological correlation between extracorporeal removal (which is quantifiable) and clinical outcome, case-reports were not excluded from analysis. When the evidence is incomplete, opinion-based arguments will be proposed following transparent methodology. Nevertheless, as explicitly mentioned by the WHO, “(…) a strong recommendation may be made in the presence of very low quality evidence given variability in values and preferences between the experts, the balance between desirable and undesirable consequences of an intervention, and resource implications.” Citation7
Although the intended objective of this initiative is to produce practice guidelines, members acknowledged that the quality of the evidence might be low for any given poison. If this is confirmed, the workgroup will preferentially use the wording “recommendations.” The work can nevertheless be considered more exhaustive than a position paper/statement because it is based on a stringent process of synthesis and methodology (AGREE instrumentCitation8) (Supplementary Appendix 1 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.683436).
Objectives
The EXTRIP workgroup will review current literature and produce recommendations on the use of ECTR in the context of poisoning. More specifically, the EXTRIP workgroup will evaluate the effects of ECTR for a set of preselected poisons in various populations (including pediatric, CKD, pregnancy) and settings (acute, acute-on-chronic and chronic poisoning), if applicable.
The effect of ECTR will be measured against current standard of care and alternative treatments.Citation9–12 Potential benefits of ECTR will be balanced with potential complications and costs (human/technical) associated with the procedure. Different types of ECTR will also be compared. Outcomes that will be measured include mortality, relevant clinical and physiological endpoints as well as the extent of extracorporeal removal of the poison.
Definitions/Terminology
Poison: A xenobiotic (exogenous chemical, including medication) or an endogenously found chemical (e.g., iron, copper, vitamins) resulting from exogenous exposure with the potential to cause toxicity.
Poisoning: Exposure to a poison causing or capable of causing toxicity, regardless of intent. It includes intoxication, toxicity, and overdose.
Adverse outcome: Significant clinical effect following poisoning. An adverse outcome can be critical (death or major end-organ damage, such as blindness in methanol poisoning) or non-critical (minor end-organ damage, such as tremors in lithium poisoning).Citation6,Citation13
Severe poisoning: Exposure to a poison causing or having the potential to cause an adverse outcome.
Extracorporeal treatment (ECTR): A treatment, occurring outside the body, which promotes poison removal by mechanisms different from endogenous pathways. ECTR includes HD, continuous renal replacement therapy, extended dialysis, peritoneal dialysis (although technically occurring in the body), hemofiltration, hemodiafiltration, hemoperfusion, therapeutic plasma exchange and albumin/“liver” dialysis.
Dialyzability: The term “dialyzability” will be used to reflect the ability of ECTR to remove a clinically significant percentage of the total body burden of the poison (i.e., ‘clinical dialyzability’). A substance may be technically dialyzable (that is, recovered in the extracorporeal circuit), but not ‘clinically dialyzable’. The term “dialyzability” is applicable to any extracorporeal modality used to remove a substance from the body. The EXTRIP workgroup's focus will be on determination of clinical dialyzability, which will be implied, unless stated otherwise, in the term “dialyzable.”
Clearance: The volume of blood (or solvent) cleared of poison per unit time, typically reported in units of mL/min. Importantly, CLEC represents solute clearance due exclusively to ECTR and is independent of endogenous systemic clearance (CLSYS; the sum of underlying renal and non-renal clearances). CLtot refers to total clearance and is the sum of CLEC and CLSYS.
Methods
Panelist selection
The workgroup includes several specialists actively involved in the care of poisoned patients. All members were selected by content expertise. One participant was chosen because of expertise in epidemiology and guideline methodology, but will not participate in the poison review. Members divulged all potential conflicts of interests prior to inclusion in the workgroup and after statements were drafted.
The workgroup will be divided into subgroups, each of which responsible to review certain poisons. Three to five members, including one nephrologist, one toxicologist and one pharmacologist/kineticist, form each subgroup. The chair of the EXTRIP initiative is Marc Ghannoum (nephrology) , while co-chairs are Bob Hoffman (medical toxicology/emergency medicine) and Thomas D. Nolin (pharmacology). The complete list of current members is included in Supplementary Appendix 2 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.683436. The workgroup is represented by several medical societies all of which have delegated a representative to the guideline process ().
Table 1. Represented societies.
The first meeting took place in Denver, Colorado, USA on October 10th 2010. The purpose of this meeting was to establish the main objectives of the workgroup and discuss the methodology structure to produce recommendations that will be both rigorous and transparent. Decisions on methodology were based on majority (50% + 1).
Criteria for publication inclusion
Types of studies. Eligibility criteria for studies will be based on their design and quality. Design studies that will be included in the systematic review of the literature are: Randomized controlled trials, non-randomized controlled trials, observational studies, case series, case reports, abstracts from scientific and clinical meetings (up to 2 years before the systematic review) animal studies and in vitro studies. The workgroup had decided to consider any original publication. Animal and in vitro studies will be accepted if the methods and results can be interpretable and correlated in humans.
Types of participants. The studied participants are patients with severe poisoning (as defined earlier). Therefore, poisonings not associated with an adverse outcome will not be evaluated, but may be included if they provide relevant toxicokinetic information.
There is no restriction on the context of the poisoning (acute, acute-on-chronic or chronic) or heterogeneity of the participants (e.g., special populations such as pediatric, chronic kidney disease, hepatic insufficiency, pregnancy).
Types of interventions. ECTR will be considered as the intervention of interest only if instituted, at least partially, for the purpose of poison removal. Therefore, studies in which ECTR was instituted exclusively for other indications will be excluded such as studies using renal replacement therapy solely for the treatment of acute kidney injury (AKI), albumin dialysis solely for the treatment of hepatic failure or extracorporeal membrane oxygenation solely for the treatment of severely hypoxemic/hypercapnic atients.
The rationale of these exclusions is that renal replacement therapy would be initiated in significant AKI, regardless of the presence or absence of poisoning. Nevertheless, if ECTR was instituted primarily for AKI but also potentially for poison removal, this publication would be included.
There will be no automatic exclusion of specific extracorporeal techniques, although some may be unpopular or unavailable in certain countries (e.g., hemoperfusion in the USA). However, ECTR will be assumed to be up-to-date with current technology and application, that is, bicarbonate dialysate and non-cellulose synthetic membranes for HD.
Types of outcome measures. Clinical outcomes (critical and non-critical as defined previously) will be reported for every reviewed poison, in order of relative importance. The workgroup has decided to report dialyzability as a separate outcome. This is explained by the observation that excellent poison removal does not equate with clinical improvement for certain poisons. Conversely, clinical improvement can sometimes be observed despite poor extracorporeal removability (as other mechanisms like acidosis correction can be responsible). The improvement of outcomes with ECTR will be balanced by the potential adverse events and complications related to ECTR.
Selection of poisons. Because a critical review of all potential poisons is impractical, the workgroup has decided to review, at least initially, a limited set of poisons. Some chemicals were discarded prior to selection: electrolytes (sodium, potassium, calcium, magnesium, phosphorus), endogenous hormones and compounds (thyroxin, cortisol, lactate, beta2-microglobulin, glutamate), radiology agents (gadolinium, iodine-based contrast). The following criteria were considered for selection:
- Frequency: Members were provided with the incidence of ECTR use in the context of poisoning, derived from the American Association Poison Control Centers database.Citation14,Citation15 Although this selection has shortcomings, namely geographical applicability, particular categorization of poisons, and imprecise ECTR indication (poison removal vs. AKI), this permitted identification of poisonings in which ECTR is often performed.
- Geography: Some poisonings display marked geographic difference in incidence. For example, paraquat poisoning is common in Asia, the Middle East, and Latin America but rare in North America. Furthermore, the prescription profile and availability of medicines can vary tremendously depending on the region. Members selected poisons that had the most universal impact on global health.
- Available evidence: Although recommendations are dependent on expert opinion, this will be informed by data obtained from the preliminary search described below. The quality of the data will help determine the strength of recommendation of ECTR for any specific poisoning.
- Relevance: Supportive care and other treatments (especially antidotes) have improved over the years, influencing potential usefulness of ECTR in poisoning. Furthermore, poisons that are associated with adverse outcomes are given preference.
With these considerations the toxicologists in the workgroup voted on the 24 most relevant poisons to review (). Supporting societies may later request the addition of one or more poisons to the list. Furthermore, more poisons will be later reviewed once recommendations for the above list are completed.
Table 2. Poison selection.
Publication selection
Preliminary search. The following databases will be used for the publication count (used to select which poisons will be reviewed by the workgroup) and to serve as a core for the specific searches ():
Medline/PubMed, EMBASE, Cochrane library (Review and Central), Conference proceedings/meeting abstracts of the EAPCCT and NACCT annual meetings (only if within 2 years of the literature search). Marc Ghannoum is the designated member for this task. Search strategy (Keywords): Toxicity OR poison* OR intoxication OR overdos*] AND [Hemoperfusion OR haemoperfusion OR hemofiltration OR haemofiltration OR hemodialysis OR haemodialysis OR hemodiafiltration OR haemodiafiltration OR dialysis OR plasmapheresis OR plasma exchange OR exchange transfusion OR Continuous renal replacement therapy (CRRT) OR renal replacement therapy OR extracorporeal therapy].
Specific searches (for each poison). This search will decide which publications are kept for review and analysis by subgroups. Databases included in the preliminary search, Google scholar and hand searching, references of editorials, review articles (including electronic publications such as UptoDate and eMedicine), book chapters, or similar literature. All members of a subgroup are expected to perform this. Search strategy (Keywords):[Commercial OR generic name for the specific poison] AND [toxicity OR poison* OR intoxication OR overdos*] AND [hemoperfusion OR haemoperfusion OR hemofiltration OR haemofiltration OR hemodialysis OR haemodialysis OR hemodiafiltration OR haemodiafiltration OR dialysis OR plasmapheresis OR plasma exchange OR exchange transfusion OR CRRT].
ECTR complications. This search will evaluate the complications of ECTR. Non-poisoning situations will be accepted but its particular context recognized, for example, the long-term risk of infectious complications of temporary catheters are not necessarily pertinent in poisoning situations. Medline/PubMed will be the databases searched by five members (nephrologists, critical care specialists, and emergency physicians) Search strategy (Keywords): [complication OR safety OR side effect OR adverse event OR harm OR adverse effect] AND [hemoperfusion OR haemoperfusion OR hemofiltration OR haemofiltration OR hemodialysis OR haemodialysis OR hemodiafiltration OR haemodiafiltration OR dialysis OR plasmapheresis OR plasma exchange OR exchange transfusion OR CRRT]
ECTR costs. This survey will be sent to various nephrologists across the world to compare the relative cost of different extracorporeal treatments available in their country. This was not intended to provide a rigorous cost analysis study or to assess cost-effectiveness of ECTR in poisoning. Rather, this will give an overall idea of the monetary costs associated with various ECTR modalities worldwide. Only the monetary costs of the technical apparatus (e.g., filter, circuit, dialysate), nursing, and physician salary will be considered for this analysis.
Pharmacokinetic data in non-poisoning situations. If toxicokinetic data for any poison is insufficiently described in publications retrieved from the specific search, pharmacokinetic publications of ECTR removal of therapeutic drugs in non-poisoning situations may be used. However, the subgroup and designated kineticist will need to consider that chronic kidney disease (CKD) and AKI patients have particular clinical characteristics, which often differ from those of poisoned patients, poison characteristics (including parameters like protein binding and volume of distribution (Vd)) may be influenced by kidney function and pharmacokinetic parameters of a drug during therapeutic use may differ from its toxicokinetics during poisoning.
Despite these differences, the workgroup believes that this search is potentially useful as the studies are often better designed (i.e., prospective, more patients) and can better explain the dialyzability of a poison. Hence, this search will be performed for a specific poison only if the data retrieved by the previous searches cannot permit a consensus on ECTR removal.
Medline/PubMed, EMBASE, Cochrane library (Review and Central) will be the databases searched by every member of the subgroup using the Keywords: [Pharmacokinetic OR drug dosage OR clearance OR dialyzability] AND [Commercial OR generic name for the specific poison] AND [Hemoperfusion OR haemoperfusion OR hemofiltration OR haemofiltration OR hemodialysis OR haemodialysis OR hemodiafiltration OR haemodiafiltration OR dialysis OR plasmapheresis OR plasma exchange OR exchange transfusion OR CRRT].
Publication exclusion. Foreign language publications will be included and translated by an appropriate resource. No publications will be excluded based on the publication date. Only original publications will be used. Reviews, editorials, book chapters, and commentaries will only be used in the ECTR complications search or if containing original data. All duplicate publications will be removed. Every subgroup can decide by consensus (two of three members) to remove publications not considered pertinent. As described above, publications describing use of RRT solely for the treatment of AKI or use of albumin dialysis solely for treatment of hepatic failure will be discarded.
Reporting by subgroup. A summary of the search strategy will be described for every poison by the responsible subgroup, according to the PRISMA statement16. It will describe in detail the number of references retrieved in the initial search. The number of doubles and excluded publications will be stated, with the reason for exclusion (relevance, very poor quality, publication type). Any disagreement in the exclusion process will be addressed explicitly.
Data extraction, synthesis, presentation, and interpretation
Data extraction. For each publication retained in the specific poison database, a group of qualified reviewers will extract all relevant data into a standardized flow sheet. The group's primary objective is to report accurately the information contained in the publication. At this stage, no calculation, inference, or interpretation should be attempted. All publications that should probably be excluded (accordingly to the previous criteria) will be marked for reevaluation by the expert subgroup. To reduce the risk of errors, two independent reviewers will evaluate each publication. To ensure uniformity, an independent individual will merge individual flow sheets into one. If observational studies or RCTs are included, the epidemiologist will assist the evaluation of the quantitative measure of effect and quality of evidence for clinical outcomes.
Evaluation of extracted data. For each publication included in the specific poison database, the subgroup will first evaluate publications marked for exclusion. A publication can be rejected if all members of the subgroup agree. If there is strong disagreement on the inclusion or exclusion of a determined publication, it will be included in the analysis. When the summary is presented to the workgroup, the arguments for and against inclusion will also be presented for transparency. Every individual publication will be assessed in regards to its quality, both for clinical outcome (methodological biases, indirectness, imprecision, and error) and for kinetic outcomes (). Finally, the effect of ECTR will be reported for each clinical outcome i.e. report descriptive value and/or comparative values (risk difference or relative risk) and for kinetic outcomes (). (See Supplementary Appendix 3 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.683436 for details).
Table 3. Scoring the quality of evidence for kinetic outcomes (individual studies*.
Summary of data. Following the previous steps, the subgroup will create a summary sheet. Data will be regrouped to allow synthesis principally by outcome, and if feasible, by intervention and by sub-population. The quality of the evidence will be summarized for each outcome (). The effect of ECTR will be summarized qualitatively or quantitatively for each outcome ( and ). Any general comments on the reviewed literature will be added to the summary sheet. At this point in the process, a conference call between the members of the subgroup to discuss the literature and to clarify any relevant point will occur.
Table 4. Summary of the effect and the quality of evidence per type of outcome.
Table 5. Summary of quality of evidence for clinical outcomes: GRADE system.Citation6
Table 6. Summary of quality of evidence for kinetic outcomes: Quality of evidence score.
Table 7. Summary of the effect for dialyzability.
Statement proposal. After reviewing and summarizing the available literature, the subgroup will propose a series of statements concerning the use of ECTR in the setting of severe poisoning. These statements must take into account the quality of evidence, the relative importance of the outcomes, expected clinical course without ECTR, the availability of other therapies, the magnitude and the precision of the effect, the balance between harm and benefits, and the costs of the procedure.
Since there have been major updates and innovations in ECTR techniques and standard of care over the years, systematic interpretation of older data is essential. The following assumptions regarding older data will be accepted by the workgroup: If ECTR appeared to improve clinical outcomes: at least the same can be assumed today. The improvement of standard of care and advent of new antidotes does not render ECTR less effective but may present alternatives to ECTR. If a poison appeared removable by ECTR: the same can be assumed today. If ECTR did not appear to enhance elimination of a poison or did not appear to improve outcome: no assumption can be made and the publication will be judged by its own merit, on a case-by-case basis.
Type of statements for proposal:
Toxicokinetic statement: Poison X is (Dialyzable, Moderately dialyzable, Slightly dialyzable, Not dialyzable) by ECTR (GRADE equivalent). EXAMPLE: Salicylates are moderately dialyzable by ECTR (A).
General statement: (We recommend/ We suggest/ it would be reasonable/ no agreement reached) to (perform/not perform) ECTR in severe poisoning with “X” (GRADE). EXAMPLE: We recommend performing ECTR in severe salicylate poisoning (1D).
Specific statements: If there is support for performing ECTR, other statements (with grade) will be submitted; indications of initiating ECTR (ingestion, level, special population, symptoms, clinical markers), when to discontinue ECTR, preferable ECTR modality, particularities of ECTR with the particular poison (timing, special population, technical).
Any other statement particular to the poison deemed significant by the subgroup (e.g., alternative therapy, antidote). EXAMPLES: “We recommend performing ECTR in the presence of coma or seizures associated with salicylate poisoning. (1C”), “We suggest performing ECTR when salicylates levels are higher than 5.0 mmol/L (69 mg/L) (2D),” “No consensus was agreed on when to discontinue ECTR for salicylate poisoning.,” “It would be reasonable to choose hemodialysis as the preferred ECTR (3D)”or “. We suggest discontinuation of urine alkalinization once hemodialysis is begun (2D).”
Once this step is completed, the subgroup will submit the following documents to the workgroup: complete publications list, merged flow sheet, summary sheet, and the proposed voting statements. At any step throughout the process, if there is strong disagreement or dissent, the issues will be brought later to the whole workgroup. Co-chairs may aid this process, if required.
Voting procedure
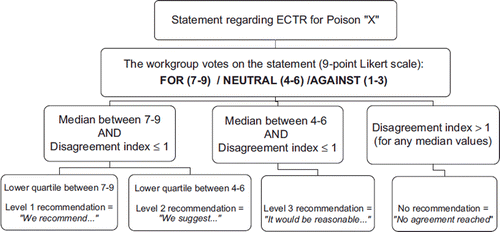
Since the majority of literature reviewed will likely be of poor quality, the majority of recommendations will be based on expert opinion. Therefore, a rigorous voting procedure is implemented to ensure transparency and reproducibility.Citation17 The modified Delphi method (i.e., an iterative consultation of experts on a given subject) was chosen to reach a formal consensus on proposed voting statements. Two rounds of consultation are scheduled.
For the first round, the subgroup submits the merged flow sheet, summary sheet, and statements to each workgroup member who then submits an anonymous vote and includes comments. The level of approval for the proposed statement is recorded on a 9-point Likert scale (with 1 being completely against and 9 being completely for the proposed statement).Citation18 Every member is encouraged to expand or challenge proposed statements.
After the first round is completed, the votes and comments will be summarized. The RAND/UCLA Appropriateness Method (a method of statistical measurement frequently used and adapted to all panel sizes) will be used to quantify the votes.Citation18 The median values will be reported and a disagreement index calculated. Median values ranging from 7 to 9 will reflect that the workgroup is in favor of the proposed statement, 4 to 6 reflects a neutral position and 1–3 reflects that the workgroup is not in favor of the statement. The disagreement index, defined as the Interpercentile Range divided by the Interpercentile Range Adjusted for Symmetry, describes the dispersion of ratings more effectively than the mean absolute deviation from the median.Citation18 Index values less than or equal to 1 indicate agreement between panellists.
A standardized form is then resubmitted to each participant with his or her vote, summary statistics, workgroup comments and modified statements. Sufficient time will be available for review. The second voting round will take place at a conference organized in June 2012. All subgroups will present the evidence a second time, that is, potential risks, costs, alternative treatments, clinical benefit, and toxicokinetic calculations of ECTR for every reviewed poison. After this presentation, the workgroup will take time to deliberate these findings and take a consensus position on each statement. Each statement is then re-voted privately. The voting procedure will result in providing strength of recommendations (). Because of the restricted number of experts voting, if dissent remains, the final decision and debate will be explained in the official recommendation document for transparency. Interpretation of each level of strength of recommendation is explained on .
Table 8. Strength of recommendation: Interpretation.
Other statements needing debate and vote
Besides reviewing individual poisons, the EXTRIP workgroup will undertake general specific objectives not expected to be supported by literature, although a literature search will be performed prior to review. This includes: providing general indications for ECTR of poisons not reviewed in the recommendation process and proposing simple toxicokinetic tools to estimate the potential effect of ECTR in a poisoning context, compare various ECTR according to their respective effectiveness, safety, cost and availability in the context of poisoning, propose standard ECTR prescriptions to improve poison removal (including timing, catheter, heparinization, filters) and limit risks of ECTR (e.g., dialysate, catheter) or propose standards/check list or data and material to be obtained in patients that undergo ECTR for poisoning to improve the quality of scientific reports (e.g., filter material and surface, blood and dialysate flow, hematocrit, pre- and post-filter concentrations of the poison, albumin serum concentration...)
Other requirements from workgroup
EXTRIP plans to discuss organizational barriers in health care centers that may not have the resources and technical expertise to adopt the recommendations. Details for improved coordination with dialysis centers will be suggested. We will also discuss the costs of implementing these recommendations; while ECTR is associated with a financial cost, recommendations will also address situations where ECTR was previously performed but no longer supported. It is possible that the present recommendations may reduce future expenses. Audits to evaluate the implementation of guidelines to monitor adherence to the recommendations via pre and post publication surveys will be proposed. A website has already been set-up (extrip-workgroup.org) to assist with organization of activities for the dissemination and promotion of recommendations such as regular publications and conferences. Members will eventually organize CME activities/conferences. Description of all levels of funding and disclosure of any potential conflicts of interests will also be done.
After the planned poison reviews, EXTRIP will prepare a timeline and process for updating the recommendations, propose future research directions, and develop a multicentric network for prospective studies, create an international registry of poisonings treated with ECTR and propose standardized data collection methodology and tools to be obtained in poisoned patients that undergo ECTR to improve the quality of scientific reports.
Writing and review process
Format of the recommendation publication. The final format of the recommendation process, for every poison, will be first an introduction (uses of the xenobiotic, toxicokinetics, manifestations of poisoning, epidemiology, natural history of the poisoning, available treatments) followed by the methodology (search strategy, when last accessed, flow diagram). The rationale for the dialyzability will then be presented (grading of dialyzability and level of evidence) with the recommendations (strength of recommendation and level of evidence) with explanations for every poison consisting of a general statement, indications of initiating ECTR, timing of ECTR, cessation of ECTR, choice of ECTR, and prescription of ECTR. Each publication will conclude with comments on future directions (if pertinent), acknowledgement, funding, and conflicts of interest.
Peer review and revision process. A first draft will be written by each subgroup, overviewed by a designated section chief, who will be lead author for the section. This draft will be submitted to the workgroup who will return comments and suggestions. The subgroup will make the necessary modifications and submit a second draft to the workgroup. Co-chairs will ensure integrity and uniformity of statements. The workgroup will then submit drafts to supporting societies and selected individuals for peer review. If necessary, the workgroup will review the proposed publication (the text of the final guidelines remains entirely under the control of the workgroup). Finally, each publication will be submitted to a recognized journal for dissemination.
Conclusion
The EXTRIP workgroup has undertaken the ambitious process of reviewing the current literature and providing recommendations on the use of extracorporeal treatments for a pre-determined set of poisonings. The final product of this worldwide initiative will be supported by the available evidence and consensus-based statements if the evidence is poor. We hope that these guidelines might standardize practice among clinicians and provide a framework of collaboration between physicians and pharmacologists who provide care for poisoned patients.
http://www.informahealthcare.com/doi/abs/10.3109/15563650.2012.683436
Download PDF (263.5 KB)Acknowlegments
We would like to acknowledge the tremendous work of our dedicated translators: Marcela Covica, Alexandra Angulo, Ania Gresziak, Samantha Challinor, Martine Blanchet, Gunel Alpman, Joshua Pepper, Lee Anderson, Andreas Betz, Tetsuya Yamada, Nathalie Eeckhout, Matthew Fisher, Ruth Morton, Denise Gemmellaro, Nadia Bracq, Olga Bogatova, Sana Ahmed, Christiane Frasca, Katalin Fenyvesi, Timothy Durgin, Helen Johnson, Martha Oswald, Ewa Brodziuk, David Young, Akiko Burns, Anna Lautzenheiser, Banumathy Sridharan, Charlotte Robert, Liliana Ionescu, Lucile Mckay, Vilma Etchart, Valentina Bartoli, Nathan Weatherdon, Marcia Neff, Margit Tischler, Sarah Michel, Simona Vairo, Mairi Arbuckle, Luc Ranger, Nerissa Lowe, Angelina White, Salih Topal, John Hartmann, Karine Mardini, Mahala Bartle Mathiassen, Anant Vipat, Gregory Shapiro, Hannele Marttila, Kapka Lazorova. We also acknowledge the important contribution from our librarians and secretarial aids: Marc Lamarre, David Soteros, Salih Topal, Henry Gaston.
Declaration of interests
KA: Advisory board (Merck); MG: Lecturer (Amgen Canada and Janssen-Ortho), advisory boards (Genzyme, Amgen Canada);RM: Consultative work (Baxter Healthcare), Travel costs unrelated to EXTRIP (Roche, Amgen); JK: Advisory board and grant (Fresenius Medical Care); DG: Consultant (Takeda), Honorarium (Genzyme); RM: Grant (Hospira); TB: Guest speaker (Gambro); KL: Stock (Amgen). The remaining authors declare that they have no competing interests.
Funding for EXTRIP was obtained from the industry. These contributions are unrestricted educational grants and are solely used to finance: retrieval and translation of publications travel expenses to EXTRIP meetings for every participant or costs related to venue or conference calls for EXTRIP meeting. Furthermore, the EXTRIP workgroup state that there is no members of the committee from industry, no honorarium offered to expert members, no industry input into scientific content, development, and publication, no industry presence at any meetings, no industry awareness or comment on the recommendations allowed and no honorarium to members of the Poison Panel or Executive committee for any role in the scientific appraisal or meeting organization process. EXTRIP members do not have direct financial relationship with the sponsors. All reimbursement claims require presentation of acceptable expenses invoices to Verdun Hospital Research Fund where financial contributions are deposited. Disclosure of competing interests of members is declared in every publication of EXTRIP. EXTRIP current sponsors are: Leo Pharma (40 000$) Janssen-Ortho (15 000$) Fresenius Canada (15 000$), Amgen Canada (10 000$) Servier (3000$).
References
- Abel JJ, Rowntree LG, Turner BB. On the removal of diffusible substances from the circulating blood by dialysis. Trans Assoc Am Physicians 1913; 58:51–54.
- Kyle LH, Jeghers H, Walsh WP, Doolan PD, Wishinsky H, Pallotta A. The application of hemodialysis to the treatment of barbiturate poisoning. J Clin Invest 1953; 32:364–371. Epub 1953/ 04/01.
- Schreiner GE. The role of hemodialysis (artificial kidney) in acute poisoning. AMA Arch Intern Med 1958; 102:896–913.
- Ghannoum M, Nolin TD, Lavergne V, Hoffman RS. Blood purification in toxicology: nephrology's ugly duckling. Adv Chronic Kidney Dis 2011; 18:160–166. Epub 2011/05/03.
- Bailey B, McGuigan M. Comparison of patients hemodialyzed for lithium poisoning and those for whom dialysis was recommended by PCC but not done: what lesson can we learn? Clin Nephrol 2000; 54:388–392. Epub 2000/12/06.
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, . Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. Epub 2004/06/19.
- Falzon D, Jaramillo E, Schunemann HJ, Arentz M, Bauer M, Bayona J, . WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011; 38:516–528. Epub 2011/08/11.
- Appraisal of Guidelines for Research & Evaluation. AGREE Instrument. London: The AGREE Collaboration; 2001.
- Goldfarb DS. Principles and techniques applied to enhance elimination. In: Nelson LS LN, Howland MA, , editor. Goldfrank's Toxicologic Emergencies, 9th ed. New York, NY: McGraw Hill; 2011: 142.
- Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol 2002; 40:415–446. Epub 2002/09/10.
- Barceloux DG, Krenzelok EP, Olson K, Watson W. American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol 1999;3 7:537–560. Epub 1999/09/25.
- Dargan PI, Wallace CI, Jones AL. An evidence based flowchart to guide the management of acute salicylate (aspirin) overdose. Emerg Med J 2002; 19:206–209. Epub 2002/04/25.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ 2008; 336:995–998. Epub 2008/05/06.
- Holubek WJ, Hoffman RS, Goldfarb DS, Nelson LS. Use of hemodialysis and hemoperfusion in poisoned patients. Kidney Int 2008; 74:1327–1334. Epub 2008/09/19.
- Holubek WJ. (internal data derived from American Association of Poison Control Centers’ National Poison Data System (NPDS)). 2011.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, . Consensus development methods, and their use in clinical guideline development. Health technology assessment 1998;2:i–iv, 1–88. Epub 1998/04/30.
- Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, . The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, CA2011. Available from: http://www.rand.org/.