Abstract
Background: This is the 30th Annual Report of the American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS). As of July 1, 2012, 57 of the nation's poison centers (PCs) uploaded case data automatically to NPDS. The upload interval was 7.58 [6.30, 11.22] (median [25%, 75%]) min, creating a near real-time national exposure and information database and surveillance system.
Methodology: We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center cases with medical outcomes of death were evaluated by a team of 34 medical and clinical toxicologist reviewers using an ordinal scale of 1–6 to assess the Relative Contribution to Fatality (RCF) of the exposure to the death.
Results: In 2012, 3,373,025 closed encounters were logged by NPDS: 2,275,141 human exposures, 66,440 animal exposures, 1,025,547 information calls, 5,679 human confirmed nonexposures, and 218 animal confirmed nonexposures. Total encounters showed a 6.9% decline from 2011, while healthcare facility (HCF) exposure calls increased by 1.2%. All information calls decreased by 14.8% and HCF information calls decreased by 1.7%, medication identification requests (Drug ID) decreased by 22.0%, and human exposures reported to US PCs decreased by 2.5%. Human exposures with less serious outcomes have decreased by 3.7% per year since 2008, while those with more serious outcomes (moderate, major, or death) have increased by 4.6% per year since 2000.
The top five substance classes most frequently involved in all human exposures were analgesics (11.6%), cosmetics/personal care products (7.9%), household cleaning substances (7.2%), sedatives/hypnotics/antipsychotics (6.1%), and foreign bodies/toys/miscellaneous (4.1%). Analgesic exposures as a class increased the most rapidly (8,780 calls/year) over the last 12 years. The top five most common exposures in children aged 5 years or less were cosmetics/ personal care products (13.9%), analgesics (9.9%), household cleaning substances (9.7%), foreign bodies/toys/ miscellaneous (7.0%), and topical preparations (6.3%). Drug identification requests comprised 54.4% of all information calls. NPDS documented 2,937 human exposures resulting in death with 2,576 human fatalities judged related (RCF of 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory).
Conclusions: These data support the continued value of PC expertise and need for specialized medical toxicology information to manage the more severe exposures, despite a decrease in calls involving less severe exposures. Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls. The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response, and situational awareness tracking. NPDS is a model system for the nation and global public health.
WARNING: Comparison of exposure or outcome data from previous AAPCC Annual Reports is problematic. In particular, the identification of fatalities (attribution of a death to the exposure) differed from pre-2006 Annual Reports (see Fatality Case Review—Methods). Poison center death cases are described as all cases resulting in death and those determined to be exposure-related fatalities. Likewise, (Exposure Cases by Generic Category) since year 2006 restricts the breakdown including deaths to single-substance cases to improve precision and avoid misinterpretation.
Introduction
This is the 30th Annual Report of the American Association of Poison Control Centers’ (AAPCC; http://www.aapcc.org) National Poison Data System (NPDS). (Citation1) On January 1, 2012, 57 regional poison centers (PCs) serving the entire population of the 50 United States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands submitted information and exposure case data collected during the course of providing telephonic patient tailored exposure management and poison information.
NPDS is the data warehouse for the nation's 57 PCs. PCs place emphasis on exposure management, accurate data collection and coding, and responding to the continuing need for poison-related public and professional education. The PC's health care professionals are available free of charge to users, 24-hours a day, every day of the year. PCs respond to questions from the public, healthcare professionals, and public health agencies. The continuous staff dedication at the PCs is manifest as the number of exposure and information call encounters exceeds 3.3 million annually. Poison center encounters either involve an exposed human or animal (EXPOSURE CALL) or a request for information with no person or animal exposed to any foreign body, viral, bacterial, venomous, or chemical agent or commercial product (INFORMATION CALL).
The NPDS Products Database
The NPDS products database contains over 400,000 products ranging from viral and bacterial agents to commercial chemical and drug products. The products database is maintained and continuously updated by data analysts at the Micromedex Poisindex® System (Micromedex Healthcare Series [Internet database]. Greenwood Village, CO: Truven Health Analytics. A robust generic coding system categorizes the product data into 1014 generic codes. These generic codes collapse into Nonpharmaceutical (558) and Pharmaceutical (456) groups. These two groups are divided into Major (68) and Minor (176) categories. The generic coding schema undergoes continuous improvement through the work of the AAPCC–Micromedex Joint Coding Group. The group consists of AAPCC members and editorial and lexicon staff working to meet best terminology practices. The generic code system provides enhanced report granularity as reflected in . The following 19 generic codes were introduced in 2012:
Table: Generic Codes Added in 2012.
Because the new codes were added at different times during the year, the numbers in for these generic codes do not reflect the entire year. For completeness certain of these categories require customized data retrieval until these categories have been in place for a year or more.
Methods
Characterization of Participating PCs and Population Served
Fifty-seven participating centers submitted data to AAPCC through December 31, 2012. Fifty-four centers (95%) were accredited by AAPCC as of July 1, 2012. The entire population of the 50 states, American Samoa, the District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands was served by the US PC network in 2012.(Citation2,Citation3)
The average number of human exposure cases managed per day by all US PCs was 6,216. Similar to other years, higher volumes were observed in the warmer months, with a mean of 6,576 cases per day in July compared with 5,583 per day in December. On average, US PCs received a call about an actual human exposure every 13.9 sec.
Call Management—Specialized Poison Exposure Emergency Providers
Most PC operations management, clinical education, and instruction are directed by Managing Directors (most are PharmDs and RNs with American Board of Applied Toxicology [ABAT] board certification). Medical direction is provided by Medical Directors who are board-certified physician medical toxicologists. At some PCs, the Managing and Medical Director positions are held by the same person.
Calls received at US PCs are managed by healthcare professionals who have received specialized training in toxicology and managing exposure emergencies. These providers include medical and clinical toxicologists, registered nurses, doctors of pharmacy, pharmacists, chemists, hazardous materials specialists, and epidemiologists. Specialists in Poison Information (SPIs) are primarily registered nurses, PharmDs, and pharmacists who direct the public to the most appropriate level of care while also providing the most up-to-date management recommendations to healthcare providers caring for exposed patients. They may work under the supervision of a Certified Specialist in Poison Information (CSPI). SPIs must log a minimum of 2,000 calls over a 12-month period to become eligible to take the CSPI examination for certification in poison information. Poison Information Providers are allied healthcare professionals. They manage information-type and low acuity (non-hospital) calls and work under the supervision of a CSPI. Of note is the fact that no nursing or pharmacy school offers a toxicology curriculum designed for PC work and SPIs must be trained in programs offered by their respective PC. PCs undergo a rigorous accreditation process administered by the AAPCC and must be reaccredited every 5 years.
NPDS—Near Real-time Data Capture
Launched on 12 April 2006, NPDS is the data repository for all of the US PCs. In 2012, all 57 US PCs uploaded case data automatically to NPDS. All PCs submitted data in near real-time, making NPDS one of the few operational systems of its kind. Poison center staff record calls contemporaneously in 1 of 4 case data management systems. Each PC uploads case data automatically. The time to upload data for all PCs is 7.58 [6.30, 11.22] (median [25%, 75%]) min creating a real-time national exposure database and surveillance system.
The web-based NPDS software facilitates detection, analysis, and reporting of NPDS surveillance anomalies. System software offers a myriad of surveillance uses allowing AAPCC, its member centers, and public health agencies to utilize NPDS US exposure data. Users are able to access local and regional data for their own areas and view national aggregate data. The application allows for increased “drill-down” capability and mapping via a geographic information system. Custom surveillance definitions are available along with ad hoc reporting tools. Information in the NPDS database is dynamic. Each year the database is locked prior to extraction of annual report data to prevent inadvertent changes and ensure consistent, reproducible reports. The 2012 database was locked on 24 October 2013 at 17:24 EDT.
Annual Report Case Inclusion Criteria
The information in this report reflects only those cases that are not duplicates and classified by the PC as CLOSED. A case is closed when the PC has determined that no further follow-up/recommendations are required or no further information is available. Exposure cases are followed to obtain the most precise medical outcome possible. Depending on the case specifics, most calls are “closed” within a few hours of the initial call. Some calls regarding complex hospitalized patients or cases resulting in death may remain open for weeks or months while data continues to be collected. Follow-up calls provide a proven mechanism for monitoring the appropriateness of management recommendations, augmenting patient guidelines, and providing poison prevention education, enabling continual updates of case information as well as obtaining final/known medical outcome status to make the data collected as accurate and complete as possible.
Statistical Methods
All Tables except and were generated directly by the NPDS web-based application and can thus be reproduced by each center. The figures and statistics in and were created using SAS JMP version 9.0.0 (SAS Institute, Cary, NC) on summary counts generated by the NPDS web-based application.
NPDS Surveillance
As previously noted, all of the active US PCs upload case data automatically to NPDS. This unique near real-time upload is the foundation of the NPDS surveillance system. This makes possible both spatial and temporal case volume and case-based surveillance. NPDS software allows creation of volume and case-based definitions. Definitions can be applied to national, regional, state, or ZIP code coverage areas. Geocentric definitions can also be created. This functionality is available not only to the AAPCC surveillance team, but also to every PC. PCs also have the ability to share NPDS real-time surveillance technology with external organizations such as their state and local health departments or other regulatory agencies. Another NPDS feature is the ability to generate system alerts on adverse drug events and other drug or commercial products of public health interest like contaminated food or product recalls. Thus NPDS can provide real-time adverse event monitoring and surveillance for resilience response and situational awareness.
Surveillance definitions can be created to monitor a variety of volume parameters or case-based definitions on any desired substance or commercial product in the Micromedex Poisindex products database and/or set of clinical effects or other parameters. The products database contains over 400,000 entries. Surveillance definitions may be constructed using volume or case-based definitions with a variety of mathematical options and historical baseline periods from 1 to 13 years. NPDS surveillance tools include the following:
Volume Alert Surveillance Definitions
Total Call Volume
Human Exposure Call Volume
Animal Exposure Call Volume
Information Call Volume
Clinical Effects Volume (signs and symptoms, or laboratory abnormalities)
Case-Based Surveillance Definitions utilizing various NPDS data fields linked in Boolean expressions
○ Substance
○ Clinical Effects
○ Species
○ Medical Outcome and others
Incoming data is monitored continuously and anomalous signals generate an automated email alert to the AAPCC's surveillance team or designated PC or public health agency staff. These anomaly alerts are reviewed daily by the AAPCC surveillance team, the PC, or the public health agency that created the surveillance definition. When reports of potential public health significance are detected, additional information is obtained via the NPDS surveillance correspondence system or phone as appropriate from reporting PCs. The PC then alerts their respective state or local health departments. Public health issues are brought to the attention of the Health Studies Branch, National Center for Environmental Health, Centers for Disease Control and Prevention (HSB/NCEH/CDC). This unique near real-time tracking ability is a unique feature offered by NPDS and the PCs.
AAPCC Surveillance Team clinical and medical toxicologists review surveillance definitions on a regular basis to fine-tune the queries. CDC, as well as State and local health departments with NPDS access as granted by their respective PCs, also have the ability to create surveillance definitions for routine surveillance tasks or to respond to emerging public health events.
Fatality Case Review and Abstract Selection
NPDS fatality cases can be recorded as DEATH or DEATH (INDIRECT REPORT). Medical outcome of death is by direct report. Deaths (indirect reports) are deaths that the PC acquired from medical examiners or media, but did not manage nor answer any questions related specifically to that death.
Although PCs may report death as an outcome, the death may not be the direct result of the exposure. We define exposure-related fatality as a death judged by the AAPCC Fatality Review Team to be at least contributory to the exposure. The definitions used for the Relative Contribution to Fatality (RCF) classification are defined in Appendix B and the methods to select abstracts for publications is described in Appendix C. For details of the AAPCC fatality review process, see the 2008 annual report.(Citation1)
Pediatric Fatality Case Review
A focused Pediatric Fatality Review team, comprised of four pediatric toxicologists, evaluated cases in patients under 18 years of age. The panel reviewed the documentation of all such cases, with specific focus on the conditions behind the poisoning exposure and on finding commonality which might inform efforts at prevention. The pediatric fatality cases reviewed exhibited a bimodal age distribution. Exposures causing death in children ≤ 5 years of age were mostly coded as “Unintentional-General”, while those in ages over 12 years were mostly “Intentional”. Often the Reason Code did not capture the complexities of the case. For example, there were few mentions of details such as the involvement of law enforcement or child protective services. While there were some complete and informative reports, in many narratives the circumstances which preceded the exposure thought responsible for the death were unclear or absent. In response to these findings, the pediatric fatality review team developed and distributed Pediatric Narrative Guidelines, with specific attention to the root cause of these cases. PCs are requested to heed these guidelines and the need for a more in-depth investigation of “causality.”
RESULTS
Information Calls to PCs
Data from 1,025,547 information calls to PCs in 2012 () was transmitted to NPDS, including calls in optional reporting categories such as prevention/safety/ education (28,019), administrative (28,638), and caller referral (52,061).
shows that All Drug ID calls decreased dramatically in mid 2009, again in late 2010 and late 2011 and continue to decrease in 2012. Law enforcement Drug ID Calls also showed a decline. The most frequent information call was for Drug ID, comprising 558,117 calls to PCs during the year. Of these, 328,858 (58.9%) were identified as drugs with known abuse potential; however, these cases were categorized based on the drug's abuse potential without knowledge of whether abuse was actually intended.
While the number of Drug Information calls decreased by 17.0% from 2011 (173,904 calls) to 2012 (144,267 calls), the distribution of these call types remained steady at 14.5% and 14.1%, respectively, of all information request calls. The most common drug information requests were in regards to therapeutic use and indications, followed by drug–drug interactions, questions about dosage, and inquiries of adverse effects. Environmental inquiries comprised 2.1% of all information calls. Of these environmental inquiries, questions related to cleanup of mercury (thermometers and other) remained the most common followed by questions involving pesticides.
Of all the information calls, poison information comprised 6.2% of the requests with inquiries involving general toxicity the most common followed by questions involving food preparation practices, safe use of household products, and plant toxicity.
Exposure Calls to PCs
In 2012, the participating PCs logged 3,373,025 total encounters including 2,275,141 closed human exposure cases (), 66,440 animal exposures (), 1,025,547 information calls (), 5,679 human confirmed non-exposures, and 218 animal confirmed non-exposures. An additional 574 calls were still open at the time of database lock. The cumulative AAPCC database now contains almost 58 million human exposure case records (). A total of 15,586,479 information calls have been logged by NPDS since the year 2001.
Table 1A. AAPCC Population Served and Reported Exposures (1983–2012).
Table 1B. Non-Human Exposures by Animal Type.
Table 1C. Distribution of Information Calls.
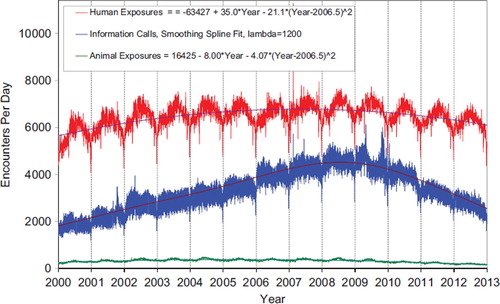
shows the human exposures, information calls, and animal exposures by day since January 1, 2001. Second order (quadratic) least squares regression of these data shows a statistically significant departure from linearity (declining rate of calls since mid 2007) for Human Exposure Calls. Information Calls are declining more rapidly than the quadratic regression this year, best described by a smoothing spline fit, and Animal Exposure Calls have likewise been declining since mid-2005.
Fig. 1. Human Exposure Calls, Information Calls, and Animal Exposure Calls by Day since January 1, 2000 Regression lines show least-squares second order regression—both linear and second order (quadratic) terms were statistically significant for Human Exposures and Animal Exposures (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
A hallmark of PC case management is the use of follow-up calls to monitor case progress and medical outcome. US PCs made 2,702,081 follow-up calls in 2012. Follow-up calls were done in 46.2% of human exposure cases. One follow-up call was made in 22.4% of human exposure cases, and multiple follow-up calls (range 2–80) were placed in 23.8% of cases.
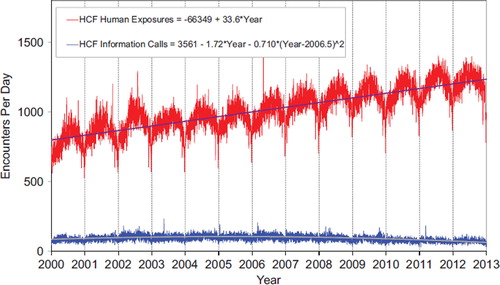
shows a graphic summary and analyses of HCF Exposure and HCF Information calls. HCF Exposure Calls did not depart from linearity (continued to increase at a steady rate), while the rate of HCF Information Calls has been declining since early 2005. This linearly increasing use of the PCs for the more serious exposures (HCF calls) is important in the face of the declining growth of all exposure and information calls. The May 2, 2006 exposure data spike on the Figure was the result of 602 children in a Midwest school reporting a noxious odor which caused anxiety, but resolved without sequelae.
Fig. 2. All Drug Identification and Law Enforcement Drug Identification Calls by Day since January 1, 2000 Smoothing Spline Fits were better than 2nd order regressions, R-Square = 0.796 for All Drug Identification Calls, R-Square = 0.632 Law Enforcement Drug ID Calls (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
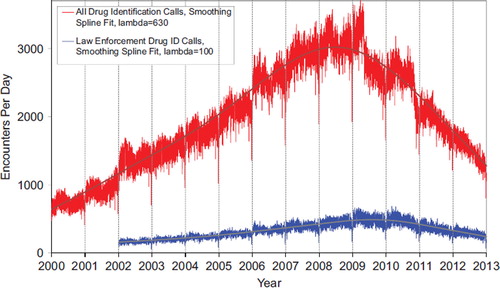
Fig. 3. HCF Exposure Calls and HCF Information Calls by Day since January 1, 2000 Regression lines show least-squares first and second order regressions—linear regression for HCF Exposure Calls (second order term was not statistically significant) and second order regression for HCF Information Calls. All terms shown were statistically significant for each of the two regressions (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
(Nonpharmaceuticals) and 22B (Pharmaceuticals) provide summary demographic data on patient age, reason for exposure, medical outcome, and use of a HCF for all 2,275,141 human exposure cases, presented by substance categories.
Column 1: Name of the major, minor generic categories, and their associated generic codes.
Column 2: No. of Case Mentions (all exposures) in gray shading, displays the number of times the specific generic code was reported in all human exposure cases. If a human exposure case has multiple instances of a specific generic code it is only counted once.
Column 3: No. of Single Exposures This column was previously named ‘No. of `Single Exposures’ and was renamed in the 2009 report for clarity. This column displays the number of human exposure cases that identified only one substance (one case, one substance).
The succeeding columns (Age, Reason, Treatment Site, and Outcome) show selected detail from these single-substance exposure cases. Death cases include both cases that have the outcome of Death or Death (indirect report). These death cases are not limited by the relative contribution to fatality.
and 22B restrict the breakdown columns to single-substance cases. Prior to 2007, when multi-substance exposures were included, a relatively innocuous substance could be mentioned in a death column when, for example, the death was attributed to an antidepressant, opioid, or cyanide. This subtlety was not always appreciated by the user of this Table. The restriction of the breakdowns to single-substance exposures should increase precision and reduce misrepresentation of the results in this unique by-substance Table. Single-substance cases reflect the majority (89.4%) of all exposures. In contrast, only 41.9% of fatalities are single-substance exposures ().
and 22B tabulate 2,662,456 substance- exposures, of which 2,032,956 were single-substance exposures, including 1,052,906 (51.8%) nonpharmaceuticals and 980,050 (48.2%) pharmaceuticals. In 19.2% of single-substance exposures that involved pharmaceutical substances, the reason for exposure was intentional, compared with only 3.7% when the exposure involved a nonpharmaceutical substance. Correspondingly, treatment in a HCF was provided in a higher percentage of exposures that involved pharmaceutical substances (29.3%) compared with nonpharmaceutical substances (15.7%). Exposures to pharmaceuticals also had more severe outcomes. Of single-substance exposure-related fatal cases, 761 (69.6%) were pharmaceuticals compared with 333 (30.4%) nonpharmaceuticals.
Age and Gender Distributions
The age and gender distribution of human exposures is outlined in . Children younger than 3 years of age were involved in 35.7% of exposures and children younger than 6 years accounted for approximately half of all human exposures (48.4%). A male predominance was found among cases involving children younger than 13 years, but this gender distribution was reversed in teenagers and adults, with females comprising the majority of reported exposures.
Caller Site and Exposure Site
As shown in , of the 2,275,141 human exposures reported, 72.5% of calls originated from a residence (own or other) but 93.6% actually occurred at a residence (own or other). Another 19.5% of calls were made from a healthcare facility. Beyond residences, exposures occurred in the workplace in 1.6% of cases, schools (1.3%), healthcare facilities (0.3%), and restaurants or food services (0.2%).
Table 2. Site of Call and Site of Exposure, Human Exposure Cases.
Table 3A. Age and Gender Distribution of Human Exposures.
Table 3B. Population-Adjusted Exposures by Age Group.
Exposures in Pregnancy
Exposure during pregnancy occurred in 7,671 women (0.3% of all human exposures). Of those with known pregnancy duration (n = 7,149), 31.5% occurred in the first trimester, 37.1% in the second trimester, and 31.4% in the third trimester. Most of them (74.1%) were unintentional exposures and 19.5% were intentional exposures. There were four deaths in pregnant females in 2012.
Chronicity
Most human exposures, 2,006,316 (88.2%) were acute cases (single, repeated, or continuous exposure occurring over 8 hours or less) compared with 559 acute cases of 2,937 fatalities (19.0%). Chronic exposures (continuous or repeated exposures occurring over > 8 hours) comprised 2.1% (47,407) of all human exposures. Acute-on-chronic exposures (single exposure that was preceded by a continuous, repeated, or intermittent exposure occurring over a period greater than 8 hours) numbered 189,737 (8.3%).
Reason for Exposure
The reason category for most human exposures was unintentional (80.1%) with unintentional general (54.9%), therapeutic error (12.3%), and unintentional misuse (5.5%) of all exposures ().
Table 4. Distribution of Agea and Gender for Fatalitiesb.
Table 5. Number of Substances Involved in Human Exposure Cases.
Table 6A. Reason for Human Exposure Cases.
Scenarios
Of the total 296,666 therapeutic errors, the most common scenarios for all ages included inadvertent double-dosing (28.7%), wrong medication taken or given (15.7%), other incorrect dose (13.6%), doses given/taken too close together (9.7%), and inadvertent exposure to someone else's medication (8.3%). The types of therapeutic errors observed are different for each age group and are summarized in .
Table 6B. Scenarios for Therapeutic Errorsa by Ageb.
Reason by Age
Intentional exposures accounted for 16.0% of human exposures. Suicidal intent was suspected in 9.9% of cases, intentional misuse in 2.6% and intentional abuse in 2.5%. Unintentional exposures outnumbered intentional exposures in all age groups with the exception of ages 13– 19 years (). Intentional exposures were more frequently reported than unintentional exposures in patients aged 13–19 years. In contrast, of the 1,190 reported fatalities with RCF 1–3, the majority reason reported for children ≤ 5 years was unintentional, while most fatalities in adults (> 20 years) were intentional ().
Table 7. Distribution of Reason for Exposure by Age.
Table 8. Distribution of Reason for Exposure and Age for Fatalitiesa.
Route of Exposure
Ingestion was the route of exposure in 83.4% of cases (), followed in frequency by dermal (7.0%), inhalation/nasal (6.0%), and ocular routes (4.3%). For the 1,190 exposure-related fatalities, ingestion (84.5%), inhalation/nasal (8.6%), and parenteral (5.0%) were the predominant exposure routes. Each exposure case may have more than one route.
Table 9. Route of Exposure for Human Exposure Cases.
Clinical Effects
The NPDS database allows for the coding of up to 131 individual clinical effects (signs, symptoms, or laboratory abnormalities) for each case. Each clinical effect can be further defined as related, not related, or unknown if related. Clinical effects were coded in 841,775 (37.0%) cases (18.0% had 1 effect, 9.5% had 2 effects, 5.1% had 3 effects, 2.2% had 4 effects, 1.0% had 5 effects, and 1.2% had > 5 effects coded). Of clinical effects coded, 78.5% were deemed related to the exposure, 9.7% were considered not related, and 11.8% were coded as unknown if related.
Case Management Site
The majority of cases reported to PCs were managed in a nonHCF (69.2%), usually at the site of exposure, primarily the patient's own residence (). 1.6% of cases were referred to a HCF but refused referral. Treatment in a HCF was rendered in 27.0% of cases.
Table 10. Management Site of Human Exposures.
Of the 613,412 cases managed in a healthcare facility, 291,414 (47.5%) were treated and released, 100,455 (16.4%) were admitted for critical care, and 67,847 (11.1%) were admitted to a noncritical care unit.
The percentage of patients treated in a HCF varied considerably with age. Only 11.6% of children ≤ 5 years and only 14.0% of children between 6 and 12 years were managed in a HCF compared with 51.2% of teenagers (13–19 years) and 37.9% of adults (age ≥ 20 years).
Medical Outcome
displays the medical outcome of human exposure cases distributed by age. Older age groups exhibit a greater number of severe medical outcomes. compares medical outcome and reason for exposure and shows a greater frequency of serious outcomes in intentional exposures.
Table 11. Medical Outcome of Human Exposure Cases by Patient Age.a
Table 12. Medical Outcome by Reason for Exposure in Human Exposuresa.
The duration of effect is required for all cases which report at least one clinical effect and have a medical outcome of minor, moderate, or major effect (n = 515,287; 22.6% of exposures). demonstrates an increasing duration of the clinical effects observed with more severe outcomes.
Table 13. Duration of Clinical Effects by Medical Outcome.
Decontamination Procedures and Specific Antidotes
and outline the use of decontamination procedures, specific physiological antagonists (antidotes), and measures to enhance elimination in the treatment of patients reported in the NPDS database. These should be interpreted as minimum frequencies because of the limitations of telephonic data gathering.
Table 14. Decontamination and Therapeutic Interventions.
Table 15. Therapy Provided in Human Exposures by Age.
Ipecac-induced emesis for poisoning continues to decline as shown in and . Ipecac was administered in only 83 (0.01%) pediatric exposures in 2012. The continued decrease in ipecac syrup use over the last 2 decades was likely a result of ipecac use guidelines issued in 1997 by the American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists and updated in 2004. (Citation5,Citation6) In a separate report, the American Academy of Pediatrics concluded not only that ipecac should no longer be used routinely as a home treatment strategy, but also recommended disposal of home ipecac stocks.(Citation7) A decline was also observed since the early 1990s for reported use of activated charcoal. While not as dramatic as the decline in use of ipecac, reported use of activated charcoal decreased from 3.7% of pediatric cases in 1993 to just 1.0% in 2012.
Table 16A. Decontamination Trends (1985–2012).
Table 16B. Decontamination Trends: Total Human and Pediatric Exposures ≤ 5 Yearsa.
Top Substances in Human Exposures
presents the most common 25 substance categories, listed by frequency of human exposure. This ranking provides an indication where prevention efforts might be focused, as well as the types of exposures PCs regularly manage. It is relevant to know whether exposures to these substances are increasing or decreasing.
Table 17A. Substance Categories Most Frequently Involved in Human Exposures (Top 25).
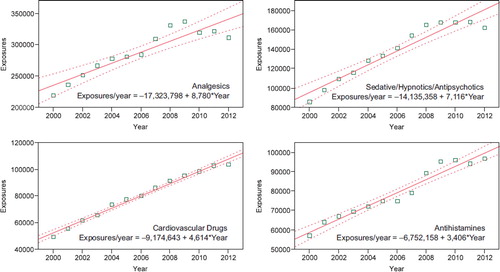
To better understand these relationships, we examined exposures per year over the last 12 years for the change over time for each of the 68 major generic categories via least squares linear regression. The exposure calls per year over this period were increasing for 37 and decreasing for 31 of the 68 categories. The change over time for the 12 yearly values was statistically significant (p < 0.05) for 53 of the 68 categories. shows the 25 categories which were increasing the most rapidly. Statistical significance of the linear regressions can be verified by noting the 95% confidence interval on the rate of increase excludes zero for all but 2 of the 25 categories. shows the linear regressions for the top four increasing categories in .
Table 17B. Substance Categories with the Greatest Rate of Exposure Increase (Top 25).
and present exposure results for children and adults, respectively, and show the differences between substance categories involved in pediatric and adult exposures.
Table 17C. Substance Categories Most Frequently Involved in Pediatric (≤ 5 years) Exposures (Top 25).a
Table 17D. Substance Categories Most Frequently Involved in Adult (≤ 20 years) Exposures (Top 25).a
reports the 25 categories of substances most frequently involved in pediatric (≤ 5 years) fatalities in 2012.
Table 17E. Substance Categories Most Frequently Involved in Pediatric (≤ 5 years) Deaths.a
reports the 25 Drug ID categories most frequently queried in 2012. Unknown is the 5th and Miscellaneous the 18th most often identified drug category. These categories include medications which could not be identified, indicating the value of Drug ID information to the AAPCC, public health, public safety, and regulatory agencies. Internet-based resources do not afford the caller the option to speak with a healthcare professional if needed. Proper resources to continue this vital public service are essential, especially since the top 10 substance categories include antibiotics as well as drugs with widespread use and abuse potential such as opioids and benzodiazepines.
Table 17F. Substance Categories Most Frequently Identified in Drug Identification Calls (Top 25).
reports the 25 substance categories most frequently reported in exposures involving pregnant patients.
Table 17G. Substance Categories Most Frequently Involved in Pregnant Exposuresa (Top 25).
Changes Over Time
Total encounters peaked in 2008 at 4,333,012 calls with 2,491,049 human exposure calls and 1,703,762 information calls. Total encounters decreased by 6.9% from 3,624,063 in 2011 to 3,373,025 in 2012. Information calls decreased by 14.8% from 1,203,282 calls in 2011 to 1,025,547 in 2012, with a 22.0% decrease in drug identification calls and a 1.7 % decrease in HCF information calls. Human exposures decreased by 2.5% from 2,334,004 to 2,275,141 cases.
shows the year-to-year change through 2000 as a percentage of year 2000 for human exposure calls broken down into cases with more serious outcomes (death, major effect, and moderate effect) and less serious outcomes (minor effect, no effect, not followed (non-toxic), not followed (minimal toxicity possible), unable to follow (potentially toxic), and unrelated effect). Since 2000, cases with more serious outcomes have increased by + 4.6% (95% CI [4.2%, 5.1%]) per year from 108,148 cases in 2000 to 170,956 cases in 2012. However, cases with less serious outcomes have consistently decreased since 2008 by 3.7% (95% CI [− 5.3%, − 2.1%]) per year from 2,339,460 in 2008 to 2,102,755 cases in 2012. This has driven the overall decrease in human exposures since 2008.
Fig. 4. Change in Encounters by Outcome from 2008 to 2012. The Figure shows the percent change from baseline for Human Exposure Calls divided among the 10 Medical Outcomes. The More Serious Exposures (Major, Moderate, and Death) increased. The Less Serious Exposures (no effect, minor effect, not followed (non-toxic), not followed (minimal toxicity possible), unable to follow (potentially toxic), and unrelated effect) decreased after 2008. Solid lines show least-squares linear regressions for the change in More Serious Exposures per year (□) and Less Serious Exposures (○). Broken lines show 95% confidence interval on the regression (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
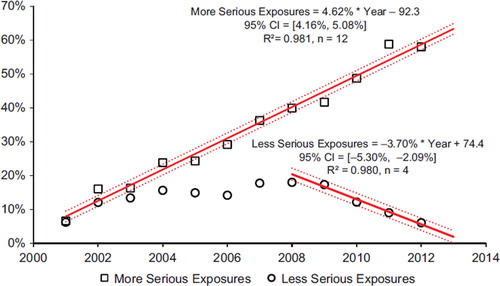
Fig. 5. Substance Categories with the Greatest Rate of Exposure Increase (Top 4). Solid lines show least-squares linear regressions for the Human Exposure Calls per year for that category (□). Broken lines show 95% confidence interval on the regression (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
Thus we see a consistent increase in exposure calls from HCFs () and for the more severe exposures (), despite a decrease in calls involving less severe exposures.
Distribution of Suicides
shows the modest variation in the distribution of suicides and pediatric deaths over the past 2 decades as reported to the NPDS national database. Within the last decade, the percent of exposures determined to be suspected suicides ranged from 30.3% to 53.9% and the percent of pediatric cases has ranged from 1.5% to 3.2%. The relatively large change seen for 2012 reflects the large increase in death (indirect reports) this year. Analyses of suicides and pediatric deaths for Direct and Indirect reports are shown in .
Table 18. Categories Associated with Largest Number of Fatalities (Top 25).a
Table 19A. Comparisons of Death Data (1985–2012).a
Table 19B. Comparisons of Direct and Indirect Death Data (2000–2012).a
Plant Exposures
provides the number of times the specific plant was reported to NPDS (N = 49,374). The 25 most commonly involved plant species and categories account for 39.7% of all plant exposures reported. The top three categories in the Table are essentially synonymous for unknown plant and comprise 12.2% (6,018/49,374) of all plant exposures. For a variety of reasons it was not possible to make a precise identification in these three groups. The top most frequent plant exposures where a positive plant identification was made were (descending order): Phytolacca americana (L.) (Botanic name), Spathiphyllum species (Botanic name), Ilex species (Botanic name), Philodendron species (Species unspecified), and Malus species (Botanical name).
Table 20. Frequency of Plant Exposures (Top 25).a
Deaths and Exposure-related Fatalities
A listing of cases () and summary of cases (, , , , , and ) are provided for fatal cases for which there exists reasonable confidence that the death was a result of that exposure (exposure-related fatalities). , , and 19 list all deaths, irrespective of the RCF. Beginning in 2010, cases with outcome of Death (Indirect Report) were not further reviewed by the AAPCC fatality review team and the RCF was determined by the individual PC review team.
Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures.
Table 22A. Demographic profile of SINGLE-SUBSTANCE Nonpharmaceuticals exposure cases by generic category.
Table 22B. Demographic profile of SINGLE-SUBSTANCE Pharmaceuticals exposure cases by generic category.
There were 1,430 deaths (indirect) and 1,507 deaths. Of these 2,937 cases, 2,576 were judged exposure-related fatalities (RCF = 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory). The remaining 361 cases were judged as follows: 79 as RCF = 4-Probably not responsible, 36 as 5-Clearly not responsible, and 246 as 6-Unknown.
Deaths are sorted in according to the category, then substance deemed most likely responsible for the death (Cause Rank), and then by patient age. The Cause Rank permits the PC to judge two or more substances as indistinguishable in terms of cause, for example, two substances which appear equally likely to have caused the death could have Substance Rank of 1,2 and Cause Rank of 1,1. Additional agents implicated are listed below the primary agent in the order of their contribution to the fatality.
As shown in , a single substance was implicated in 89.4% of reported human exposures, and 10.6% of patients were exposed to 2 or more drugs or products. The exposure-related fatalities involved a single substance in 498 cases (41.9%), 2 substances in 290 cases (24.4%), 3 in 166 cases (14.0%), and 4 or more in the balance of the cases.
In , the Annual Report ID number [bracketed] indicates that the abstract for that case is included in Appendix C. The letters following the Annual Report ID number indicate: i = Death (Indirect report) (occurred in 1,386, 53.8% of cases), p = prehospital cardiac and/or respiratory arrest (occurred in 423 of 2,576, 16.4% of cases), h = hospital records reviewed (occurred in 431, 16.7% of cases), a = autopsy report reviewed (occurred in 1,733, 67.3% of cases). The distribution of NPDS RCF was 1 = Undoubtedly responsible in 569 cases (22.1%), 2 = Probably responsible in 1,805 cases (70.1%), 3 = Contributory in 202 cases (7.8%). The denominator for these percentages in is 2,576.
All fatalities—all ages
presents the age and gender distribution for these 1,190 exposure-related fatalities (excluding death (indirect)). The age distribution of reported fatalities is similar to that in past years with 73 (6.1%) of the fatalities in children (< 20 years old), 1,114 of 1,190 (93.6%) of fatal cases occurring in adults (age ≥ 20 years) and 3 (0.3%) of fatalities occurring in Unknown Age patients. Although children ≤ 5 years old were involved in the majority of exposures, the 21 fatalities comprised just 1.8% of the exposure-related fatalities. Most (70.0%) of the fatalities occurred in 20–59-year-old individuals.
lists each of the 2,576 human fatalities (including death (indirect report)) along with all of the substances involved for each case. Please note: the substance listed in Column 3 of (alternate name) was chosen to be the most specific generic name based upon the Micromedex Poisindex product name and generic code selected for that substance. Alternate names are maintained in the NPDS for each substance involved in a fatality. The cross-references at the end of each major category section in list all cases that identify this substance as other than the primary substance. This Alternate name may not agree with the AAPCC generic categories used in the summary Tables (including ).
lists the top 25 minor generic substance categories associated with reported fatalities and the number of single-substance exposure fatalities for that category—miscellaneous sedative/hypnotics/antipsychotics, miscellaneous cardiovascular drugs, opioids, and acetaminophen combination products, lead this list followed by miscellaneous stimulants and street drugs, acetaminophen alone, miscellaneous alcohols, miscellaneous antidepressants, and selective serotonin reuptake inhibitors (SSRI). Note that is sorted by all substances to which a patient was exposed (i.e., a patient exposed to an opioid may have also been exposed to 1 or more other products) and shows single-substance exposures in the right hand column.
The first ranked substance () was a pharmaceutical in 2,142 (83.2%) of the 2,576 fatalities. These 2,142 first ranked pharmaceuticals included the following:
946 analgesics (178 methadone, 138 oxycodone, 133 acetaminophen/hydrocodone, 103 acetaminophen, 102 morphine, 53 fentanyl, 49 salicylate, 33 tramadol, and 24 acetaminophen/oxycodone)
559 stimulants/street drugs (325 heroin, 90 methamphetamine, 87 cocaine, and 14 amphetamines (hallucinogenic))
182 cardiovascular drugs (33 amlodipine, 21 metoprolol, 20 verapamil, 16 diltiazem (extended release), 13 diltiazem, 12 cardiac glycoside, and 10 atenolol)
149 antidepressants (38 amitriptyline, 19 bupropion, 14 citalopram, 12 bupropion (extended release), 11 doxepin, and 9 venlafaxine)
110 sedative/hypnotic/antipsychotics (35 quetiapine, 27 alprazolam, 9 benzodiazepine, 8 clonazepam, 7 diazepam, and 5 pentobarbital)
The exposure was acute in 1,467 (56.9%), A/C = acute on chronic in 260 (10.1%), C = chronic exposure in 87 (3.4%), and U = unknown in 762 (29.6%).
A total of 1,247 tissue concentrations for 1 or more related analytes were reported in 554 cases. Most of these (1,178) involved fatalities with RCF 1–3, and are listed in , while all tissue concentrations are available to the member centers through the NPDS Enterprise Reports. These 122 analytes included 212 acetaminophen, 93 ethanol, 82 salicylate, 35 morphine, 32 carboxyhemoglobin, 28 alprazolam, 28 diphenhydramine, 26 methadone, 24 ethylene glycol, 21 nordiazepam, 21 valproic acid, 20 bupropion, 20 diazepam, and 19 oxycodone.
Route of exposure was ingestion only in 1,511 cases (58.7%), parenteral in 104 cases (4.0%), and inhalation/nasal in 128 cases (5.0%). Most other routes were combination routes or unknown.
The Intentional exposure reason was suspected suicide in 786 cases (30.5%), abuse in 1,196 cases (46.4%), and misuse in 52 cases (2.0%). Unintentional exposure reason was environmental in 84 cases (3.3%), therapeutic error in 18 cases (0.7%), and misuse in 52 cases (2.0%). Adverse drug reaction was the reason in 45 (1.7%).
Pediatric fatalities—age ≤ 5 years
Although children younger than 6 years were involved in the majority of exposures, they comprised 46 of 2,937 (1.6%) of fatalities. These numbers are similar to those reported since 1985 (, all RCFs, and includes indirect deaths). (RCF 1–3, excludes indirect deaths) shows the percentage fatalities in children ≤ 5 years related to total pediatric exposures was 21/1,102,307 = 0.00191%. By comparison, 1,114/859,742 = 0.13% of all adult exposures involved a fatality. Of these 21 pediatric fatalities, 15 (71.4%) were reported as unintentional and 3 (14.3%) were coded as resulting from malicious intent ().
The 34 fatalities in children ≤ 5 years old in (includes death, indirect reports, and RCF 1–3) included 19 pharmaceuticals and 15 nonpharmaceuticals. The first ranked substances associated with these fatalities included smoke (7), antifreeze (ethylene glycol) (2), carbon monoxide (2), disc battery (2), lithium (2), morphine (2), and tramadol (2), and 15 other substances (1 each).
Pediatric fatalities—ages 6–12 years
In the age range 6–12 years, there were 7 reported fatalities, 4 of which were unintentional environmental, 1 was unintentional therapeutic error, 1 was intentional suspected suicide, and 1 unknown reason (). The 9 fatalities listed in (includes death, indirect reports, and RCF 1–3) included 3 carbon monoxide, 1 acetaminophen, 1 methadone, and 1 salicylate.
Adolescent fatalities—ages 13–19 years
In the age range 13–19 years, there were 45 reported fatalities including 38 intentional, 3 unintentional, and 4 unknown reason (). The 81 fatalities listed in (includes death, indirect reports, and RCF 1–3) included 71 pharmaceuticals and 10 nonpharmaceuticals. The first ranked pharmaceuticals associated with these fatalities included methadone (8 cases), heroin (6 cases), alprazolam (4 cases), oxycodone (4 cases), acetaminophen/hydrocodone (3 cases), morphine (3 cases), salicylate (3 cases), acetaminophen (2 cases), bupropion (2 cases), bupropion (extended release) (2 cases), drug unknown (2 cases), methamphetamine (2 cases), oxymorphone (2 cases), phenylethylamine (2 cases), quetiapine (2 cases), and the balance 1 substance each. The first ranked nonpharmaceuticals associated with these fatalities included carbon monoxide (3 cases), freon (2 cases), smoke (2 cases), ethanol (1 case), methanol (1 case), and selenous acid (1 case).
Pregnancy and Fatalities
A total of 30 deaths of pregnant women have been reported from the years 2000 through 2012. The majority (26 of 30) were intentional exposures (misuse, abuse, or suspected suicide). There were four deaths in pregnant women reported to NPDS in 2012.
AAPCC Surveillance Results
A key component of the NPDS surveillance system is the variety of monitoring tools available to the NPDS user community. In addition to AAPCC national surveillance definitions, 35 PCs utilize NPDS as part of their surveillance programs. Six state health departments plus CDC run surveillance definitions in NPDS. Since Surveillance Anomaly 1, generated at 2:00 pm EDT on 17 September 2006, over 210,000 anomalies have been detected. More than 1100 were confirmed as being of public health significance with PCs working collaboratively with their local and state health departments and in some instances CDC on the public health issues identified.
At the time of this report, 354 surveillance definitions run continuously, monitoring case and clinical effects volume and a variety of case-based definitions from food poisoning to nerve agents. These definitions represent the surveillance work by many PCs, state health departments, the AAPCC, and the Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, CDC.
Automated surveillance continues to remain controversial as a viable methodology to detect the index case of a public health event. Uniform evaluation algorithms are not available to determine the optimal methodologies.(Citation8) Less controversial is the benefit to situational awareness that NPDS can provide.(Citation9) Typical NPDS surveillance data detects a response to an event rather than event prediction. This aids in situational awareness and resilience during and after a public health event.
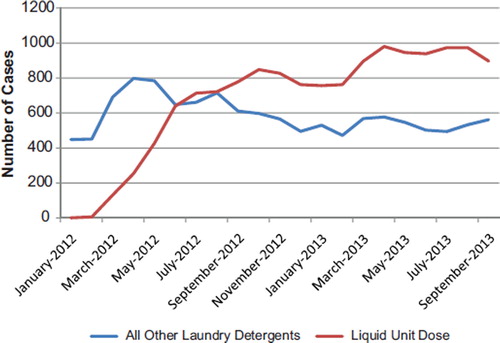
A current example of the involvement of the PC system and NPDS can be seen in the following. In February 2012, manufacturers began commercial distribution of unit dose liquid laundry detergent packets in the US. On May 4, 2012, a PC medical director reported two cases of unusual toxicity in toddlers with unit dose liquid laundry detergent packets exposures. From May 15–18, 2012, a number of other PCs reported additional cases of unusual toxicity. On May 17, 2012, AAPCC issued a press release and activated a temporary code to better capture the number of unit dose liquid laundry detergent packet cases using NPDS. In a May 23, 2012, ABC national news story on unit dose liquid laundry detergent toxicity in children using PC data, the manufacturer of one of the products announced a new child-proof container was planned. The American Cleaning Institute then partnered with AAPCC in June 2012 on a national campaign on the safe use of unit dose liquid laundry detergent packets.
A dramatic and sustained increase in number of calls about children exposed to unit dose liquid laundry detergent packets as a single substance has been noted and continues into 2013, while calls concerning all other laundry detergents have dropped (). The severity of exposures to unit dose liquid laundry detergent products as a single substance appears to be higher than older laundry formulations with, a 5-fold increase in major outcomes and a 2-fold increase in moderate outcomes. Some of the clinical effects showing increased frequency in these cases were esophageal injury, coma, respiratory depression, respiratory arrest, acidosis, dyspnea, and bronchospasm. Please note that the data for 2013 are considered preliminary because it is possible that a PC may update a case anytime during the year before the year is locked, if new information is obtained.
Fig. 6. Unit Dose Liquid Laundry Detergent Exposures, Jan 2012—Sep 2013. The Figure shows the number of calls received for single-substance human pediatric poison exposure calls to Unit Dose Liquid Laundry Detergents (![]()
Discussion
The exposure cases and information requests reported by PCs in 2012 do not reflect the full extent of PC efforts which also include poison prevention activities and public and healthcare professional education programs.
NPDS exposure data may be considered as providing “numerator data”, in the absence of a true denominator, that is, we do not know the number of actual exposures that occur in the population. NPDS data covers only those exposures which are reported to PCs.
NPDS 2000–2012 call volume data clearly demonstrate a continuing decrease in exposure calls. This decline has been apparent and increasing since mid-2007 and reflects the decreasing use of the PC for less severe exposures. However, in contrast, during this same period, exposures with a more severe outcome (death, major, and moderate) and HCF calls have continued a consistent increase. Possible contributors to the declining PC access include declining US birth (especially since exposure rates are much higher in children ≤ 5 years of age), increasing use of text rather than voice communication, and increased use of and reliance on internet search engines and web resources. To meet our public health goals, PCs will need to understand and meet the public's 21st century communication preferences. We are concerned that failure to respond to these changes may result in a retro-shift with more people seeking medical care for exposures that could have been managed at home by a PC. Likewise minor exposures may progress to more severe morbidity and mortality because of incorrect internet information or no telephone management. The net effect could be more severe poisoning outcomes because fewer people took advantage of PC services, with a resultant increased burden on the national healthcare infrastructure as may be reflected in the increased number of cases managed in a healthcare facility this year.
NPDS statistical analyses indicate that all analgesic exposures including opioids and sedatives are increasing year over year. This trend is shown in and . NPDS data mirrors CDC data that demonstrates similar findings.(Citation9) Thus NPDS provides a real-time view of these public health issues without the need for data source extrapolations.
One of the limitations of NPDS data has been the perceived lack of fatality case volume compared with other reporting sources. However, when change over time is studied, NPDS is clearly consistent with other public health fatality analyses. One of the issues leading to this concern is the fact that medical record systems seldom have common output streams. This is particularly apparent with the various electronic medical record systems available. It is important to build a federated approach similar to the one modeled by NPDS to allow data sharing, for example, between hospital emergency departments and other medical record systems including medical examiner offices nationwide. Enhancements to NPDS can promote interoperability between NPDS and electronic medical records systems to better trend poison-related morbidity and mortality in the US and internationally.
Summary
Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls.
Changes in encounters in 2012 shown in include the following:
Total encounters (all exposure and information calls) decreased by 6.9%;
All information calls decreased by 14.8%, Drug ID calls decreased by 22.0%, and human exposures decreased by 2.5%;
HCF information calls decreased by 1.7%, while HCF exposures increased by 1.2%;
Human exposures with less serious outcomes decreased by 2.7%, while those with more serious outcomes (minor, moderate, major, or death) decreased by 0.5% not withstanding an overall 4.6% yearly increase since 2000;
The categories of substance exposures increasing most rapidly are analgesics, followed by sedative/hypnotics/antipsychotics, cardiovascular drugs, and antihistamines.
These data support the continued value of PC expertise and need for specialized medical toxicology information to manage the more severe exposures, despite a decrease in calls involving less severe exposures. PCs must consider newer communication approaches that match current public communication patterns in addition to the traditional telephone call.
The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response, and situational awareness tracking. NPDS is a model system for the nation and global public health.
Disclaimer
The American Association of Poison Control Centers (AAPCC; http://www.aapcc.org) maintains the national database of information logged by the country's regional PCs serving all 50 United States, Puerto Rico and the District of Columbia. Case records in this database are from self-reported calls: they reflect only information provided when the public or healthcare professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure, etc.), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s).
Notes
This report is published under contractual arrangement with AAPCC and does not undergo external peer-review.
References
- National Poison Data System: Annual reports 1983–2012 [Internet]. Alexandria (VA): American Association of Poison Control Centers;. Available from: http://www.aapcc.org/annual-reports/
- US Census Bureau. Table 1. Annual Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2010 to July 1, 2012 (NST-EST2012-01) [downloaded 2013 Oct 23] http://www.census.gov/popest/data/state/totals/2012/index.html
- US Census Bureau: International Data Base (IDB) Demographic Indicators for: American Samoa, Federated States of Micronesia, Guam, Virgin Islands, [downloaded 2012 Oct 26]: http://www.census.gov/population/international/data/idb/region.php
- US Census Bureau: State Characteristics Datasets: Annual Estimates of the Civilian Population by Single Year of Age and Sex for the United States and States: April 1, 2010 to July 1, 2012[downloaded 2013 Oct 23]: http://www.census.gov/popest/data/state/asrh/2012/SC-EST2012-AGESEX-CIV.html
- Position statement: ipecac syrup. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:699–709.
- American Academy of Clinical Toxicology European Association of Poisons Centres and Clinical Toxicologists. Position Paper: Ipecac Syrup. J Toxicol Clin Toxicol 2004; 42: 133–143.
- American Academy of Pediatrics Policy Statement. Poison treatment in the home. Pediatrics 2003; 112:1182–1185.
- Savel TG, Bronstein A, Duck, M, Rhodes MB, Lee, B, Stinn J, Worthen, K. Using Secure Web Services to Visualize Poison Center Data for Nationwide Biosurveillance: A Case Study [Internet]. Online Journal of Public Health Informatics 2010; 2:1–9; [downloaded 2012 Oct 30] http://ojphi.org/htbin/cgiwrap/bin/ojs/index.php/ojphi/article/view/2920/2505
- Centers for Disease Control and Prevention. QuickStats: Number of Poisoning Deaths* Involving Opioid Analgesics and Other Drugs or Substances – United States, 1999—2007. MMWR Morb Mortal Wkly Rep. 2010; 59:1026.
- McGraw-Hill's AccessMedicine, Laboratory Values of Clinical Importance (Appendix), Harrison's Principles of Internal Medicine 17e. McGraw-Hill Professional, 2008 [cited 2010 Nov 1]. Available from: http://www.accessmedicine.com/.
- Goldfrank's Toxicologic Emergencies, Ninth Edition, McGraw-Hill Companies, 2010.
- Dart RC, editor. Medical Toxicology, Third Edition. Philadelphia, Lippincott, Williams & Wilkins, 2004.
Appendix A—Acknowledgments
The compilation of the data presented in this report was supported in part through the US Centers for Disease Control and Prevention AAPCC Contract 200-2011-41767.
The authors wish to express their appreciation to the following individuals who assisted in the preparation of the manuscript: Katherine W. Worthen and Laura J. Rivers.
The authors express their sincere gratitude to the staff at the AAPCC Central Office for their support during the preparation of the manuscript: John Fiegel, MS, CAE, Interim Executive Director, Beth Doggette, and the entire staff.
Poison Centers
We gratefully acknowledge the extensive contributions of each participating PC and the assistance of the many healthcare providers who provided comprehensive data to the PCs for inclusion in this database. We especially acknowledge the dedicated efforts of the SPIs who meticulously coded 3,373,025 calls made to US PCs in 2012.
As in previous years, the initial review of reported fatalities and development of the abstracts and case data for NPDS was the responsibility of the staff at the 57 participating PCs. Many individuals at each center participated in the fatality case preparation. These toxicology professionals and their centers are the following:
Alabama Poison Center
Perry Lovely, MD, ACMT
John Fisher, PharmD, DABAT, FAACT
Lois Dorough BSN, RN, CSPI
Arizona Poison and Drug Information Center
Keith Boesen, PharmD, CSPI
F. Mazda Shirazi, MS, MD, PhD, FACEP
Arkansas Poison and Drug Information Center
Henry F. Simmons, Jr., MD
Pamala R. Rossi, PharmD
Howell Foster, PharmD
Banner Good Samaritan Poison and Drug Information Center
Daniel Brooks, MD
Belinda Sawyers, RN, CSPI
Jane Klemens, RN, CSPI
Sharyn Welch, RN
Blue Ridge Poison Center
Christopher P. Holstege, MD
Nathan P. Charlton, MD
William Rushton, MD
Luke Hardison, MD
California Poison Control System—Fresno/Madera Division
Richard J. Geller, MD, MPH
California Poison Control System—Sacramento Division
Timothy Albertson, MD, PhD
Justin Lewis, PharmD, CSPI
California Poison Control System—San Diego Division
Richard F. Clark, MD
Lee Cantrell, PharmD
Michael Durracq, MD
Jennifer Cullen, MD
Landon Rentmeester, MD
California Poison Control System—San Francisco
Kent R. Olson, MD
Susan Kim-Katz, PharmD
Raymond Ho, PharmD
Kathryn Meier, PharmD
Sandra Hayashi, PharmD
Suad A. Al-Abri, MD
Gabriela Cordero-Schmidt, MD
Freda Rowley, PharmD
Ilene Anderson, PharmD
Jo Ellen Dyer, PharmD
Beth Manning, PharmD
Ben Tsutaoka, PharmD
Carolinas Poison Center
Michael C. Beuhler, MD
Marsha Ford, MD
Anna Rouse Dulaney, PharmD
William Kerns II, MD
Christine M. Murphy, MD
Steven J Walsh, MD
Central Ohio Poison Center
Hannah Hays, MD
Marcel J. Casavant, MD, FACEP, FACMT
Henry Spiller, MS, DABAT, FAACT
Jason Russell, DO
Devin Wiles DO
Kaitlyn Day
Central Texas Poison Center
Ryan Morrissey, MD
S. David Baker, PharmD, DABAT
Children's Hospital of MI Regional Poison Center
Cynthia Aaron, MD
Lydia Baltarowich, MD
Aimee Nefcy, MD
Bram Dolcourt, MD
Susan C. Smolinske, PharmD
Matthew Hedge, MD
Cincinnati Drug and Poison Information Center
Shan Yin, MD, MPH
Sara Pinkston, RN
Connecticut Poison Center
Charles McKay, MD
Kathy Hart MD
Bernard C. Sangalli, MS
Florida/USVI Poison Information Center—Jacksonville
Thomas Kunisaki, MD, FACEP, ACMT
Florida Poison Information Center—Miami
Jeffrey N. Bernstein, MD
Richard S. Weisman, PharmD
Florida Poison Information Center—Tampa
Alfred Aleguas, Jr., BS Pharm, PharmD, DABAT
Cynthia R. Lewis-Younger, MD, MPH
Pam Eubank, RN, CSPI
Shirley Rendon, MD, CSPI
Judy Turner, RN, CSPI
Georgia Poison Center
Robert J. Geller, MD
Brent W. Morgan, MD
Ziad Kazzi, MD
Stella Wong, DO
Gaylord P. Lopez, PharmD
Stephanie Hon, PharmD
Adam Pomerleau, MD
Justin Arnold, DO
Alaina Steck, MD
Melissa Halliday, MD
Molly Boyd, MD
Hennepin Regional Poison Center
Deborah L. Anderson, PharmD
Jon B. Cole, MD
JoAn Laes, MD
Benjamin S. Orozco, MD
David J. Roberts, MD
Laurie Willhite, PharmD, CSPI
Illinois Poison Center
Michael Wahl, MD
Sean Bryant, MD
Indiana Poison Center
James B. Mowry, PharmD
Gwenn Christianson, MSN, CSPI
R. Brent Furbee, MD
Iowa Poison Control Center
Sue Ringling, RN
Linda B. Kalin, RN
Edward Bottei, MD
Kentucky Regional Poison Center
George M. Bosse, MD
Barbara M Chenault RN CSPI
Louisiana Poison Center
Mark Ryan, PharmD
Thomas Arnold, MD
Maryland Poison Center
Suzanne Doyon, MD, FACMT
Mississippi Poison Control Center
Robert Cox MD, PhD, DABT, FACMT
Christina Parker, Rn, CSPI
Missouri Poison Center at SSM Cardinal Glennon
Children's Medical Center
Anthony Scalzo, MD, FACMT, FAAP, FAACT
Shelly Enders, PharmD, CSPI
National Capital Poison Center
Cathleen Clancy, MD, FACMT
Nicole Reid, RN, BA, BSN, MEd, CSPI
Nebraska Regional Poison Center
Claudia Barthold, MD
Ronald I. Kirschner, MD
New Jersey Poison Information and Education System
Steven M. Marcus, MD
Bruce Ruck, PharmD
New Mexico Poison and Drug Information Center
Steven A. Seifert, MD, FAACT, FACMT
Blaine E. (Jess) Benson, PharmD, DABAT
New York City Poison Control Center
Maria Mercurio-Zappala, MS, RPh
Robert S. Hoffman, MD
Lewis Nelson, MD
Rana Biary, MD
Nicholas Connors, MD
Mai Takematsu, MD
Betty Chen, MD
Lauren Shawn, MD
Hong Kim, MD
North Texas Poison Center
Brett Roth MD, ACMT, FACMT
Melody Gardner, RN, MSN, MHA, CCRN
Northern Ohio Poison Center
Lawrence S. Quang, MD
Adrianne Grendzynski, RN, BSN, CSPI
Danielle Richardson, RN, BSN, CSPI
Susan Scruton, RN, BSN, CSPI
Northern New England Poison Center
Jane Clark
Tamas Peredy, MD
Oklahoma Poison Control Center
William Banner, Jr., MD, PhD, ABMT
Scott Schaeffer, RPh, DABAT
Oregon Poison Center
Zane Horowitz, MD
Sandra L. Giffin, RN, MS
Palmetto Poison Center
William H. Richardson, MD
Jill E. Michels, PharmD
Pittsburgh Poison Center
Michael Lynch, MD
Rita Mrvos, BSN
Edward P. Krenzelok, PharmD
Puerto Rico Poison Center
José Eric Dîaz-Alcalá, MD
Andrés Britt, MD
Elba Hernández, RN
Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
Michele Burns Ewald, MD, MPH
Dennis Wigandt, PharmD
May Yen, MD
Diana Felton, MD
Regional Poison Control Center—Children's of Alabama
Erica Liebelt, MD, FACMT
Michele Nichols, MD
Sherrel Brooks, RN, CSPI
Ann Slattery DrPH DABAT
Diane Smith, RN, CSPI
Rocky Mountain Poison and Drug Center
Alvin C. Bronstein, MD, FACEP, FACMT
Beau Braden DO, MPH, MS
Janetta L. Iwanicki, MD
Joseph Maddry, MD
Daniel Sessions, MD
Sam Wang, MD
Shireen Banerji, PharmD, DABAT
Carol Hesse RN, CSPI
Regina R. Padilla
South Texas Poison Center
Cynthia Abbott-Teter, PharmD
Douglas Cobb, RPh
Miguel C. Fernandez, MD
George Layton, MD
C. Lizette Villarreal, MA
Southeast Texas Poison Center
Wayne R. Snodgrass, MD, PhD, FACMT
Jon D. Thompson, MS, DABAT
Jean L. Cleary, PharmD, CSPI
Tennessee Poison Center
John G. Benitez, MD, MPH
Saralyn Williams, MD
Donna Seger, MD
Texas Panhandle Poison Center
Shu Shum, MD
Jeanie E. Jaramillo, PharmD
Cristie Johnston, RN, CSPI
The Poison Control Center at the Children's Hospital of Philadelphia
Fred Henretig, MD
Kevin Osterhoudt, MD
University of Kansas Hospital Poison Control Center
Tama Sawyer, PharmD, DABAT
Stephen Thornton, MD
Upstate NY Poison Center
Jeanna M. Marraffa, PharmD
Alexander Garrard, Pharm.D.
Christine M. Stork, PharmD
Timothy Wiegand, MD
Utah Poison Control Center
B. Zane Horowitz, MD
Tom Martin, MD, MPH, FACEP
Virginia Poison Center
Rutherfoord Rose, PharmD
Kirk Cumpston, DO
Brandon Wills, DO
Paul Stromberg, MD
Washington Poison Center
William T. Hurley, MD, FACEP, FACMT
Curtis Elko, PharmD
David Serafin, CPIP
West Texas Regional Poison Center
Stephen W. Borron, MD, MS, FACEP, FACMT
Salvador H. Baeza, PharmD, DABAT
Hector L. Rivera, RPh, CSPI
West Virginia Poison Center
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACT
Anthony F. Pizon, MD, ABMT
Wisconsin Poison Center
David D. Gummin, MD
Lori Rohde, RN, CSPI
Amy E. Zosel MD
AAPCC Fatality Review Team
The Lead and Peer review of the 2012 fatalities was carried out by the 34 individuals listed here including 4 who reviewed the pediatric cases [Peds]. The authors and the AAPCC wish to express our appreciation for their volunteerism, dedication, hard work, and good will in completing this task in a limited time.
Alfred Aleguas Jr, PharmD, DABAT, Florida Poison Information Center—Tampa
Anna Rouse Dulaney*, PharmD, DABAT, Carolinas Poison Center
Ann-Jeannette Geib*, MD, Robert Wood Johnson Med School, New Brunswick, NJ
Bernard C Sangalli*, MS, DABAT, Connecticut Poison Center
Charles McKay, MD, Connecticut Poison Center
Christine Murphy, MD, Department of Emergency Medicine, Division of Medical Toxicology, Carolinas Medical Center/Carolinas Poison Center, Charlotte, NC
Curtis Elko, CSPI, Washington Poison Center, Seattle
Cynthia Lewis-Younger, MD, MPH, Florida Poison Information Center—Tampa
Daniel E. Brooks, MD, Banner Good Samaritan Medical Center, Phoenix
David D Gummin, MD, Wisconsin Poison Center
Diane Calello, MD, New Jersey Poison Information and Education System [Peds]
Elizabeth J Scharman, PharmD, DABAT, BCPS, FAACT, West Virginia Poison Center
Gar Chan, MD, Launceston General Hospital, Tasmania, Australia
Henry Spiller, MS, DABAT, FAACT, Central Ohio Poison Center, Columbus
Jan Scaglione, PharmD, DBAT, Cincinnati Drug and Poison Information Center
Jeffrey S Fine, MD, NYU School of Medicine/Bellevue Hospital [Peds]
Jennifer Lowry, MD, Clin Pharm & Med Tox, Children’s Mercy Hospital, Kansas City, MO [Peds]
Jill E. Michels, PharmD, DABAT, Palmetto Poison Center, SC
John McDonagh, MD, Hartford, CT
Karen E Simone, PharmD, DABAT, Northern New England PC, Maine Medical Center
Kathy Hart, MD, Connecticut Poison Control Center
L Keith French, MD, Oregon Poison Center
Maria Mercurio-Zappala, RPh, MS, DABAT, FAACT, NYC Poison Control Center
Mark Su, MD, FACEP, FACMT, North Shore University Hospital, NY
Mike Levine*, MD, Banner Good Samaritan Medical Center, Phoenix
Nathanael McKeown*, DO, Oregon Poison Center
Rachel Gorodetsky, PharmD, D’Youville College School of Pharmacy, University of Rochester Medical Center
Rais Vohra*, MD, California Poison Control System, Fresno/Madera
Robert B Palmer, PhD, DABAT, Rocky Mountain Poison and Drug Center, Denver, CO
Robert Goetz, PharmD, DBAT, Cincinnati Drug and Poison Information Center
Steven M. Marcus, MD, NJ Poison Information and Education System, [Peds]
Susan Smolinske, PharmD, Children's Hospital of Michigan RPCC, Detroit
Timothy Wiegand, MD, Director of Toxicology, University of Rochester, Medical Center and Strong Memorial Hospital; Consultant Toxicologist, SUNY Upstate Poison Center
William Hurley, MD, Washington Poison Center, Seattle
* These reviewers further volunteered to read the top-ranked 200 abstracts and judged to publish or omit each.
AAPCC Micromedex Joint Coding Group
Chair: Elizabeth J. Scharman, Pharm.D., DABAT, BCPS, FAACT
Alvin C. Bronstein, MD, FACEP, FACMT
Rick Caldwell
Christina Davis, PharmD
Sandy Giffin, RN, MS
Kendra Grande, RPh
Katherine M. Hurlbut, MD
Wendy Klein-Schwartz, PharmD, MPH
Fiona McNaughton
James Mowry, PharmD
Susan C. Smolinske, PharmD
AAPCC Rapid Coding Team
Chair: Alvin C. Bronstein, MD, FACEP, FACMT
Elizabeth J. Scharman, Pharm.D., DABAT, BCPS, FAACT
Jay L. Schauben, PharmD, DABAT, FAACT
Susan C. Smolinske, PharmD
AAPCC Surveillance Team
NPDS surveillance anomalies are analyzed daily by a team of ten medical and clinical toxicologists working across the country in a distributed system. These dedicated professionals interface with the HSB/NCEH/CDC and the PCs on a regular basis to identify anomalies of public health significance and improve NPDS surveillance systems:
Alfred Aleguas, Pharm D, DABAT
S. David Baker, PharmD, DABAT
Director, Alvin C. Bronstein, MD, FACEP, FACMT
Douglas J. Borys, PharmD, DABAT
John Fisher, PharmD, DABAT, FAACT
Jeanna M. Marraffa, PharmD, DABAT
Maria Mercurio-Zappala, RPH, MS, DABAT, FAACT
Henry A. Spiller, MS, DABAT, FAACT
Richard G. Thomas, Pharm D, DABAT
Regional Poison Center Fatality Awards
Each year the AAPCC and the Fatality Review team recognized several regional PCs for their extra effort in their preparation of fatality reports and prompt responses to reviewer queries during the review process. The awards were presented at the October 2013, North American Congress of Clinical Toxicology meeting in Atlanta, GA.
First Center to Complete all Cases (December 15, 2012, last of their 16 cases)
Nebraska Regional Poison Center (Omaha)
Largest Number with Autopsy Reports (57 of 79 cases)
Carolinas Poison Center (Charlotte)
Highest Percentage with Autopsy Reports (80% of 15 cases)
Central Ohio Poison Center (Columbus)
Largest Number of INDIRECT cases (759 of 1409 total cases reported for 2012)
Maryland Poison Center (Baltimore)
Highest Overall Quality of Reports (12.5 of possible 22 for 16 cases)
University of Kansas Hospital Poison Control Center (Kansas City)
Greatest improvement in Overall Quality of Reports (2.81 increase from last year)
University of Kansas Hospital Poison Control Center (Kansas City)
Most Abstracts Published in last year's Annual report (9 of the 68 published narratives) Maryland Poison Center (Baltimore)
Most Helpful Regional Poison Center Staff (based on survey of AAPCC review team) Missouri Regional Poison Center (St. Louis)
Honorable mention
Carolinas Poison Center (Charlotte)
Appendix B—Data Definitions
Reason for Exposure
NPDS classifies all calls as either EXPOSURE (concern about an exposure to a substance) or INFORMATION (no exposed human or animal). A call may provide information about one or more exposed person or animal (receptors).
SPIs coded the reasons for exposure reported by callers to PCs according to the following definitions:
Unintentional general: All unintentional exposures not otherwise defined below.
Environmental: Any passive, non-occupational exposure that results from contamination of air, water, or soil. Environmental exposures are usually caused by manmade contaminants.
Occupational: An exposure that occurs as a direct result of the person being on the job or in the workplace.
Therapeutic error: An unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person, or administration of the wrong substance. Only exposures to medications or products used as medications are included. Drug interactions resulting from unintentional administration of drugs or foods which are known to interact are also included.
Unintentional misuse: Unintentional improper or incorrect use of a nonpharmaceutical substance. Unintentional misuse differs from intentional misuse in that the exposure was unplanned or not foreseen by the patient.
Bite/sting: All animal bites and stings, with or without envenomation, are included.
Food poisoning: Suspected or confirmed food poisoning; ingestion of food contaminated with microorganisms is included.
Unintentional unknown: An exposure determined to be unintentional, but the exact reason is unknown.
Suspected suicidal: An exposure resulting from the inappropriate use of a substance for reasons that are suspected to be self-destructive or manipulative.
Intentional misuse: An exposure resulting from the intentional improper or incorrect use.
Intentional abuse: An exposure resulting from the intentional improper or incorrect use of a substance where the patient was likely attempting to gain a high, euphoric effect or some other psychotropic effect, including recreational use of a substance for any effect.
Intentional unknown: An exposure that is determined to be intentional but the specific motive is unknown.
Contaminant/tampering: The patient is an unintentional victim of a substance that has been adulterated (either maliciously or unintentionally) by the introduction of an undesirable substance.
Malicious: Patients who are victims of another person’s intent to harm them.
Withdrawal: Inquiry about or experiencing of symptoms from a decline in blood concentration of a pharmaceutical or other substance after discontinuing therapeutic use or abuse of that substance.
Adverse Reaction Drug: Unwanted effects due to an allergic, hypersensitivity, or idiosyncratic response to the active ingredient(s), inactive ingredient(s) or excipient of a drug, chemical, or other drug substance when the exposure involves the normal, prescribed, labeled or recommended use of the substance.
Adverse Reaction Food: Unwanted effects due to an allergic, hypersensitivity, or idiosyncratic response to a food substance.
Adverse Reaction Other: Unwanted effects due to an allergic, hypersensitivity, or idiosyncratic response to a substance other than drug or food.
Unknown Reason: Reason for the exposure cannot be determined or no other category is appropriate.
Medical Outcome
No effect: The patient did not develop any signs or symptoms as a result of the exposure.
Minor effect: The patient developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. A minor effect is often limited to the skin or mucus membranes (e.g., self-limited gastrointestinal symptoms, drowsiness, skin irritation, first-degree dermal burn, sinus tachycardia without hypotension, and transient cough).
Moderate effect: The patient exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Usually, some form of treatment is indicated. Symptoms were not life-threatening, and the patient had no residual disability or disfigurement (e.g., corneal abrasion, acid–base disturbance, high fever, disorientation, hypotension that is rapidly responsive to treatment, and isolated brief seizures that respond readily to treatment).
Major effect: The patient exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement (e.g., repeated seizures or status epilepticus, respiratory compromise requiring intubation, ventricular tachycardia with hypotension, cardiac or respiratory arrest, esophageal stricture, and disseminated intravascular coagulation).
Death: The patient died as a result of the exposure or as a direct complication of the exposure.
Not followed, judged as nontoxic exposure: No follow-up calls were made to determine the outcome of the exposure because the substance implicated was nontoxic, the amount implicated was insignificant, or the route of exposure was unlikely to result in a clinical effect.
Not followed, minimal clinical effects possible: No follow-up calls were made to determine the patient's outcome because the exposure was likely to result in only minimal toxicity of a trivial nature. (The patient was expected to experience no more than a minor effect.).
Unable to follow, judged as a potentially toxic exposure: The patient was lost to follow-up, refused follow-up, or was not followed, but the exposure was significant and may have resulted in a moderate, major, or fatal outcome.
Unrelated effect: The exposure was probably not responsible for the effect.
Confirmed nonexposure: This outcome option was coded to designate cases where there was reliable and objective evidence that an exposure initially believed to have occurred actually never occurred (e.g., all missing pills are later located). All cases coded as confirmed nonexposure are excluded from this report.
Death (indirect report): Death (indirect report) are deaths that the poison center acquired from medical examiner or media, but did not manage nor answer any questions about the death.
Relative Contribution to Fatality
The definitions used for the RCF classification were as follows:
Undoubtedly responsible—In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES actually caused the death.
Probably responsible—In the opinion of the CRT the Clinical Case Evidence suggests that the SUBSTANCES caused the death, but some reasonable doubt remained.
Contributory—In the opinion of the CRT the Clinical Case Evidence establishes that the SUBSTANCES contributed to the death, but did not solely cause the death. That is, the SUBSTANCES alone would not have caused the death, but combined with other factors, were partially responsible for the death.
Probably not responsible—In the opinion of the CRT the Clinical Case Evidence establishes to a reasonable probability, but not conclusively, that the SUBSTANCES associated with the death did not cause the death.
Clearly not responsible—In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES did not cause this death.
Unknown—In the opinion of the CRT the Clinical Case Evidence is insufficient to impute or refute a causative relationship for the SUBSTANCES in this death.
Appendix C—Abstracts of Selected Cases
Selection of Abstracts for Publication
The abstracts included in Appendix C were selected for publication in a 3-stage process consisting of qualifying, ranking, and reading. Qualifying was based on the RCF— only RCF = 1-Undoubtedly Responsible, 2-Probably Responsible, or 3-Contributory were eligible for publication. Fatalities by Indirect report were excluded beginning with the 2008 annual report. Ranking was based on the number of substances (1/N) and weighted case score. The case weighting factors were the averages chosen based on review team recommendations in 2006. Each case score was multiplied by the respective factors to obtain a weighted publication score: Hospital records * 4.4 + Postmortem * 7.6 + Blood levels * 6.9 + Quality/Completeness * 6.4 + Novelty/Educational value * 6.0. Scores were normalized (z-score) within each reviewer before the final weighting: 33% for 1/N and 67% for weighted case scores.
The top-ranked abstracts (200 + ties) were each read by individual reviewers (See Appendix A) and the two managers (Cantilena and Spyker). Each reader judged each abstract as “publish” or “omit” and all abstracts receiving five or more of eight publish votes were selected, further edited, and cross-reviewed by the two managers.
Abstracts
Abstracts of the cases were selected (see Selection of Abstracts for Publication, above) from the human fatalities judged related to be an exposure as reported to US PCs in 2012. A structured format for abstracts was required in the PC preparation of the abstracts and was used in the abstracts presented. Abbreviations, units, and normal ranges omitted from the abstracts are given at the end of this appendix.
Case 57. Ethanol (non-beverage) ingestion: undoubtedly responsible.
Scenario/Substances: A 41 y/o female had been abusing ethanol, her partner confronted her about it, and she stated she drank denatured alcohol. She became unresponsive and was brought to the ED.
Physical Exam: Not provided
Laboratory Data: ABG-pH 6.5, Na 144, Cl 106, HCO3 < 10, methanol 329 mg/dL.
Clinical Course: The patient was unresponsive upon arrival to ED, BP 62/45, HR 42, pupils fixed and dilated. She was intubated, given sodium bicarbonate boluses, started on dopamine and norepinephrine which resulted in BP 80/60 and HR 110. Fomepizole was started and folate 50 mg q 4 hrs was given. Due to hypotension, patient started on CRRT rather than hemodialysis. The patient was able to be weaned off dopamine and norepinephrine, but her neurologic exam remained unimproved. Follow-up laboratory data: ABG-pH 7.31/pCO2 21/pO2 189/HCO3 11/BE -19, she continued on a sodium bicarbonate drip. CRRT was discontinued on Day 2 due to hypotension, and she sustained three episodes of cardiac arrest. Repeat methanol was 136 mg/dL. By Day 3, methanol level was less than 20 mg/dL and fomepizole was discontinued, but she continued on multiple vasopressors and mechanical ventilation. She continued to be unresponsive. Based on the prognosis, the family opted for institution of comfort measures and she expired on Day 5.
Autopsy Findings: Final cause of death: 1) methanol, 2) Chronic ETOH. Manner of death: Accidental.
Case 107. Acute methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 50 y/o male was found unresponsive in his home with numerous empty bottles of ethanol. EMS noted RR 4, intubation was performed before transport. The patient also received NS and 1 ampule of bicarbonate for peaked T waves. History from the scene was significant for possible methanol ingestion.
Past Medical History: Hypertension, depression, anxiety, prior myocardial infarction, and stent placement, history of seizure thought to be alcohol-related, 60 + pack-year smoking history, medications: lisinopril, bupropion, nitroglycerin, and alprazolam.
Physical Exam: Unresponsive, intubated, BP 116/65, HR 92, RR 22, T 36°C.
Laboratory Data: pH 6.53, Na 142, K 8.2, Cl 104, BUN 17, Cr 1.4, Ca 6.8, serum osmolarity 518 mosm/kg: serum lactate 8.7 mmol/L, UDS negative.
Clinical Course: Shortly after arrival to the ED, the patient had a generalized seizure and hypotension. Two amps of bicarbonate were given by IV push. Fomepizole and bicarbonate were administered, follow-up pH 7.3. Hemodialysis was attempted but the filter repeatedly clogged with blood clots. The patient had a myocardial infarction early in the hospitalization. Methanol was measured at 110, but unclear when the sample was obtained, Na was 172 and the patient was placed on DDAVP. Hemodialysis was repeated.
Post dialysis the patient was non-responsive, CT head showed massive brain swelling and edema throughout the cerebral hemispheres, cerebellum, and brainstem, with virtually complete effacement of the basilar cisterns and sulci. Brain blood flow was assessed as absent and consistent with brain dealth on Day 3. Organs were harvested for donation and life-support was withdrawn.
Autopsy Findings: External examination only.
Case 190. Acute methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 31 y/o male consumed 32 ounces of windshield washer fluid. The patient complained to EMS of visual disturbance.
Past Medical History: Alcoholism
Physical Exam: Prehospital BP 120/84, HR 65, RR 18 and shallow, O2 sat 91%. Initially the patient was confused with slurred speech, but became progressively unresponsive, GCS 3.
Laboratory Data: Intial ABG-pH 6.8/pCO2 56/pO2 138/HCO3 6, Na 143, K 6.7, Cl 108, AST 59, ALT 70, Glu 487, lactate 11, anion gap 40, osm gap 164, methanol 91 mg/dL. Serum acetaminophen, ethanol, ethylene glycol, and salicylate were not detected. After the initial dialysis the ABG-pH 7.33/pCO2 39/HCO3 20, pO2 68. On Day 2 Ca, Mg, and phosphorus were normal; troponin T 0.05, CKMB 6.2, serum osm 293, and methanol 47 mg/dL. After the second 4-hour dialysis, methanol: 16 mg/dL.
Clinical Course: Upon arrival to the ED the patient was noted to be unresponsive with periods of apnea. Pupils were dilated and unreactive, he was endotracheally intubated. At 12 hrs after arrival he was opening eyes to voice. Right-sided motor paralysis was noted. At 24 hrs, T 35.4°C, BP 138/93, HR 81, O2 sat 97% with spontaneous respiratory effort, sedated, intubated on a ventilator, pupillary response normal, able to follow commands moving left side. On Day 2 the patient desaturated into the 80s% with increasing airway pressures. Post intubation the patient became hypotensive and was treated with dopamine, but progressed to PEA arrest lasting 30 min with wide QRS complexes. During the arrest, the patient received 4 mg epinephrine, bicarbonate, Mg, and vasopressin with return of spontaneous circulation. A post-arrest cooling protocol was initiated. In the ICU, the patient completed the 24-hour post-arrest cooling protocol while paralyzed and intubated. Vasopressors including dopamine, norepinephrine, insulin, and vasopressin were weaned. The patient received folate and fomepizole and was dialyzed. The patient was sedated with propofol, fentanyl, and midazolam. Approximately 36 hrs after arrival the patient experienced oxygen desaturations requiring increased ventilatory support. A bronchoscopy-obtained sputum specimen revealed no bacteria, but the patient was treated with vancomycin. A head CT performed on Day 3 showed bilateral basal ganglia infarcts with hemorrhage on the left. The patient was given furosemide and an inferior vena cava filter was placed. He became progressively more difficult to ventilate and expired on Day 6.
Autopsy Findings: Results not available.
Case 201. Acute antifreeze (ethylene glycol) ingestion: undoubtedly responsible.
Scenario/Substances: A male in his 80s was found down by family members. He was last seen 7 hrs before being found. The family suspected antifreeze may have been ingested as they found an open bottle in the kitchen. Police on the scene found two glasses that contained residual antifreeze.
Physical Exam: Unresponsive male. VS: BP 190/94, HR 85. Pupils 3 mm, slowly reactive.
Laboratory Data: ABG-pH 6.86 / pCO2 61/pO2 47, Serum Osmolality 473 mOsm/kg, lactate 3.6, albumin 4.7, alk phos 115, ALT 17, AST 25, direct bili 0.1, INR 1.2, PTT 48.6. Ethylene glycol 829 mg/dL.
At 3 hrs post presentation, ABG-pH 6.93/pCO2 13/pO2 141, at 6 hrs post presentation pH 7.2.
Clinical Course: The patient was intubated in the ED, received a dose of fomepizole, was admitted to the ICU, and started on dialysis. At 12 hrs post presentation the patient completed 6 hrs of dialysis, but had hemoptysis. Based on the prognosis, the family opted for comfort measures only and the patient expired approximately 19 hrs post presentation.
Autopsy Findings: No autopsy performed.
Case 233. Acute hydrochloric acid ingestion: undoubtedly responsible.
Scenario/Substances: A 69 y/o female intentionally ingested a glass of muriatic acid. She was found by her husband and brought to the ED.
Clinical Course: In the ED she had difficulty speaking and reported mild stomach pain. BP 112/78, HR 78, O2 sats 96% on nasal cannula. She was given IV ranitidine and pantoprazole. Over the next 20 hrs, the patient developed hypotension requiring multiple vasopressors, elevation of BUN and Cr. Chest and abdominal xrays showed no perforation. She had increasing respiratory difficulty and required intubation. Dialysis was begun after K increased to 7.0 and urine output was minimal. The patient developed multiorgan failure. Based on the prognosis, the family opted for institution of comfort measures and she expired.
Autopsy Findings: Not performed.
Case 234. Acute cyanide ingestion: undoubtedly responsible
Scenario/Substances: An 85 y/o male presented to the ED hypotensive with agonal respirations. He was a chemist by profession, and was noted to have medication bottles in his bedroom labeled KCN.
Past Medical History: The patient had been seen in the ED on the day prior to presentation for a urinary tract infection.
Laboratory Data: Initial ABG-pH 7.191 / pCO2 51.7 / pO2 45.7 / HCO3 19.3, COHb 12.3%.
Clinical Course: In the ED he was unresponsive and hypotensive. Systolic BP was 70, later decreased to 40, corneal reflexes absent. Patient smelled of ammonia. He was noted to be acidemic on laboratory workup. His code status was determined to be DNR. He did not receive cyanide antidotal therapy. The patient expired shortly after arrival to the ED.
Autopsy Findings: Marked coronary artery atherosclerosis, hypertensive cardiovascular disease, remote and resolving myocardial infarcts, and remote strokes. Toxicology results: cyanide 2.8 mg/L. Cause of death: suicide by cyanide toxicity.
Case 235. Acute ammonia ingestion and dermal: contributory.
Scenario/Substances: An 87 y/o male had a syncopal episode at home. Wife tried to revive him with ammonia and spilled some of the ammonia on his face and into his mouth.
Physical Exam: Pharyngeal edema, drooling, no reported respiratory distress. BP 146/83, HR 104, RR 21, T 36°C, O2 sat 95% (on face mask),
Clinical Course: Patient treated with oxygen, steroids, and albuterol treatments. His respiratory status declined over the next 24 hrs. He reportedly developed copious amounts of secretions and had upper airway sloughing. His underlying pulmonary function was unknown. He suffered respiratory distress and asystolic arrest within 36 hrs of admission, unresponsive to CPR efforts.
Autopsy Findings: ME death abstract included the following: Multifactorial cardiovascular disease, ammonia gas exposure was a contributory factor.
Case 243. Acute drain cleaner ingestion: undoubtedly responsible.
Scenario/Substances: A 32 y/o male was brought to the ED by EMS a few minutes after ingesting an unknown quantity of industrial strength drain cleaner at home. The patient was vomiting copious amounts of blood during transport and in the ED.
Past Medical History: Seizure disorder, permanent dysarthria, and ataxia after a previous bupropion overdose. He had been treated and discharged home following an aspirin overdose 2 days prior to this admission.
Physical Exam: BP 115/70, HR 75, afebrile, erythematous oral mucosa with bloody secretions. First and second degree cutaneous burns to lips, tongue, soft palate, hands, and buttocks.
Laboratory Data: ABG-pH 7.35 / pCO2 37 / HCO3 20, Na 137, K 3.9, Cr 1.1, Hgb 15, Hct 45.8, AST 22, ALT 31, salicylate 23.2 mg/L.
Clinical Course: The patient complained of difficulty swallowing and breathing and abdominal pain. No decontamination was performed. He was intubated for airway protection and admitted to the ICU. An esophagogastro-duodenoscopy was attempted, but was stopped before visualization of the complete esophagus due to severely necrotic tissue. The patient became increasingly agitated and self-extubated on Day 6. For the first 9 days of hospitalization the patient's CxR showed no evidence of perforation. Treatments included prophylactic antibiotics, IV proton pump inhibitor drip, benzodiazepines, and fentanyl. On Day 7 the patient developed intermittent atrial fibrillation and atrial flutter, which was controlled on an amiodarone drip. An esophagram was attempted on Day 7 without complete visualization of the esophagus. A CT scan on Day 13 revealed a fistula between the esophagus and proximal left main bronchus. He was reintubated, but surgical repair of the fistula was judged unlikely to be successful. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 15. Cause of death was respiratory failure, secondary to tracheoesophageal fistula secondary to caustic ingestion.
Autopsy Findings: Not performed.
Case 260. Acute hydrogen peroxide ingestion: undoubtedly responsible
Scenario/Substances: An 85 y/o male who regularly used 35% hydrogen peroxide for oxygen therapy, had been in his usual state of good health prior to the evening of admission. His wife noted he was in the bathroom taking his diluted hydrogen peroxide. She heard a crash, found him sitting on a chair, pale and diaphoretic with an altered mental status. She brought him to the bed, went to go get help from a neighbor, returned to find him unresponsive with vomitus next to him. EMS was summoned and transported him to the ED.
Past Medical History: AF controlled with metoprolol, left eye blindness, reflux esophagitis, dementia, questionable history of necrotic bowel.
Physical Exam: Unresponsive, abdominal distention, in AF with a regular ventricular rhythm, HR 140s.
Laboratory Data: WBC 103, lactate 2.3, troponin 0.05, hepatic enzymes unremarkable.
Clinical Course: He was intubated, converted to sinus tachycardia with diltiazem. A chest, abdominal, and pelvic CT showed pneumomediastinum, air around the esophagus, portal air in the liver, and scant amount of pneumoperitoneum, along with pneumomobilia and aspiration pneumonitis. He transferred to a tertiary care hospital. The patient remained unresponsive—no cough during suctioning and no response to nail bed pressure. Babinski reflex was positive, GCS 5, coarse crackles throughout his lungs. HR 67, BP 132/52, RR 17, O2 sat 99%, and T 36°C. MRI showed multiple cerebral infarcts. Neurology felt his prognosis for neurologic recovery was very poor. Based on the prognosis, comfort measures were instituted and he expired on Day 3.
Autopsy Findings: Not done.
Case 261. Acute formaldehyde/methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 52 y/o male tried to commit suicide by first cutting his left wrist and then drinking an 8 oz bottle of a holding tank deodorant containing 11% methanol and 35%. formaldehyde. EMS started an IV and placed him on high flow oxygen. He vomited multiple times during transport and required suction en route to the ED.
Past Medical History: Hypertension, prior suicide attempt 2 weeks earlier. He had mentioned several times that he wanted to kill himself.
Laboratory Data: Hgb 16.6, Hct 49.7, WBC 27.8, AST 75, ALT 45, bilirubin 0.5, platelets 260, UDS was negative for ethanol, acetaminophen, and salicylates.
Clinical Course: In the ED, BP 97/49, HR 92, RR 24, O2 sat 96% on room air. He had no oral burns. He became hypertensive, agitated, and began thrashing around. They were preparing to intubate him when he became cyanotic and bradycardic. He was resuscitated with sodium bicarbonate, atropine, and epinephrine. CPR was continued for 25 min, but he expired.
Autopsy Findings: Cause of death: Acute formaldehyde and formic acid toxicity. Blood samples from post-mortem femoral blood: formaldehyde 3.1 mg/L, formic acid 433 mg/L; vitreous humor: formaldehyde 3.97 mg/L, humor formic acid 690 mg/L; urine: formaldehyde 4.59 mg/L, formic acid 5501 mg/L; focal hepatocellular steatosis, corrosive fixation of the enteric mucosa, pulmonary edema and congestion, congestive brain swelling, global. Other results included incised wounds and nick abrasions to the left forearm and wrist, focal necrotizing granulomatous Coccidioides immitis pneumonitis, hypertensive cardiovascular disease and cardiomegaly.
Case 285. Acute hydrogen sulfide inhalation/nasal, dermal: undoubtedly responsible.
Scenario/substance: A 26 y/o male was monitoring the filling of a tanker with liquid sludge from a hog processing plant. He was standing on a catwalk on top of the tanker. The unusable pork by-product had been treated with chemicals to compact the product prior to disposal. He was noted earlier to have been wearing his hardhat and harness. Just before the accident the tanker driver observed him to have removed his helmet and harness and hold his head. He collapsed into the port on top of the tanker trailer that was being filled. He was discovered unresponsive, wedged waist deep head first in the hole. The estimated exposure time was 10—15 min. Of note, 1-year prior there was a report of a “blast of gas” occurring when workers opened the valve to start this process and a worker had been temporarily overcome, but recovered.
Past Medical History: No medical history, no medications or recent illnesses.
Physical Exam: Unresponsive male in asystole cardiac arrest. He was pronounced in the field but resuscitation was continued upon ED arrival.
Clinical Course: The patient was intubated and CPR performed for 40 min. IV fluids were given. In the ED, the patient was decontaminated for hydrogen sulfide with clothing removal and sterile washing of the body. He expired approximately 1 hour after exposure.
Autopsy findings: Dark purplish colored skin discoloration about the upper chest, neck, and face along with some pinkish red discoloration. Petechial hemorrhages were noted on the eyelids and sclera of both eyes. An unusual dark reddish discoloration was noted in the upper airway mucosa (uvula, epiglottis, larynx, trachea, and bronchi). No color change was noted on the brain beyond slight edema. No trauma was noted.
Cause of death: The result of thiosulfate (hydrogen sulfide) toxicity due to closed space exposure to animal processing by-products. Post-mortem heart blood thiosulfate 4.8 mg/L.
Case 302. Acute helium inhalation/nasal: undoubtedly responsible.
Scenario/Substances: A 42 y/o male was found down by family near a hose connected to 2 unmarked tanks, later identified as containing helium.
Past Medical History: Obesity.
Laboratory Data: Post-mortem blood and UDS were negative.
Autopsy Findings: Patient succumbed to asphyxia due to suffocation by plastic bag and inhalation of helium. Post-mortem testing was unremarkable.
Case 303. Acute hydrogen sulfide inhalation: undoubtedly responsible.
Scenario/Substances: A 42 y/o male was found passed out in his car with the windows rolled up and the engine running. Open on the passenger seat were lime sulfur, liquid sulfur, bleach, and toilet bowl cleaner. EMS got high readings for hydrogen sulfide outside and inside the car. He was intubated and EMS noted burns of his mouth and throat. He was decontaminated at the scene and transported to the ED. He had also taken multiple tablets of clonazepam.
Past Medical History: The patient had a history of ethanol abuse, depression, and prior suicide attempts. He was not compliant with his medications. At the time of his death he was going through alcohol withdrawal. The patient's girlfriend reported he had read an article in the newspaper regarding suicide using a mixture of chemicals that had the same effect as carbon monoxide but was more rapid.
Physical Exam: Unresponsive, pupils fixed and dilated, HR 92, BP 160/108, RR 23, O2 sat 100%, T 35.5°C.
Laboratory Data: Glu 138, HCO3 10, BUN 9, Cr 0.8, lactate 5.9, ammonia < 10, ethanol 285, serum acetaminophen and salicylate were not detected. Head CT showed decreased attenuation throughout the brain, most severe throughout the cerebral cortex, in the caudate nuclei, the putamen and the globus pallidus bilaterally, as well as in the posteromedial thalami bilaterally. This was consistent with a severe global anoxic injury. There was also subarachnoid hemorrhage seen in the inferior portions of both sylvan fissures, as well as in the perimesencephalic cistern.
Clinical Course: The patient was unresponsive and intubated, but was breathing on his own. His HR was progressively rising. His lactate was 16 and he was given several amps of bicarbonate. He then went into VT, then VF and could not be resuscitated.
Autopsy Findings: Cause of Death: Polydrug intoxication combined with inhalation of toxic chemical mixture. Manner of Death: Suicide. Subclavical blood: 7-Aminoclonazepam 18.2 ng/mL, fentanyl 0.6 ng/mL (therapeutic 1–3), ethanol 0.092% (w/v), diphenhydramine 1540 ng/mL (therapeutic 30–300), metoprolol 194 ng/mL (30–350), caffeine positive.
Case 308. Carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: A 43 y/o male, last seen in the morning, was found by his girlfriend when she returned in the afternoon and called EMS. The carbon monoxide detector alarm was sounding, a gas-powered generator was running in a woodworking area in the basement of the home, and the patient was seated in a chair with a suicide note, a family photograph, and other personal belongings nearby. When EMS arrived, the female was also overcome by carbon monoxide and was transported to ED. Firefighters measured a carbon monoxide concentration over 900 ppm in the upstairs of the home.
Autopsy Findings: Pink discoloration of skin consistent with carboxyhemoglobinemia was noted. Carboxyhemoglobin concentration in cardiac blood was 73.9%. Citalopram and caffeine were also detected but quantitative assays were not performed. Cause of death was carbon monoxide poisoning and the ME ruled manner of death suicide.
Case 315. Acute nitrogen gas inhalation: undoubtedly responsible
Scenario/Substances: A 45 y/o male working at a chemical company as a sandblaster was inadvertently hooked up to a nitrogen tank instead of an oxygen tank. He was later found in cardiac arrest 2 hrs after the mistake was made. EMS initiated CPR and intubated the patient. The patient received a paralytic, but it is not clear if he received it before or after the intubation.
Physical Exam: The patient was comatose, HR 70–90s, BP 92/56, RR 12 (on ventilator), T 33.1°C.
Laboratory Data: ABG-pH 7.19/pCO2 23/pO2 518/HCO3 8.5, Na 135, K 3.7, Cl 100, Ca 7.6, Glu 335, BUN 10, Cr 1.3, methemoglobin 15.3%, troponin 0.46 ng/mL.
Clinical Course: In the ED, the patient began to waken and breathe over the ventilator so he was placed on a midazolam drip. ECG showed AF treated with a diltiazem infusion, which was soon discontinued. He was admitted to the ICU where he was cooled, paralyzed, and sedated with midazolam and fentanyl per the post-cardiac arrest hypothermia protocol, IV bicarbonate infusion was given for his metabolic acidosis. Head CT was negative, EEG showed possible seizure activity so he was given a loading dose of levetiracetam. He continued to have seizures treated with phenobarbital and propofol. AF continued and diltiazem was administered then discontinued. His neurologic condition deteriorated and he developed fixed and dilated pupils. Norepinephrine was required to maintain his BP. On Day 4 an MRI documented anoxic brain injury. On Day 7 he was declared brain dead and expired the same day.
Autopsy Findings: Not available. The Occupational Safety and Hazard Administration completed an investigation and fined the company citing multiple workplace safety violations.
Case 384. Chronic lead ingestion: undoubtedly responsible.
Scenario/Substances: A 53 y/o female presented to ED complaining of generalized weakness and paralysis.
Past Medical History: End stage renal disease on hemodialysis, hypertension, poly-substance abuse, pica, and hepatitis C. Meds: amlodipine, epogen, hydralazine, neurontin, fosrenal, lisinopril, metoprolol, naprosyn, and omeprazole.
Physical Exam: Paralysis and loss of sensation in arms and legs.
Laboratory Data: Blood lead 113 mcg/dL, Hgb 10.5.
Clinical Course: Patient was admitted to the medicine service and continued on hemodialysis. EMG showed peripheral neuropathy with moderate axonal damage. Neurology opined her symptoms were toxicology related. Blood lead 113 mcg/dL reported on Day 4. Exposure source was unclear. BAL was administered IM q 4 hrs. Abdominal xray showed fragments of radio-dense material mixed with stool. Patient developed bowel incontinence of foul-smelling loose stool. Whole bowel irrigation initiated. Lead decreased to 54 mcg/dL; zinc protoporphyrin > 300 mcg/dL. Succimer initiation was delayed due to radio-dense material on KUB. Additional history during admission revealed a history of pica. She had eaten dirt around her home for years, but she recently moved across state lines. Patient was started on oral succimer 500 mg q 8 hrs, but it was held due to radio-dense material reappearing on abdominal xray. She developed respiratory failure and was intubated, continued to decline, and died, reportedly secondary to complications from septic shock and possible aspiration. It was later discovered that the patient had dirt with her in the hospital and had continued to ingest it during admission.
Autopsy Findings: Autopsy declined by family.
Case 402. Acute freon and marijuana exposure: undoubtedly responsible.
Scenario/Substances: A 42 y/o male with altered mental status was found in a big box store near five empty cans of freon-powered duster. He had been seen in the ED for the same exposure 2 days prior.
Laboratory Data: Initial labs included elevated BUN, Cr, and liver enzymes, Ca 5.3, K 3.9.
Past Medical History: Alcoholism, illicit drug use, huffing.
Clinical Course: In the ED the patient was anxious and, tremulous, HR 120. About 80 min after arrival the patient experienced a witnessed VF arrest that deteriorated into asystole. Resuscitative efforts were continued for 40 min but were unsuccessful.
Autopsy Findings: Not available, Antemortem blood 1, 1-difluoroethane 2.8 mcg/mL, acetone 1.5 mg/dL, delta-9 THC 1.2 ng/mL, delta-9 carboxy THC 19 ng/mL.
Case 405. Acute Freon inhalation/nasal: undoubtedly responsible.
Scenario/Substances: A 47 y/o male developed dyspnea after huffing five cans of keyboard duster containing 1, 1 difluoroethane over a 5-hour period. He was brought into the ED by EMS.
Past Medical History: Depression, ethanol abuse, narcotic abuse, and chronic huffing.
Laboratory Data: ECG sinus tachycardia.
Clinical Course: Anxious male, BP 90/54, HR 132–155, RR 34–36, T 36.6 C, and O2 sat 94%. Skin: cool, mouth was dry. The patient developed ventricular fibrillation that did not respond to resuscitation. The patient expired 1 hour after presentation.
Autopsy Findings: Medical Examiner determined the cause of death to be due to 1, 1 difluoroethane toxicity. Other significant conditions include severe atherosclerotic coronary disease, bilateral pulmonary emphysema with pulmonary hypertension, and a fibroblastic meningioma. Post-mortem heart blood level of 1, 1 difluoroethane was 0.94 mg/L. Blood ethanol was negative. No other toxicologic anaylsis was performed.
Case 413. Acute mushroom (cyclopeptides) ingestion: undoubtedly responsible.
Scenario/Substances: A 54 y/o female ingested suspected amanita mushrooms that she had foraged herself. She developed vomiting and diarrhea 12 hrs after the ingestion which lasted for 2 days. On the second day she became obtunded and was transported to the ED.
Laboratory Data: Na 141, Cl 116, K 6.2, HCO3 18, Hgb 21, Cr 3.0, BUN, 54, Ca 7.4, AST 513, ALT 435, bilirubin 1.6, INR 1.8. Day 2: platelets 61, INR 3.9, AST 1583, ALT 1901, bilirubin 3.5, anion gap 14, BUN 49 Cr 2.46, Ca 6.2, CK 4635 CKMB 95.9, troponin 1.65. Day 3: AST 5186, ALT 2489, bilirubin 5.3, INR 3.0. Day 9: INR 2.2 (down from a peak of 9.9) after 6 units of plasma, AST 223, ALT 609, phosphorus 3.7.
Clinical Course: In the ED, the patient was unresponsive. Her pupils were dilated and her respirations were agonal. She was intubated, sedated, and ventilated. IV fluids with bicarbonate and activated charcoal were administered, and she was admitted to ICU. HR 65, BP 86/52. That evening her gallbladder was aspirated. The patient was transferred to a tertiary care facility, admitted to the ICU, and administered silibinin and N-acetylcysteine. On Day 2, HR 120–130, urine output decreased, and she was started on CVVHD, hypotension required maximum doses of three vasopressors. She received IV antibiotics, calcium gluconate, and magnesium. She received FFP on Day 5. On Day 9, she was responsive to pain only, CxR showed pneumonia, she was in AF. She was added to the transplant list. On Day 13, no liver had become available and the patient expired.
Autopsy Findings: External exam performed. Cause of death was liver failure secondary to ingestion of toxic mushrooms. Manner of death was accidental.
Case 417. Acute mushroom (cyclopeptides) ingestion: undoubtedly responsible.
Scenario/Substances: A housekeeper at a Board and Care Home for elderly dementia patients collected and cooked wild (Amanita) mushrooms into a sauce for consumption by herself and six residents of the home. Three days later, one of these residents, an 87 y/o female was brought to the ED with lethargy, able to open her eyes and communicate with her daughter.
Past Medical History: Dementia patient with living will/ Do Not Resuscitate status.
Physical Exam: BP 107/51, HR 99, T 36°C, O2 sat 97% on 2 L O2.
Laboratory Data: AST 6365, ALT 3855, biirubin 4.9, alk phos 141, CK 47, Ca 9.6, PTT 54.4, INR 5.8.
Clinical Course: She was admitted and given IV fluids and initially received comfort care only. The patient's family requested more aggressive treatment and the patient received IV N-acetylcysteine. Modest output amber colored urine was observed. The patient expired later on the day of admission.
Autopsy Findings: Cause of death: fulminant hepatic failure due to ingestion of poisonous mushrooms. Other significant conditions included dementia, acute esophagitis, and atherosclerotic cardiovascular disease. Specific autopsy findings: Massive hepatic necrosis, spot-like hemorrhages involving serosal surfaces and mesentery, hemopericardium, hemorrhagic erosive esophagitis, atherosclerosis of the aorta and coronary arteries, calcification of the mitral valve, mild pulmonary congestion and edema with atelectasis, CNS changes suggestive of Alzheimers disease.
Case 418. Acute mushroom (cyclopeptides) ingestion: undoubtedly responsible.
Scenario/Substances: A housekeeper at a Board and Care Home for elderly dementia patients collected and cooked wild (Amanita) mushrooms into a sauce that she consumed with six residents of the home. Three days later, one of the residents, a 90 y/o male was brought to the ED with lethargy, nausea, vomiting, and diarrhea.
Past Medical History: Alzheimer's disease/dementia, renal failure, BPH, anemia, anxiety, and a history of urinary tract infections.
Physical Exam: Awake, alert, and responsive. BP 113/58, HR 123, O2 sat 95% on room air.
Laboratory Data: AST 360, ALT 329, t-bili 0.7, alk phos 63, urine was dark amber in color. Day 2: AST 5300, ALT 4400, t-bili 1.7, alk phos 87, and ammonia 34.
Clinical Course: N-acetylcysteine was started IV (IV NAC) for 21 hrs then discontinued. Day 2 AF occurred and was treated with metoprolol, which was stopped due to hypotension. IV NAC was restarted. Follow-up laboratory data:
WBC 8.4, Hgb 13.9, platelets 83K, Ca 8.3, INR 2.5, AST/ALT > 5000, and ALT peaked on Day 3 at 6200. On Day 7, platelets 9, T. bili 4.2, the patient developed C. diff infection and antibiotics were given, IV NAC continued. The patient's conservator decided to change to comfort care. On Day 8 the patient was transferred to a skilled nursing facility where he expired the following day.
Autopsy Findings: Autopsy cause of death as fulminant hepatic failure due to toxic mushroom poisoning. No other information provided.
Case 421. Acute malathione ingestion: contributory.
Scenario/substance: A 42 y/o male ingested 32 oz malathion, was decontaminated at the scene, became unresponsive during transport to the ED, and was intubated by EMS.
Past Medical History: Disruptive and paranoid behavior, family history of schizophrenia and Huntington's Chorea.
Physical Exam: Intubated, mechanically ventilated, and sedated with midazolam. BP 101/79, HR 97, RR 16, T 36°C, O2 sat 85% on 100% FiO2. Pupils were pinpoint, equal, and reactive to light, Oral cavity with large amount of secretions, Lungs clear.
Laboratory Data: ABG-pH 7.4 / pCO2 36 / pO2 548 UDS negative; acetaminophen and salicylates were not detected.
Clinical Course: Atropine, pralidoxime, and diazepam were administered in the ED. One liter of gastric contents were obtained via NG tube. The patient was admitted to the ICU and had severe diarrhea. The patient was treated with IV infusions of atropine and pralidoxime. On Day 3 he was extubated but continued to have periods of agitation and severe hypertension treated with haloperidol and multiple antihypertensive medications, his body fluids continued to have the odor of pesticide. On Day 4 the patient had a cardiac arrest and was re-intubated. Atropine was restarted for increased oral secretions and loose stools. The patient continued to have periods of restlessness and agitation, secretions diminished markedly and breath sounds were clear. On Day 10 fever and pulmonary infiltrate coincided with random jerking movements that were not seizure activity by EEG. On Day 17 Comfort measures were instituted and he expired on Day 20.
Autopsy Findings: Autopsy not performed.
Case 424. Acute borate ingestion: undoubtedly responsible.
Scenario/Substances: A 60 y/o female ingested roach tablets in an apparent suicide attempt. The patient sent an e-mail to a relative that stated she was planning to commit suicide using roach poison. The relative called a neighbor who found the patient unresponsive with shallow breathing and bluish emesis. A box containing boric acid from which an estimated 30–40 tablets were missing was found near the patient. Endotracheal intubation for airway support was performed prior to transport.
Physical Exam: Unresponsive, agitated female mechanically ventilated. BP 68/40, HR 116, RR 16, T 34.9 C. Poor distal perfusion, tachycardia with irregular heartbeat. Lungs with clear breath sounds. Skin warm, flushed, and dry with a pressure ulcer noted on her back. Sloughing of eyelids noted.
Laboratory Data: ABG-pH 7.09/pCO2 37.1/pO2 432. WBC 10.5, Hgb 11.5, Hct 34.7, platelets 178, Ca 8, total protein 5.4, albumin 2.5, t-bili 0.3, alk phos 60, AST 51, ALT 35, serum osmolality 314. UDS positive for benzodiazepines. Ethanol, acetaminophen, Li, and salicylate were not detected. PT 25.7, INR 2.41, PTT 64.9, CK 7533, troponin 0.01, CKMB 61.3, and CKMB index 0.8.
Clinical Course: In the ICU, bloody stool and excoriated skin was noted. The patient had pulseless electrical activity and resuscitation with CPR, epinephrine, and sodium bicarbonate was applied with success. Subsequent episodes of hypotension continued. Based on the prognosis, the family opted for institution of comfort measures and the patient expired approximately 12 hrs after admission.
Autopsy Findings: Cause of Death: Boric acid ingestion. Antimortem blood: caffeine positive, lidocaine positive, diazepam 240 ng/mL, nordiazepam 240ng/mL, oxazepam 22ng/mL, temazepam 24ng/mL, zolpidem 4.2 ng/mL, and boron 79,000 mcg/L.
Case 431. Acute selenous acid ingestion: undoubtedly responsible.
Scenario/Substances: 17 y/o male presented to the ED after ingestion of instant gun blueing agent.
Clinical Course: Intubated, unresponsive, vomiting, and incontinence of stool and urine. BP 80/20, HR 122. Patient remained hypotensive without evidence of peritonitis or gastrointestinal perforation. Within 2 hrs of presentation, the patient had asystole. Resuscitation attempts were unsuccessful and the patient expired.
Autopsy Findings: Cause of death : acute selenium toxicity. Stomach contents and lung parenchyma had a metallic odor, there was denuding of the mucosa of the larynx, epiglottis, esophagus, stomach, and duodenum. Lungs showed severe pulmonary congestion. There were effusions of multiple body cavities.
Case 432. Acute nicotine parenteral: undoubtedly responsible.
Scenario/Substances: A 29 y/o male was found by EMS to be in cardiopulmonary arrest with a suicide note indicating that he had intravenously injected himself with eLiquid.
Past Medical History: Depression
Laboratory Data: K 2.4, Glu 401, Cr 2.0, WBC 21.8, CK 977, troponin 0.34, and lactate 7.2. UDS was positive for amphetamine.
Serum acetaminophen, ethanol, ethylene glycol, methanol, and salicylate were not detected.
Clinical Course: He was intubated and resuscitated in the ED, with a GCS 3, but developed seizures resistant to treatment with lorazepam, phenobarbital, phenytoin, and leviteracetam. Head CT was unremarkable. He was transferred to a tertiary care hospital where the seizures were controlled with propofol. Therapeutic hypothermia was initiated, but he never regained consciousness. He was diagnosed with anoxic encephalopathy and declared brain dead on Day 5.
Autopsy Findings: An autopsy was not performed as he was an organ donor. Comprehensive serum drug testing done on specimens collected on presentation detected only lidocaine, which he received during his resuscitation, nicotine 2000 ng/mL, and nicotine's primary metabolite, cotinine 2100 ng/mL.
Case 440. Acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances: A 10 y/o female with a decreased level of consciousness, agitation, and dehydration was transported to the ED by family members. Reasons for symptoms unknown. An empty acetaminophen bottle was found at her bedside.
Laboratory Data: On admission acetaminophen 102 mcg/mL (time of ingestion unknown), AST 700, ALT 1100. 18 hrs post arrival: AST 3900, ALT 6000.
Clinical Course: Intravenous NAC was initiated and patient was transported via helicopter to a pediatric ICU. Upon arrival she was intubated and placed on a ventilator. Head CT results were inconclusive. At 18 hrs after initial presentation the patient's ammonia level was elevated and liver enzymes continued to rise, she was placed on the liver transplant list. Her condition continued to deteriorate over the next 12 hrs at which time brain death studies were performed, she was taken off the ventilator and expired shortly thereafter.
Autopsy Findings: Complete autopsy shows subacute large duodenal ulcer and liver necrosis compatible with toxic hepatitis due to acetaminophen. Postmortem toxicological studies including hospital blood samples obtained, confirm the toxic levels of acetaminophen. No other drugs were detected. Cause of death: acute hepatic failure due to massive hepatic necrosis from acute acetaminophen intoxication. The underlying natural disease of large duodenal ulcer would be a participating factor to her death. The manner of death was ruled to be an accident.
Case 446. Acute acetaminophen/hydrocodone, alprazolam ingestion: undoubtedly responsible.
Scenario/Substances: A 16 y/o male posted on social media that he wanted to hurt himself. He was last seen awake when his mom awoke and found him crouching by her bed, near her medications, which were acetaminophen/hydrocodone and alprazolam. When mom awoke the next morning she noted that 26 of the acetaminophen/hydrocodone and 29 of the alprazolam were missing. She awoke the patient and asked him about this, but he denied taking any medications. She noted that his speech was slurred. He went back to sleep. About 13.5 hrs post-ingestion mom noted his hand looked white and he was not breathing. EMS was called and found him to be asystolic. CPR was started and he received epinephrine, atropine, vasopressin, and sodium bicarbonate. He was transported to the ED.
Past Medical History: Attention deficit hyperactivity disorder, not currently on any medications, depression with suicidal ideation and previous suicide attempts, marijuana and tobacco use.
Physical Exam: At the initial hospital the patient was intubated, received IV fluids, and had a CT of his brain. The CT showed diffuse cerebral edema and loss of gray–white differentiation. The patient was then transferred to a tertiary care facility.
Laboratory Data: ABG-pH 7.08 / pCO2 75 / pO2 335/ BE 9.
Clinical Course: The patient had not received any pain or sedation medications. At 17.5 hrs a brain death exam showed no brain reflexes, including no corneal reflexes. His pupils were 6 mm, dilated, and nonreactive bilaterally. He also had negative doll's eyes, negative cold caloric exam, and negative gag. An arterial line and a central venous line were placed. He initially had significant hypertension (BP 170/110), then had precipitous drops in his BP. He was treated with milrinone and low-dose dopamine. His CxR showed a right upper lobe collapse and a right lower lobe effusion. He required high ventilator settings to maintain oxygenation. He developed lactatic acidosis, shock liver, and prerenal dehydration. An excessive urine output was treated with vasopressin. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 2.
Autopsy Findings: No autopsy was performed. Coroner's cause of death: cerebral anoxia due to polydrug intoxication. Post-mortem drug levels: serum alprazolam 176 ng/mL (therapeutic range: 10–40), THC 1.1 ng/mL, THC-COOH 26.4 ng/mL, hydrocodone 249 ng/mL (therapeutic range: 10–40), acetaminophen 30 mcg/mL (therapeutic range 10–30), caffeine positive; Urine concentrations: alprazolam 1420 ng/mL, a-OH-alprazolam > 2500 ng/mL, hydrocodone > 10, 000 ng/mL, hydromorphone 1839 ng/mL, carboxy-THC 139 ng/mL.
Case 456. Acute salicylate ingestion: undoubtedly responsible.
Scenario/Substances: An 18 y/o female ingested 500 salicylate 325 mg tablets 6 hrs before arrival to the ED in an attempted suicide. Prior to arrival to the ED, the patient complained of ringing in her ears, vomited without pill fragments in the emesis.
Past Medical History: Bipolar disorder, schizoaffective disorder, ADHD, history of multiple suicide attempts.
Physical Exam: Awake, alert, and oriented. BP 138/74, HR 151, RR 24, T 37.7°C, O2 sat 99% on room air. Lungs clear, skin warm and dry.
Laboratory Data: ABG-pH 7.41 / pCO2 26 / pO2 130.
Salicylate 90 mg/dL, lactate 4.5 mmol/L, acetaminophen and ethanol were not detected. HCG and UDS negative. AG 6.0, AST 16, ALT 18, INR 2.1.
Clinical Course: The patient received a bolus then continuous infusion of sodium bicarbonate and was admitted to the ICU. Her lethargy and confusion worsened, follow-up salicylate 94.3 mg/dL. Hemodialysis was performed 12 hrs after arrival to the hospital, salicylate 67 mg/dL. At 8 hrs post dialysis the patient has respiratory distress and becomes unresponsive, is intubated and mechanically ventilated, salicylate 132 mg/dL. The patient has a cardiac arrest, resuscitation efforts are unsuccessful and the patient expired < 24 hrs after admission to the hospital.
Autopsy Findings: Cause of death was salicylate intoxication. The manner of death is suicide. The liver showed vascular congestion in the midzonal region. Antemortem blood showed salicylic acid 654 mg/L, salicylic acid was positive in urine.
Case 496. Unknown, salicylate, doxylamine, acetaminophen, ibuprofen ingestion: undoubtedly responsible.
Scenario/substance: A 22 y/o male presented with altered mental status, weakness, hearing impairment, and fever. He admitted taking aspirin and ibuprofen in an apparent suicide attempt.
Physical Exam: Diaphoretic, BP 123/71, HR 120, RR 30, T 38.8°C (rectal).
Laboratory Data: ABG-pH 7.53/pCO2 19.4/pO2 107.4, K 5.0, HCO3 14, Cr 1.6, glucose 130. Salicylate 111 mg/dL, acetaminophen 12.6 mcg/mL, INR 1.9. A later ABG after intubation but just before or around the time of his arrest demonstrated a pH 7.24 / pCO2 52, / pO2 152.
Clinical Course: He initially did not provide a history of drug overdose, was evaluated for possible sepsis, and given antibiotics and IV fluids. When the salicylate level became known a bicarb drip was started and he was transferred to tertiary hospital for dialysis. At the second HCF he was obtunded and was intubated and allowed to maintain a high minute ventilation of > 20 L/min), but was sedated with propofol. A dialysis catheter was placed; shortly after transfer to ICU he became hypotensive and bradycardic progressing to cardiac arrest with ventricular tachycardia, ventricular fibrillation then asystole. Resuscitation was unsuccessful and the patient expired.
Autopsy Findings: The medical examiner determined the cause of death to be due to salicylate toxicity. Post-mortem iliac vein blood demonstrated doxylamine 0.35 mg/L. Liver demonstrated doxylamine at less than 2.0 mg/kg; benzodiazepines, cocaine metabolite, ethanol, and gabapentin/pregabalin were not detected.
Case 538. Unknown, salicylate, alprazolam ingestion: undoubtedly responsible.
Scenario: A 26 y/o pregnant female was discovered at home with altered mental status and agitation. A nearly empty bottle of aspirin was found next to her. Naloxone 0.4 mg and D50 were given prior to transport without effect.
Past Medical History: Depression, substance abuse (crack and methadone), and prior suicidal overdoses.
Physical Exam: Groaning, agitated female with fixed stare to her right. BP 152/73, HR 140, RR 48, O2 sat 99% on 80% FiO2.
Laboratory Data: ABG-pH 7.03/pCO2 70/pO2 213
WBC 23.1, Ca 8, Salicylate level, 90 mg/dL, UDS positive for benzodiazepines, barbiturates, and THC. Acetaminophen was not detected. Urine pregnancy test positive.
Clinical Course: Because of her focal neurological findings, initial diagnostic studies were done to rule out an intracranial event and were negative. When the salicylate returned, alkaline therapy was started and hyperventilation was implemented by changing ventilator settings. Emergent dialysis was planned, fetal ultrasound was performed prior to attempting dialysis. During dialysis catheter placement, sudden hypotension and cardiac arrest occurred. Resuscitation attempts were unsuccessful and the patient expired 90 min after arrival.
Autopsy Findings: Medical examiner determined the cause of death as salicylate toxicity. Post-mortem vena cava blood showed alprazolam 0.008 mg/L, lorazepam 0.026mg/L, and salicylates 39 mg/dL. The following substances were not detected: cocaine metabolite, ethanol, gabapentin/pregabalin, opiates/opioids, and organic acids; organic bases or organic neutrals were detected in aortic blood. Autopsy findings included moderate pulmonary congestion and an intrauterine pregnancy.
Case 563. Acute salicylate ingestion: probably responsible.
Scenario/Substances: A 27 y/o male came to the ED stating he took ˜60 aspirin to see what would happen. He arrived ˜5 hrs post ingestion awake and alert. He complained of difficulty breathing. He denied suicidal ideation, headache, lightheadedness, dizziness, and visual or auditory hallucinations.
Past Medical History: Depression treated with aripiprazole.
Physical Exam: BP 136/84, HR 111, RR 16, O2 sat 98% on room air. Patient was diaphoretic, alert, and oriented x 3.
Laboratory Data: ABG-pH 7.34 / pCO2 28 / pO2 130, Na 137, K 4.1, Cl 107,Glu 146, Cr 1.45, BUN 19, Ca 8.5, INR 1.2, PTT 23, ECG sinus tachycardia QRS 86, and QTc 408. Serum ethanol and acetaminophen were not detected, salicylate > 100 mg/dL.
Clinical Course: In the ED he received a bolus of NS along with 1 ampule of sodium bicarbonate, activated charcoal with sorbitol. Prior to admission to ICU the patient became acutely psychotic, aggressive with staff, biting, and staring off into space. Intubation was attempted after 1 amp of bicarb given prior to rapid sequence intubation along with versed. The patient became hypoxic and bradycardic, received ACLS meds and CPR, the rhythm was restored and patient intubated. After admission to ICU, the patient's HR was in the 180s with a wide complex, treated with adenosine. At 6 hrs after admission, the patient became febrile to 40.5°C. He developed asystole, ACLS failed to restore circulation and he died.
Autopsy Findings: Not done.
Case 629. Acute colchicine and omeprazole ingestion: undoubtedly responsible.
Scenario/Substances: A 31 y/o male intentionally ingested 160 tablets of colchicine 0.6 mg and 30 tablets of omeprazole 20 mg. He immediately reported the ingestion and was transported to the ED with 1 episode of emesis prehospital.
Past Medical History: Asthma, depression, pericarditis, previous overdoses including in the last 6 months.
Laboratory Data: WBC 2.8, INR 1.0, AST 25, ALT 61 alk phos 60; serum acetaminophen, ethanol, and salicylate were not detected.
Clinical Course: On arrival to the ED, he was awake, alert, and oriented, BP 125/76, HR 75, RR 16, O2 sat 99% on room air, T 37°C, pupils 3–4 mm, equal, and reactive. Heart and lungs unremarkable, bowel sounds present. He received 50 g activated charcoal without sorbitol for the estimated 1.3 mg/kg ingestion of colchicine. On Day 1 he remained awake and alert with frequent nausea and vomiting despite ondansetron and metoclopramide. WBC count rose over the first day to a peak of 32. ALT 4216, AST 4435. The patient's status deteriorated over the next 3 days with progressive worsening of hemodynamic status. The WBC count began to decrease at ˜48 hrs after ingestion to 15.7. Multi-system organ failure with renal toxicity and hepatotoxicity ensued, The patient became unresponsive and expired on Day 3.
Autopsy Findings: Final Pathologic Diagnoses: 1) acute centrilobular necrosis, 2) acute renal failure, 3) pericardial effusion, 4) pleural effusions bilaterally, 5) no evidence of pericarditis, 6) GI System: 120 mL of brown thick partially digested food with 2 round tan-blue pills seen. Colchicine (hospital blood) 49 ng/mL. Cause of Death: Complications of acute colchicine intoxication. Manner of Death: Suicide.
Case 689. Acute ibuprofen ingestion: undoubtedly responsible.
Scenario/Substances: A 34 y/o female, last seen 11 hrs earlier, was found unresponsive and incontinent of urine. A suicide note dated the previous day was later found.
Past Medical History: The patient had been treated for poison ivy with steroids and another medication.
Physical Exam: HR 120–130, systolic BP 102, ECG sinus tachycardia, otherwise normal intervals.
Laboratory Data: ABG-pH 7.1 / HCO3 9.9
Clinical Course: The patient arrived to the ED with pink residue on her mouth. A nasal trumpet was placed. A few hrs later the patient was still unresponsive and was intubated with propofol. Systolic BP was 105. A half-empty 1000-tablet bottle of ibuprofen was found. Serum acetaminophen, salicylate, and ethanol were not detected. UDS was negative, lactate 6.7, BUN 18, Glu 126, and serum osmolality 302. She had received a 200 mEq bolus + 150 mEq of sodium bicarbonate in 2L D5W. Her systolic BP was in the 100s, pH 6.83 / pCO2 25, lactate 10.8, CKMB < 0.5, Cr 1.8. A hemodialysis catheter and NG tube were placed; the NG yielded 350 ml of pink pasty material. Hemodialysis was started, but stopped after 1.25 hrs due to persistent hypotension despite fluid boluses and phenylephrine. She had scant urine output. She remained unresponsive, pupils 2–3 mm and non-reactive, BP 70/20, HR 80s. ECG showed inverted P waves and ST depression. Her NG tube drained 500 ml of fluid. ABG- pH 7.14/pCO2 27/pO2 252/HCO3 10.6/ BE 18.4, Na 147, K 4.1, Cl 104/HCO3 12, and CK 285. Her condition declined over the next few hrs, systolic BP 50–70 on maximum doses of phenylephrine, she tolerated only an additional 30 min of hemodialysis, ABG-pH 6.8/HCO3 2, lactate 26.4. Based on the prognosis, the family opted for institution of comfort measures and she expired ˜24 hrs post-arrival.
Autopsy Findings: The anatomical diagnosis included severe pulmonary edema, pale kidneys, gastric contents consisting of brown-thin liquid with white settling particles. The final cause of death was complications of ibuprofen toxicity, with manner suicide. Qualitative toxicological findings included cardiac blood positive for ibuprofen, ibuprofen-related compounds, pramoxine, and opiates. Toxicological findings: cardiac blood ibuprofen 262 mg/L, morphine (free) 860 ng/mL, vitreous morphine (free) 170 ng/mL. The post-mortem ibuprofen concentration > 24 hrs after ingestion was > 10 times the expected peak therapeutic concentration for ibuprofen.
Case 710. Fentanyl (transdermal) ingestion: probably responsible.
Scenario/substance: A 36 y/o male was found unresponsive by a friend in a car. A short time before he had chewed and swallowed a 25 mcg fentanyl patch. He was pulseless and apneic. CPR was initiated and EMS called. He was successfully resuscitated.
Past Medical History: COPD, drug abuse, Hepatitis C, status post cholecystectomy.
Physical Exam: BP 117/68, HR 103, RR 22 rpm, O2 sat 100% on 100% FiO2. GCS 3, flaccid, pupils non-reactive. Lungs: clear, Abdomen: not distended.
Laboratory Data: ABG-pH 7.01 / pCO2 51 / pO2 172, CK 14,012, ALT 670, AST 826, INR 1.3, phos 8.2, troponin peak 8.59. Ethanol 102 mg/dL, salicylate and acetaminophen were not detected. Toxic alcohol screen was negative. Abdominal xray was negative for the patch. Initial lactate was 10.5, falling to 2.5 in 4 hours.
Clinical Course: The patient was intubated and given epinepherine and vasopressin. Naloxone infusion was started without response. Albuterol nebulizer was given at the first HCF. The patient received D50 for hypoglycemia and a single dose of activated charcoal. The patient was treated with the hypothermia protocol due to the cardiac arrest and transferred to tertiary care hospital where he was sedated with propofol and fentanyl infusions. Follow-up laboratory data showed ALT 1616, AST 1868, CK 23, 167, INR 1.3, phosphorus 8.2 mg/dL, peak troponin level 8.59 on Day 2. N-acetyl cysteine infusion was started following an oral loading dose. One dose of fomepazole was administered empirically based on the patient's metabolic acidosis. On Day 4, 10 mg naloxone was given without response. The patient had acute renal failure with diabetes insipitus and pulmonary atalectasis. Over the next 7 days he demonstrated severe anoxic brain injury and brain herniation determined by neuro exam and CT of the head. Comfort care was instituted, the patient expired on Day 7.
Autopsy Findings: Post-mortem toxicology was not performed for fentanyl due to its use therapeutically during the hospital course. No autopsy was performed. Medical examiner determined the cause of death to be due to anoxic brain injury due to presumed fentanyl overdose.
Case 727. Acute-on-chronic fentanyl (transdermal), diazepam, gabapentin ingestion, dermal: undoubtedly responsible.
Scenario/substance: A 37 y/o male was found unresponsive by his daughter. When EMS arrived, a 100 mcg/hr fentanyl patch was removed from his skin. HR 114, BP 138/95, RR 5, and GCS 3. He was given 1 mg naloxone IV by EMS and was alert and oriented on arrival to the ED.
Past Medical History: Multiple surgeries and chronic pain following a motor vehicle collision. Medications: diazepam, gabapentin, cyanocobalamin, calcium with vitamin D, methadone, acetaminophen/hydrocodone, and transdermal fentanyl. His fentanyl dose had been increased from 75 mcg/hr to 100 mcg/hr within the last 48 hrs.
Physical Exam: He had a 2 cm hematoma to the right frontal scalp.
Laboratory Data: Serum acetaminophen, ethanol, and salicylate were not detected. Head CT was unremarkable.
Clinical Course: He was fed and observed until 4 hrs after initially being found. He was then sent home, with a 50 mcg/hr fentanyl patch placed 10 min prior to discharge. At this time his RR 13. He was found dead by his family on his bathroom floor < 12 hrs after discharge.
Autopsy Findings: A full autopsy was not performed. Post-mortem subclavian blood: fentanyl 64 ng/mL, diazepam 0.24 mg/L, nordiazepam 035 mg/L, gabapentin 15 mg/kg. The toxicology screen did not analyze for methadone. Cause of death: asphyxia due to hypoventilation from combined fentanyl and diazepam toxicity.
Case 827. Acute acetaminophen/diphenhydramine ingestion: undoubtedly responsible
Scenario/Substances: EMS brought a 43 y/o female to the ED after an ingestion of 96 tablets of acetaminophen/ diphenhydramine in a suicide attempt earlier that day. A suicide note was found at the scene. She denied co-ingestants. She was reportedly drowsy en route to the hospital and given 1 dose of naloxone with questionable improvement. Her blood glucose level was also checked en route and found to be 17. She was given 25 grams of dextrose, after which she became more alert.
Past Medical History: Anxiety, depression, previous suicide attempt, and breast implants. Medications: citalopram.
Physical Exam: BP 86/34, HR 96, RR16. Her temperature could not be obtained initially as patient was screaming. Later she was awake, but not oriented with diffuse abdominal tenderness, no jaundice noted.
Laboratory Data: PT 67.5, INR 8.66, platelets 259, WBC 15.2, Hgb 10.6, acetaminophen 38.7 mcg/mL, salicylates were not detected, Glu 89, BUN 19, Cr 2.2, Ca 8.3, Na 135, K 3.6, Cl 97, HCO3 18, anion gap 23, UDS positive for opiates only, albumin 3.6, bilirubin 3.4 (total) 1.8 (direct), alk phos 112, ALT 12,275, AST 15,309, Lactate 6.
Clinical Course: She arrived at the ED agitated. BP was 121/41 after a fluid bolus. She was admitted to the hospital, but due to worsening mental status and abnormal laboratory studies, she was transferred to a tertiary care facility on Day 2. Shortly after transfer she exhibited worsening mental status and increasing agitation. She was started on IV N-acetylcysteine. She was tachypneic (RR 30–50), was intubated, placed on a ventilator. Head CT showed cerebral edema. She had hematuria and hemorrhage from both orbits. Propofol and midazolam infusions were started due to persistent tachypnea and tachycardia, presumed related to pain. Repeat head CT on Day 3 that showed increasing cerebral edema with mass effect, INR 9.2, Cr 4.56, HCO3 12, ALT 4,876, AST 4,912, ammonia 186. She was given vitamin K, FFP, and prothrombin complex concentrates. On Day 4 the patient continued to do poorly, CVVHD was started. A subarachnoid bolt was placed and her ICPs were noted to increase to 60 cm H2O throughout the evening, during which time she was noted to lose all reflexes. Her ICP was unresponsive with intermittent rapid shallow breathing. INR was reduced to 2.5 AST 3,038, ALT 3,334, Cr 3.5, ammonia 141 after lactulose. On Day 5 she remained unresponsive with no reflexes and intermittent rapid shallow breathing. On Day 6 the patient continued to do poorly with ICPs in the 50–60 cm H2O range, for which she was given 3% saline. Based on the prognosis, the family opted for institution of comfort measures and she expired on Day 8.
Autopsy Findings: Cause of death: diffuse centrilobular liver necrosis due to intoxication by acetaminophen. Manner of death: suicide. Other findings included cerebral edema, cerebellar tonsillar herniation, and pulmonary edema. There was no detectable acetaminophen in post-mortem femoral blood.
Case 946. Acute-on-chronic, colchicine ingestion: undoubtedly responsible.
Scenario/Substances: A 48 y/o depressed male with history of gout took an overdose of his colchicine.
Past Medical History: Gout, alcoholism, colchicine overdose.
Laboratory Data: Ca < 5 mg/dL, Glu 497, Cr 5, bilirubin 4.1, alk phos 275, AST 791, ALT 142, ABG-pH 7.26 / pO2 68, ammonia 51 mmol/L.
Clinical Course: In the ED, HR 130, BP 97/60, RR 20, O2 sat 93% on room air. Hypotension was treated with IV fluids and vasopressors. He was found to have pancreatitis and pancytopenia due to the colchicine. He required ventilatory support, enteral nutrition, antibiotics, and bicarbonate drip due to renal insufficiency. The patient's condition continued to deteriorate, requiring more vasopressors. He developed oliguria, Cr 5. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 7.
Autopsy Findings: Autopsy was not performed, cause of death: toxic overdose of colchicine, manner of death: suicide.
Case 958. Acute colchicine ingestion: undoubtedly responsible.
Scenario/Substances: A 48 y/o male took 58 tablets of colchicine 0.6 mg with suicidal intent. About 1 hour later he developed abdominal pain and subsequently had many episodes of vomiting and diarrhea. He presented to the hospital approximately 13 hrs post ingestion.
Past Medical History: Gout, hypothyroidism, thyroid goiter, sleep apnea, severe obesity, and recurrent major depression, rare alcohol use. Medications: levothyroxine, aripiprazole, fluoxetine, allopurinol, and colchicine 0.6 mg twice a day.
Physical Exam: BP125/97, HR 103, RR 22, T 37°C. Alert, chronically ill appearing, disheveled, anxious, in mild distress, complaining of 9/10 abdominal pain and some nausea. Abdomen: mild, diffuse abdominal tenderness.
Laboratory Data: ABG-pH 7.37 / pCO2 32 / pO2 88, AST 119, ALT 58,, alk phos 170, CK 555, troponin 0.07, WBC 18, Hgb 16.2, platelets 290, salicylate, acetaminophen, and ethanol were not detected, CxR normal, ECG showed normal sinus rhythm.
Clinical Course: The patient was given a fluid bolus, transferred to the ICU and placed on oxygen. Abdominal pain, vomiting, and diarrhea continued. He developed a fever, worsening metabolic acidosis, and deteriorating renal function, and required intubation due to worsening hypoxia. On Day 2, he had a cardiac arrest, was resuscitated with recovery of HR and BP but remained hypotensive despite vasopressors. Hemodialysis was attempted but terminated due to hypotension. Based on the prognosis, the family opted for comfort measures only and the patient expired on Day 3.
Autopsy Findings: No autopsy was performed.
Case 1247. Acute salicylate ingestion: probably responsible.
Scenario/Substances: A 60 y/o male ingested 400 tablets of aspirin in the morning and presented to the ED at mid-day.
Past Medical History: Hypertension, on losartan; depression and bipolar disorder on duloxetine, trazadone, lamotrigine; prior suicide attempts.
Physical Exam: BP 159/90, HR 124, RR 39, complaining of tinnitus, and exhibiting some tremor.
Laboratory Data: Na 150, BUN 13, Cr 1.4. ABG- pH 7.46/pCO2 30/pO2 71. UDS positive for benzodiazepines. Serum salicylate 79.7 mcg/mL @ ˜1.5 and 128 mcg/mL @ ˜7.7 hrs after admission.
Clinical Course: Patient was treated with IV bicarbonate, but expired prior to transfer to a facility where dialysis was available.
Autopsy Findings: ME report showed post-mortem salicylate 690 mcg/mL, nordiazepam 0.094 mcg/mL; diazepam, trazadone, and lamotrigine were present.
Case 1389. Chronic dabigatran ingestion: probably responsible.
Scenario/Substances: A 77 y/o male was admitted to the hospital ICU with acute GI bleeding and hypotension. He was on dabigatran etexilate mesylate and had recently had his dose lowered from 150 mg/day to 75 mg/day.
Past Medical History: Atrial fibrillation.
Physical Exam: This patient had bleeding from several sites, including oozing from his triple-lumen internal jugular central IV site and bright red blood per rectum.
Laboratory Data. PTT > 200, PT 148, Cr 2.8, ABG-pH 7.0.
Clinical Course: He was endotracheally intubated and placed on mechanical ventilation. He remained hypotensive despite vasopressors with metabolic acidosis and oliguria. He received 4 units of FFP and 4 units of packed RBCs and was started on hemodialysis. The patient died on Day 2. The cause of death was acute GI bleeding with hypovolemic shock.
Autopsy Findings: Not performed.
Case 1418. Acute-on-chronic, carbamazepine, cyclic antidepressant, unknown ingestion: undoubtedly responsible.
Scenario/Substances: A 50 y/o male ingested 24 grams of carbamazepine and 13.5 grams of clomipramine in a suicide attempt.
Past Medical History: Seizure disorder, depression. Medications: carbamazepine and clomipramine.
Physical Exam: Obtunded, not responsive to stimuli, BP 120/80, HR 77.
Laboratory Data:
Ca 8.6, lactate 1.4, CK < 0.05, AST 27, ALT 43. Serum acetaminophen, ethanol, and salicylate were not detected. Blood clomipramine 1600 ng/mL, norclomipramine 1046 ng/mL. ECG: PR 160, QRS 110, QTc 471, HR 81.
Clinical Course: Upon arrival to the ED, the patient was intubated with sedation and paralyzed with vecuronium. Within the next 5 hrs, he had a seizure and was hypotensive, treated with phenylephrine then vasopressin and norepinephrine were added. He was initially given 1 ampule of sodium bicarbonate. At that time ABG-pH of 7.17 / pCO2 69. In the subsequent 5 hrs, he was able to be weaned off phenylephrine, remaining on vasopressin, norepinephrine, and sodium bicarbonate. At that time his pupils were fixed and dilated. Serum carbamazepine 35.4 mcg/mL (upon arrival), 32.1 (12 h after arrival), 18.2 (15 hr later), 26.8 (24 hr later), 22.6 (8 hr later), 23.7 (16 hr later), 23.6 (11 hr later)
The patient received multi-dose activated charcoal for ˜3 days. Hypoactive bowel sounds were noted, and WBI was not performed. He expired on Day 8.
Autopsy Findings: A hospital autopsy findings: pulmonary edema, acute cerebral infarct in the left posterior cerebral artery, and evidence of a 6 cm drug bezoar in the stomach, with diffuse pill fragments surrounding it. Charcoal was found in the stomach, duodenum, proximal jejunum, skipping the rest of small intestine until the terminal ileum and continuing to the transverse colon, with nothing in the remainder. No charcoal stools were noted during the hospital course. Cause of death was mixed drug intoxication via suicide.
Case 1425. Bupropion (extended release), aripiprazole ingestion: undoubtedly responsible.
Scenario/Substances: A 17 y/o female reported to her parents that she had overdosed on bupropion. EMS was called, but she denied the ingestion to them and refused transport. She had a seizure 3 hrs after initial EMS call followed by cardiac arrest. She was transported to the ED.
Past Medical History: Depression
Physical Exam: HR 100, BP 116/56 (on norepinephrine), Pupils 5–6 mm and non-reactive, no response to painful stimuli.
Laboratory Data: ABG-pH 7.28 / pCO2 53, serum acetaminophen and salicylates were not detected.
Clinical Course: The patient presented to the ED in cardiac arrest following witnessed seizure activity. She was intubated, had return of spontaneous circulation, and was started on a norepinephrine infusion for hypotension. Acidemia was treated with a sodium bicarbonate infusion. A therapeutic hypothermia protocol was initiated. Head CT showed massive cerebral edema. The patient was transported to the PICU at a tertiary care facility. She received intralipid, but her BP continued to decline and she required dopamine infusion in addition to norepinephrine. She had no evidence of brain activity and was ultimately declared brain dead.
Autopsy Findings: Toxicology revealed a bupropion level of 0.21 mg/L. Cause of death: hypoxic encephalopathy due to acute bupropion toxicity.
Case 1437. Acute-on-chronic bupropion (extended release), methylphenidate, polyethylene glycol ingestion: probably responsible.
Scenario/Substances: A 24 y/o male had two witnessed grand mal seizures at home and was brought to the ED with the history from family members of a recent social media posting stating that he ingested 90 bupropion 300 mg extended release tablets and 10 methylphenidate tablets to get attention. The patient was dazed and tremulous. Lorazepam 2 mg was given, IV fluids were started and he received another 2 mg lorazepam.
Past Medical History: ADHD, Tourette syndrome, development delay, depression, anxiety, and previous suicide attempts.
Physical Exam: HR 160s. pupils dilated.
Clinical Course: The patient was given 4 mg lorazepam and admitted to ICU. After lorazepam, BP 131/89, HR 110. The patient remained agitated and confused. An NG tube was placed, whole bowel irrigation was administered, the patient vomited and possibly aspirated. Two hrs later, another seizure occurred and he had a cardiac arrest and could not be resuscitated.
Autopsy Findings: Death was attributed to acute bupropion and methylphenidate ingestion. Blood concentrations from ante-mortem hospital samples showed bupropion 1300 ng/mL, hydroxybupropion 1300 ng/mL, methylphenidate 47 ng/ml, ritalinic acid 1000 ng/mL, and qualitative caffeine positive.
Case 1531. Acute bupropion (extended release), ethanol, venlafaxine ingestion: undoubtedly responsible.
Scenario/Substances: A 53 y/o male arrived to the ED late in the evening with the police after he reported that he ingested 20–30 bupropion extended-release 300 mg tabs and police reported he had had at least 9 large beers.
Past Medical History: Alcoholism, depression, and bipolar disorder. Medications: bupropion, valproic acid, and gabapentin.
Physical Exam: BP 124/80, HR 80, RR 24, T 37°C, O2 sat 100% on room air. The patient was alert and complained of nausea.
Laboratory Data: Na 137, K 3.9, Cl 102, HCO3 21, BUN 6, Cr 0.9, ethanol 350 mg/dL; serum valproate, acetaminophen, and salicylate were not detected. ECG and QRS were unremarkable.
Clinical Course: He received ondansetron and IV crystalloid for nausea. A few hrs after admission (early in the morning of Day 2, still in the ED) the patient developed status epilepticus and was intubated and ventilated with oxygen. He was placed on a propofol infusion and the seizures stopped. Neurology was consulted and started the patient on phenytoin and levetiracetam. On Day 2 the patient deteriorated further, he was responsive only to painful stimuli. He was requiring norepinephrine, epinephrine, and vasopressin for hypotension. Continuous EEG monitoring confirmed seizure activity had stopped. Propofol was discontinued in light of hypotension. On Day 8, the patient was no longer responsive to painful stimuli. EEG showed no further seizure activity and the patent remained on three vasopressors and no sedatives. On Day 9, based on prognosis, comfort measures were instituted and he expired.
Autopsy Findings: Cause of death: acute multiple drug intoxication from bupropion, venlafaxine, and ethanol. Manner of death: suicide. A total of 8–12 tablet remnants were recovered from the patient's small bowel. Also noted was diffuse softening of the brain due to anoxic/ischemic encephalopathy, and signs of fluid overload: diffuse pulmonary edema, ascites (750 mL), and bilateral pleural effusions (350 mL each). GC/MS Basic UDS obtained by the Coroner's office was positive for bupropion, bupropion metabolite, nicotine, norvenlafaxine, and venlafaxine. Hospital blood was sent by the coroner and showed a bupropion level of 416 ng/mL, a hydroxybupropion level of 514 ng/mL, norvenlafaxine level of 312 ng/mL, and venlafaxine level of 258 ng/mL. Hospital blood samples also showed the blood ethanol level was 298 gm%. No post-mortem blood samples were analyzed.
Case 1542. Acute-on-chronic vilazodone, alprazolam, salicylate, escitalopram, and duloxetine ingestion: undoubtedly responsible.
Scenario/Substances: A 55 y/o male was found unresponsive, unknown down time, with empty bottles of vilazodone 10 mg (21 missing), and alprazolam 0.5 mg (100 missing), prescribed for him the day before. He was despondent over a pending divorce.
Past Medical History: Depression, past suicide attempts, alcohol abuse. Medications: escitalopram, aripiprazole, and clonazepam on previous admission.
Physical Exam: BP 70/30, HR 38, atrial flutter, RR 5–6, T 40°C, pupils 5 mm, emesis in mouth and pharynx, nystagmus, extremities mottled, mild tremor in feet and rigidity in lower extremities, diaphoresis, O2 sat 84% on 100% oxygen.
Laboratory Data:
Lactate 4.5, AST 56, ALT 184, CK 7736, CxR: hazy infiltrates. Serum acetaminophen and ethanol were not detected, salicylates 26 mg/dL.
Clinical Course: He was intubated and given paralytics to control hyperthermia (T 42.5°C) 1 hour after arrival. He also received 1 dose of activated charcoal and 2 mg of naloxone. Dysrhythmias were treated with lidocaine and amiodarone, hypotension with norepinephrine, epinephrine, and dopamine, and bradycardia with atropine. Repeat CK 8,000. En route to transfer to a tertiary care center, he had a cardiorespiratory arrest, was given CPR, and had vomited around the tube with coffee-ground emesis. His HR after resuscitation was 60 paced, QRS 152, QT 430. Serum pH was 7.09. He shortly thereafter went into VT, could not be resuscitated and expired within 4 hrs of arrival to the ED.
Autopsy Findings: Autopsy found heart blood levels of salicylate 11 mg/dL, duloxetine 930 ng/mL, and citalopram/escitalopram 1200 ng/mL, pulmonary edema and listed cause of death as drug overdose via suicide. There was no cerebral edema.
Case 1605. Acute hydroxychloroquine, metformin, acetaminophen/diphenhydramine, hydroxyzine, cocaine, alprazolam, and pregabalin ingestion: undoubtedly responsible.
Scenario/Substances: A 33 y/o female was brought to the ED unresponsive, with depressed respirations, bradycardic, and hypotensive. Naloxone was given in the field with no response. Her list of medications and empty pill bottles included hydroxychloroquine sulfate 200 mg, metformin 500 mg, pregabalin 150 mg, acetaminophen/diphenhydramine, and naproxen 375 mg. Her family provided additional history that the patient was prescribed pregabalin, but had not taken it for at least a day, no naproxen was taken but the patient did crack cocaine sometime earlier that day.
Past Medical History: Polycystic ovarian syndrome, previous suicide attempts, polydrug user including oxymorphone, fentanyl, and other opioids.
Physical Exam: Unresponsive, BP 40, HR 40, pupils dilated.
Laboratory Data: K 2.5, acetaminophen 100 mcg/ml, UDS positive for benzodiazepines, cocaine, opiates, and propoxyphene. ECG: QRS 150, QTc 478. 3 hrs after presentation to the ED: pH 7.0 then 5 hrs later: pH 6.96, K 1.4, and lactate 7.4. At 11.5 hrs after presentation, K 2.4, pH 6.8, INR 1.8, and lactate 21.5.
Clinical Course: Patient was intubated and given norepinephrine, diazepam, and sodium bicarbonate in the ED. HR increased to the 80s and BP increased to 80s systolic. She was minimally responsive to painful stimuli, pupils remained dilated and nonreactive. Sodium bicarbonate constant infusion and NAC was started. She continued to receive epinephrine and norepinephrine, BP 100s, HR90s. At 7 hrs after presentation, her condition declined. Antibiotics were started for a suspected aspiration. At 9 hrs after presentation, left bundle branch block developed, a hemodialysis catheter was placed, and hemodialysis started approximately 12 hrs after presentation to ED. At that time of dialysis the patient was awake, alert, able to see. After 6 hrs of dialysis, and while receiving sodium bicarbonate and hyperventilation, her condition deteriorated. The patient expired approximately 24 hrs after presentation to the ED.
Autopsy Findings: Cause of Death: Acute diphenhydramine, metformin, cocaine, alprazolam, and pregabalin intoxication. Manner of Death: Suicide (administered overdose of drugs) Antemortem blood alprazolam 0.052 mg/L, cocaine metabolite (benzoylecgonine) 0.230 mg/L: cocaine metabolite (ecgonine methyl ester) 0.013 mg/L, diphenhydramine 2.45 mg/L, serum pregabalin 0.44 mcg/mL, and metformin 140 mcg/mL.
Case 1614. Acute-on-chronic theophylline ingestion: probably responsible.
Scenario: A 79 y/o female presented to the ED from a nursing home with seizures, AF, tachycardia, hypotension, and agonal respirations.
Past Medical History: COPD and respiratory insufficiency.
Laboratory Data: Na 123, K 2.6, Mg 1.6, theophylline 40 mcg/mL.
Clinical Course: Female patient actively seizing with agonal respirations. BP 80, HR 210 (AF). The patient had attempted chemical and electrical cardioversion without success. BP continued to deteriorate and amiodarone and norepinephrine were given, followed by phenylephrine. The patient was intubated and received activated charcoal via nasogastric tube and lorazepam and levetiracetam for seizure activity. Hypertonic saline was given for hyponatremia and hemodialysis was initiated. Follow-up theophylline 29 mcg/mL, unclear when the sample was obtained with respect to hemodialysis. Based on the prognosis, the family opted for institution of comfort measures and the patient expired approximately 10 hrs after admission.
Autopsy: Not performed.
Case 1622. Acute flecainide, clonidine, fluoxetine, potassium chloride, ethanol, lorazepam, cocaine, clonazepam, lisinopril, and furosemide ingestion: undoubtedly responsible.
Scenario/Substances: A 28 y/o male in an apparent suicide attempt ingested his mother's medications after attacking her. He was later found collapsed and unresponsive by his mother, who reported he may have been down for as long as 40 min. She called EMS, who found the patient in cardiac arrest. They began CPR, gave two doses of naloxone and two doses of epinephrine. This patient had access to flecainide 100 mg, clonidine 0.1mg, fluoxetine 10 mg, potassium 10 mg, clonazepam 0.5 mg, lisinopril 40 mg, and furosemide 20 mg (number taken unknown).
Past Medical History: Depression, personality disorder, polysubstance abuse, previous suicide attempt.
Physical Exam: Comatose, no spontaneous circulation.
Laboratory Data: Na 136, K 3.4, HCO3 23, Glu 231, BUN 6, Cr 1.32, ABG-pH 7.33 / pCO2 31 / pO2 440, ethanol 218 g/L, UDS positive for cocaine.
Clinical Course: CPR was performed for over 1 hour in the ED, the patient was put on a ventilator with oxygen. The patient was placed on cardiopulmonary bypass. He developed status epilepticus treated with benzodiazepines. After seizures were controlled he exhibited posturing. He failed to improve with a hypothermia protocol and with sodium bicarbonate. His course was complicated by cardiogenic shock, repeated arrhythmias and a compartment syndrome of his left lower extremity upon removal of a cardiopulmonary catheter treated with emergency fasciotomy on the day of admission. Brain death was declared on Day 3.
Autopsy Findings: The death in this case was the end result of acute intoxication by flecainide and was suicidal in nature.
Case 1637. Acute verapamil ingestion: undoubtedly responsible.
Scenario/Substances: A 39 y/o female ingested 90 tablets of verapamil 240 mg in an apparent suicide attempt. She called EMS approximately 2 hrs later and was transported to the ED.
Past Medical History: Depression, hypertension. Medications: clonazepam, amphetamine, dextroamphetamine, verapamil, lamotrigine, duloxetine, and aripiprazole.
Physical Exam: Awake, alert, and oriented. BP 75/39, HR 59. Respirations and bowel sounds described as normal.
Laboratory Data:
Ca 9.4. Ethanol, acetaminophen, and salicylates were not detected.
Clinical Course: The patient became less responsive shortly after arrival in the ED and was intubated. Activated charcoal was administered via NG tube and she was given 100 units of insulin to initiate the hyperinsulinemia euglycemia protocol. Calcium gluconate was given IV. Norepinephrine infusion was started just prior to air transport to a tertiary care facility. Upon arrival BP 91/47, HR 30 with a junctional rhythm. Glucose 240 treated with insulin by infusion. The patient received several grams of calcium chloride. Bradycardia and hypotension continued. Intralipid 20% bolus and infusion was initiated and the patient was transferred to the ICU. On Day 2 progressive heart block was treated by pacemaker placement. Follow-up laboratory showed ionized Ca 1.9 and pH 7.18. Calcium gluconate and sodium bicarbonate separate infusions were initiated. Whole bowel irrigation was started 24 hrs post ingestion, but was discontinued due to 3 L gastric residuals. BP support with pressors increased late in the day, CxR showed volume overload. Insulin was increased and another bolus of 20% Intralipid was given with poor response. The patient's condition worsened and she was placed on ECMO, CVVH was being planned. Due to further decline in respiratory status and her overall prognosis, her family opted for comfort measures, and she expired 48 hrs after ingestion.
Autopsy Findings: Not available.
Case 1651. Acute flecainide, bupropion (extended release), and ethanol ingestion: undoubtedly responsible.
Scenario/Substances: A 43 y/o female intentionally ingested unknown amounts of flecainide and buporprion. EMS noted evidence of copious emesis and the patient was in cardiac arrest. Paramedics resuscitated for 30 min.
Past Medical History: Bipolar disorder, polysubstance abuse, hypertension, arthritis, pacemaker, and previous intentional overdoses.
Clinical Course: During the resuscitation the patient demonstrated VT, VF, and PEA (HR 20–30). Her resuscitation included intermittent CPR, intubation, epinephrine, magnesium, bicarbonate, and lipid rescue. The patient died ˜45 min after ED arrival.
Autopsy Findings: Autopsy demonstrated pulmonary vascular congestion and stomach contents with numerous partially dissolved white round pills. Post-mortem aortic blood: positive for atropine, benzodiazepines, caffeine, and other organic bases as present, ethanol 220 mg/dL and doxylamine < 0.25 mg/L. Post-mortem iliac vein blood: bupropion, 2.5 mg/L, diazepam 0.034 mg/L, flecainide 27 mg/L, nordiazepam 0.027 mg/L, threobupropion 2.1 mg/L. Post-mortem liver: bupropion 2.5 mg/kg, threobupropion 11 mg/kg. Cause of death: bupropion, flecainide, and ethanol toxicity.
Case 1674. Acute diltiazem (extended release) and amlodipine ingestion: undoubtedly responsible.
Scenario/Substances: A 50 y/o female was found retching and poorly responsive by her spouse. She told her husband that she had taken an overdose of 14 diltiazem 180 mg extended release and 14 amlodipine (unknown strength) about 1.5 hrs before in an apparent suicide attempt. EMS found the patient minimally responsive, hypotensive, and bradycardic with evidence of vomiting. En route to ED she became unresponsive, was given 0.5 mg atropine and IV fluids.
Past Medical History: Depression, cardiac disease, angina, high cholesterol, prior cerebral aneurism rupture with right hemiparesis complicated by cardiac arrest. Medications: diltiazem, amlodipine, simvastatin, conjugated estrogens. Current smoker 0.5 pack per day for 1 year.
Physical Exam: In the ED the patient was, bradycardic (HR 31), BP 52/35, had agonal breathing, and was unresponsive with sluggishly reactive pupils.
Laboratory Data: Na 137, K 5.3, Cl 101, HCO3 17, BUN 90, Cr 1.6, glucose 201, WBC 21.9. AST 52, ALT 60, CK 61, CKMB 1.7. Serum acetaminophen, ethanol, and salicylate were not detected. UDS was positive only for methadone.
Clinical Course: In the ED she was intubated, given IV fluids, glucagon, calcium gluconate, and norepinephrine. An external pacemaker was placed. Gastric lavage was performed and activated charcoal was given. Upon admission to the ICU, the patient was paced, but remained hypotensive. In addition to norepinephrine and glucagon, she was given 10 U insulin IV followed by 6 U/hr and a single dose of sodium bicarbonate (44 mEq). The patient developed hyperkalemia and was given sodium polystyrene sulfonate via NG tube and IV sodium bicarbonate. Repeat Na 142, K 8.6, Cl 108, total CO2 10, BUN 16, Cr 2.07, Glucose 87–> 299, Ca 12.6, albumin 3.3–> 1.5. Echocardiogram showed poor LV function. There was no clinical response to maximal vasopressors (dopamine, epinephrine, and norepinephrine) nor to 20% lipid emulsion (bolus and infusion). Her systolic BP remained in the 40–50 range. The patient expired approximately 15 hrs after the reported overdose.
Autopsy Findings: There was no autopsy. Antemortem hospital blood (time not specified) amlodipine 230 ng/mL (therapeutic reference range 3–11 ng/mL) and diltiazem 1800 ng/mL (therapeutic reference range 50–200 ng/mL).
Case 1731. Acute-on-chronic sitagliptin, citalopram, alprazolam, diltiazem (extended release), metoprolol (extended release) ingestion: undoubtedly responsible.
Scenario/substance: A 64 y/o female ingested diltiazem (240 mg, #11), metoprolol (50 mg, #18), sitagliptin (25 mg #6), citalopram (#20), and alprazolam. The patient left a suicide note that listed the medications and the amounts she took in an apparent suicide attempt. Patient was found unresponsive at her home and brought to the ED.
Past Medical History: Depression, hypertension, chronic renal insufficiency on dialysis. Ingested medications were the patient's current medications.
Physical Exam: Alert and oriented on arrival. BP 142/84, HR 83.
Laboratory Data: ECG: NSR at 63, normal intervals. Acetaminophen, salicylate, and ethanol were not detected. UDS positive for benzodiazepines.
Clinical Course: IV fluids were started and the patient received activated charcoal. Approximately 1 hour after ED arrival, BP 53 systolic, HR 40 with a junctional rhythm. One milligram IV glucagon and IV calcium gluconate was administered, with improvement in BP to 96 systolic. Recurrent hypotension was treated with dopamine and she was transferred to a tertiary care center. At the second HCF, HR was 30–40, systolic BP was 80–110. IV fat emulsion (400 ml of 20%) was given and she was intubated and a glucagon infusion was started as was norepinephrine while dopamine continued. The patient also received a sodium bicarbonate infusion and IV high dose insulin with IV D10. Continuous renal replacement therapy dialysis was initiated, BP improved to 127/66 with a HR of 51 (junctional rhythm) with occasional PVCs. Pupils were dilated and unresponsive. Acidemia developed and she became anuric. Thrombocytopenia was noted (platelets 70). On Day 3 insulin/glucose therapy stopped. On Day 5 tachycardia, hypertension, and AF were noted, pressor therapy was discontinued. Poor perfusion of her feet was noted, amputation was considered. On Day 6 The patient was agitated, and moving all extremities. Neurological consultation noted poor prognosis. The patient expired on Day 12.
Autopsy Findings: Cause of death was suicide involving mixed drug (diltiazem, metoprolol, sitagliptin, citalopram) toxicity.
Case 1757. Chronic cardiac glycoside ingestion: probably responsible.
Scenario/Substances: A 73 y/o female with a high digoxin level presented to the ED with nausea, vomiting, and AF. She underwent scheduled hemodialysis one day prior to ED presentation.
Past Medical History: AF, congestive heart failure, renal failure, and remote history of left mastectomy.
Physical Exam: BP 142/88, HR 121.
Laboratory Data: K 4.5, digoxin 4.7 ng/mL.
Clinical Course: She was given promethazine for nausea. Four vials of digoxin antibodies were given 14 hrs later in the CCU, the patient became hypotensive and was given norepinephrine infusion. On pressors: BP 137/105, HR 111, RR 31, O2 sat 94%. Follow-up laboratory:
The patient expired approximately 26 hours after admission.
Autopsy Findings: Not performed.
Case 1795. Acute nifedipin ingestion: undoubtedly responsible.
Scenario/Substances: An 11 m/o male was found playing with opened bottle of grandparent's nifedipine 10 mg capsules. Up to 60 pills were missing.
Physical Exam: Unresponsive, HR of 30 which improved to 50 with bagging.
Clinical Course: The patient was in respiratory arrest upon presentation to the ED, was intubated and shortly afterwards went into full cardiac arrest. Resuscitation efforts were unsuccessful.
Autopsy Findings: Postmortem Examination: Acute nifedipine intoxication, No gross injury or natural disease at autopsy. Cause of Death: (Forensic Pathologist) acute nifedipine intoxication. Toxicology Laboratory Report: GC/MS Acidic Drug screen: Post-mortem blood, heart 1000 ng/mL.
Case 1812. Acute yohimbine ingestion: contributory.
Scenario/substance: A 48 y/o male took an entire bottle of a multi-botanical male enhancement product containing yohimbine, tribus, Korean ginseng, cnidium monnier, eluthercoccus, xanthroparmelia scarbosa, horny goat weed, velvet deer title, damian, muira puama, pumpkin seed, stinging nettle root, astragalus root, licorice root, L-arginine, ho shou wu extract, boron, foliate, zinc, DHEA, pregnanolone, black pepper, piper longum, and ginger. He collapsed later during the filming of an intimate encounter between himself and others. A participant called EMS, but CPR was not performed during the ˜10 min which elapsed before EMS arrived.
Past Medical History: Remote history of left lower extremity amputation, diabetes, and unspecified vascular disease.
Physical Exam: Male in cardiac arrest wearing a buprenorphine patch.
Laboratory Data: pH 7.33, Na 133, K 3.4, Mg 1.7. AST 42, ALT 22, INR 1.0, Troponin 1.34 (peak 4.58), and CK 27.8. Acetaminophen was not detected.
Clinical Course: The patient arrived in cardiac arrest wearing a buprenorphine patch, was resuscitated, intubated, placed on therapeutic hypothermic protocol, and an intra-aortic balloon pump was placed. Cardiac catheterization showed multivessel disease (100% left anterior descending artery and 85% right coronary artery occlusions) and an echocardiogram showed an ejection fraction of 25%. Post resuscitation, BP 130/80, HR 108, O2 sat 99%. The patient was sedated with midazolam and fentanyl and paralyzed with cisatracurium and given an insulin drip. His pupils were 5 mm, his urine output was 15–23 mL/hr, and he was experiencing decorticate posturing. Day 3: the patient was re-warmed and demonstrated status epilepticus and was given levatiracetam, propofol as well as benzodiazepines. He became bradycardic and hypotensive with continued seizures. Based on the prognosis, the family opted for institution of comfort measures on Day 5 and the patient expired later that day.
Autopsy Findings: ME initially determined the probable cause of death to be due to herbal stimulant overdose, further review suggested it was due to hypertension and atherosclerotic disease. Ante mortem toxicology of serum (peripheral specimen) demonstrated no ethanol and no yohimbine.
Case 1815. Acute calcium parenteral: undoubtedly responsible.
Scenario/Substances: A 5-month-old male with multiple medical problems received 10 times the therapeutic dose of IV calcium chloride while preparing for a transfer to another tertiary care hospital for a heart transplant.
Past Medical History: Included congenital hypoplastic left heart syndrome, mitral stenosis, and aortic atresia; s/p median sternotomy with placement of pulmonary artery bands and pulmonary artery catheter; s/p Norwood procedure with Blalock–Taussig shunt; s/p left parieto–occipital hemorrhage with posterior third ventricle extension; renal failure requiring peritoneal dialysis.
Physical Exam: The patient was an edematous, intubated white male with a Grade II/VI systolic murmur, bilateral rhonchi and rales, and hepatosplenomegaly.
Laboratory Data: Serum ionized calcium levels during patient's hospital course ranged from 0.9 to 1.49 and was 1.16 ˜3 hrs prior to receiving the calcium chloride infusion. The ionized Ca was 3.68 immediately post calcium chloride infusion.
Clinical Course: At time of planned transfer, the patient developed respiratory distress and hypotension to 50s/40s and received 1 gram of calcium chloride intravenously. The child's weight was 5 kg and the order was for 20 mg/kg, that is 100 mg. However, the patient received 10 mL of 10% calcium chloride. The patient became bradycardic and hypoxic shortly after this with subsequent atrio–ventricular dissociation. VF developed ˜5 to 10 min after calcium chloride infusion. Resuscitation efforts were unsuccessful and patient died ˜1.5 hrs after the calcium chloride infusion.
Autopsy Findings: An autopsy was performed and concluded that death was due to acute hypercalcemia in the setting of a patient with complications of hypoplastic left heart syndrome. Post-mortem examination of the heart revealed no signs of surgical complications. Multiple splenic infarcts were present. Post-mortem neuropathology revealed acute and chronic subdural hemorrhages, chronic hematoma of supracerebellar cisternae, and microscopic infarcts of right frontal cortical white matter and left thalamus.
Case 1816. Acute atropine/diphenoxylate ingestion: undoubtedly responsible.
Scenario/Substances: A 2 y/o female was found with a bottle of grandmother's atropine/diphenoxylate. Family removed 16 tablets from her mouth—they did not know how many pills were swallowed.
Past Medical History: Normal, healthy child.
Clinical Course: In the ED she was a normal appearing 2 y/o, HR 135, BP 103/45. She remained normal during 4 hours of observation and was discharged home. The following morning the child's mother found her unarousable and foaming at the mouth with yellow secretions. She called their pediatrician, then brought her to the ED ˜2 hours after finding that she would not awaken. In the ED she was intubated with succinocholine and etomidate for agonal respirations and acute mental status changes. Systolic BP 50, head CT showed no bleeding, but low attenuation in both cerebellar hemispheres suggestive of infarction. She was transported by helicopter to a tertiary care pediatric hospital. During flight she was sedated with versed and fentanyl and received an albuterol treatment for wheezing. She remained unresponsive to painful stimuli, pinpoint pupils, over breathing the ventilator, GCS 3, T 37.3°C, HR 139, BP 103/70, RR 24, Biox 99%, AST 119, ALT 36, and ammonia 29.
Hypocalcemia was treated with calcium gluconate 50 mg/kg. CxR suggested aspiration, cultures were done, and she was started on piperacillin and tazobactam. EEG showed severe diffuse slowing, no epileptiform discharges, and no electrographic seizures; MRI was suggestive of anoxic injury in the watershed areas. During the early AM of Day 2 the patient developed fixed and dilated pupils. She had briefly withdrawn to painful stimuli, but that reaction stopped. She did not gag when she was suctioned and her cranial nerve reflexes were absent. She was suspected to have diffuse cerebral edema with beginnings of hydrocephalus due to obstruction of the 4th ventricle and there was concern for impending herniation. She was on dopamine at 12 mcg/kg/min, and received 2 NS boluses, but her hypotension was refractory to dopamine. She had a significant urine output, consistent with diabetes insipidus. She was started on vasopressin at 0.5 milliunits/kg/h and a 0.45 NS fluid replacement. Labs at ˜21.5 hrs post-ingestion showed urine diphenoxylate negative and blood diphenoxylate 6.1 ng/mL. Comprehensive UDS was positive for fentanyl, normeperidine, cotinine, caffeine, and theobromine. The patient expired on Day 3, ˜31 hours post ingestion.
Autopsy Findings: Not performed.
Case 1824. Acute metformin ingestion: undoubtedly responsible.
Scenario/Substances: A 38 y/o male presented to the ED 3 hrs after a self-reported ingestion of 120 metformin 500 mg tablets with suicidal intent.
Past Medical History: Depression, bipolar disorder, and diabetes mellitus.
Physical Exam: The patient presented awake, alert, and oriented, BP 103/37, HR 77, O2 sat 96% on room air.
Laboratory Data: Glu 344, BUN 21, Cr 3.5, PT 35.6, INR 3.82, lactate 26, total bilirubin 0.6, AST 1682, ALT 1980, acetaminophen and ethanol were not detected, ABG-pH 6.9/pCO2 63/CO2 12.
Clinical Course: At ˜8 hrs after the ingestion, the patient reported nausea and abdominal and back spasms. At 12 hrs post-ingestion, he developed lactic acidosis and had a cardiopulmonary arrest. He was endotracheally intubated and placed on a ventilator, while ACLS resuscitation measures were applied. He arrested twice during transport to a tertiary care center. At 22 hrs post-ingestion, he was unresponsive, receiving vasopressin, norepinephrine, and epinephrine, while on CRRT. At 25 hrs, based on the prognosis, the family opted for institution of comfort measures and he expired.
Autopsy Findings: Antemortem blood metformin 310 μg/mL, post-mortem metformin 160 μg/mL. Cause of death: 1) Complications of metformin toxicity, self-reported history of consumption of a large amount of metformin, elevated metformin concentration in antemortem blood, clinical history of severe lactateosis, agitation, and bowel incontinence, clinical history of multiple episodes of cardiopulmonary arrest, subsequent development of anoxic encephalopathy and multisystem organ failure, moderate bilateral pulmonary edema, and acute bronchopneumonia. Atherosclerotic cardiovascular disease, moderate atherosclerosis of coronary arteries and aorta, cardiomegaly (510 g), moderate fatty liver.
Case 1845. Chronic metformin ingestion: contributory.
Scenario/Substances: A 66 y/o female patient presented to the ED with nausea, vomiting, and diarrhea.
Past Medical History: Diabetes. Medications: metformin.
Physical Exam: Initially normal vital signs, mild abdominal tenderness to palpation. On later examination, marked tachypnea and hyperpnea, benign abdomen.
Laboratory Data: Anion gap 16, UA 2 + glucose.
Follow-up laboratory data: Cr 1.4, anion gap 22, glucose 318, lactate 13.2 mmol/L, salicylate 2.1 mg/dL, venous BG- pH 7.0 / pCO2 29 / pO2 46 / HCO3 7 / BE -25, UDS negative, beta hydroxybutyrate 21.9, blood culture x2 negative, urine culture positive for Klebsiella pneumoniae.
Clinical Course: Initial mild anion gap metabolic acidosis was thought due to gastroenteritis and dehydration. However, after IV fluid hydration with 4 liters of NS, repeat labs showed worsening acidosis and anion gap. Patient had no history of acute ingestion of metformin, although the possibility was raised she had taken additional doses with her GI symptoms. She had no complaints of abdominal pain in ED, CT scan with contrast done after initial lactate of 13.2 mmol/L. Repeat labs showed Cr 1.4, lactate 19 mmol/L, worsening acidosis (pH 6.98), low salicylate, negative ethylene glycol/methanol. She was given supportive care with IV fluids and started on insulin (0.1 unit/kg/hr) on arrival to the ICU. Sodium bicarbonate infusion and CVVH were started while a dialysis catheter was placed. The patient maintained BP and HR until shortly prior to starting CVVH. She had a VF/VT cardiac arrest ˜11 min after starting CVVH treated with epinephrine, defibrillation, and amiodarone bolus with conversion to PEA arrest. She then received epinephrine ×3, sodium bicarbonate ×4 with brief return of spontaneous circulation followed rapidly by return of nonperfusing VF/VT. She could not be resuscitated and expired on Day 1.
Autopsy Findings: Cause of death: complications of severe lactatic acidosis, possibly due to metformin. Contributory conditions: diabetes mellitus, coronary artery sclerosis, urinary tract infection. No metformin levels performed.
Case 1873. Acute baclofen ingestion: undoubtedly responsible.
Scenario/Substances: A 57 y/o male was found unresponsive in his vehicle with a prescription bottle missing 120 tablets of baclofen, 20 mg.
Past Medical History: Multiple sclerosis, confined to a wheelchair.
Physical Exam: Unresponsive, shaking male. Systolic BP 177, HR 60.
Laboratory Data: Acetaminophen was not detected.
Clinical Course: The patient was intubated soon after arrival and transferred to a tertiary care facility, admitted to the ICU on propofol with intermittent doses of benzodiazepines. Seven hrs after admission to the ICU, the patient had a 35 min generalized tonic–clonic seizure treated with benzodiazepines. He subsequently had repeated episodes of seizures and was given valproic acid. Hypotension with systolic BP 50–60 was treated with dopamine. Intermittent seizures continued for 72 hrs and progressed to continuous subclinical seizures on EEG. Hypotension required treatment with epinephrine, dopamine, and phenylephrine. Supportive care only was instituted on Day 5 and the patient expired.
Autopsy Findings: Baclofen overdose, Post-mortem baclofen blood concentration 8.7 mcg/mL (timing of sample not provided).
Case 1922. Acute-on-chronic clozapine ingestion: undoubtedly responsible.
Scenario/Substances: A 40 y/o male took approximately 30 tabs of his clozapine, 100 mg in an apparent suicide attempt. He called EMS who noted a seizure and bradycardia during transport.
Past Medical History: Schizophrenia, bipolar disorder, anxiety.
Laboratory Data: ABG-pH 7.10 / pCO2 30 / pO2 285. UDS positive for benzodiazepines, ethanol, lithium, salicylates, and acetaminophen were not detected. ECG showed QT prolongation.
Clinical Course: Agitated, diaphoretic, poorly responsive GCS 6, BP 114/73, 112 RR 36, T 36.2°C, O2 sat 96% on room air. Pupils were equal and reactive to light bilaterally.One amp of sodium bicarbonate was given with improvement of the QTc. Intubation was performed for respiratory distress. Systolic BP declined to 60s treated with norepinephrine. Gastric lavage was performed and activated charcoal administered. ARDS ensued and antibiotics were given. Bronchial alveolar lavage showed few oral flora, blood cultures negative. Day 2 K 2.4. On Day 3 two generalized seizures occurred, treated effectively with lorazepam and a loading dose of fosphenytoin. On Days 4, 5, and 6 intermittent seizures continued. On Day 7 Norepinephrine weaned off with adequate BP. On Day 8 fever continued, intermittent seizure activity, baclofen was administered. On Day 9 the patient was febrile and hypotensive with systolic BP 70. Based on the prognosis, the family opted for institution of comfort measures and the patient expired shortly thereafter.
Autopsy Findings: Findings: Death as a result of clozapine intoxication due to intentional overdose.
Manner of Death: Suicide
Case 1993. Hallucinogenic amphetamine ingestion: probably responsible.
Scenario/substance: A 17 y/o male was found seizing in woods after ingesting two doses of a psychopharmaceutical that was reported to be LSD. Soon afterwards his friends noted that he was exhibiting bizarre behavior.
Past Medical history: Healthy, on no medications.
Physical Exam: BP 120/80, HR 160, T 40–40.6°C, O2 sat, 100% on ventilator. Not rigid.
Laboratory Data: Initial pH 6.8, after resuscitation, venous blood pH 7.26 / pCO2, 45 / pO2, 40 / HCO3 20, lactate, 1.91, CK > 50, CKMB > 300, troponin 2.3, AST 1419, ALT 324, anion gap 9, Glu 290, Ca 7.5, Mg 3.7, serum acetaminophen, ethanol, and salicylate were not detected, PT 11.7, INR 1.21, UDS positive for THC and benzodiazepines. Head CT showed cerebral edema.
Clinical Course: Patient seized for 30–45 min in the woods, then seized for 20 min en route to hospital and continued to have tonic–clonic seizures for an additional 1.5 hrs in the HCF. He was intubated and administered lorazepam 16 milligrams, midazolam 10 milligrams, and fosphenytoin. Seizures were slowed when he received phenobarbital 15 mg/kg. He was given antibiotics. IV fluids were NS with 3 amps of sodium bicarbonate. He developed hypertension treated with a nitroprusside infusion. He was sedated with propofol. On Day 3, Cr 8, BUN 49 K 6.9. He became anuric and hemodialysis was performed. MRI showed anoxic injury with hyperintensity with restricted perfusion and brain damage. Pupils became pinpoint and minimally responsive. Patient remained minimally responsive, herniated, and expired on Day 7.
Autopsy Findings: Based on his clinical course and similar cases elsewhere, the most likely substance was 2C-I-NBOMe. The case was discussed with medical examiner, no samples from early in his hospitalization were available for analysis.
Case 2014. Chronic cocaine, promethazine ingestion, inhalation/nasal: probably responsible.
Scenario/Substances: A 21 y/o male had a 2-day history of symptoms consistent with a viral illness, including nausea and vomiting. He self-medicated with 2 doses of oral promethazine approximately 4 hrs apart. Two hours after the second promethazine dose, he became agitated and was thrashing, and became unresponsive. A bag of white powder found at his house later and was presumptive positive for cocaine by law enforcement.
Physical Exam: Comatose male. BP 127/59, HR 94, RR 40, afebrile with O2 sat of 100%. Skin showed rash on his chest.
Laboratory Data: ABG-7.43 / pCO2 < 20 / pO2 115.
UDS positive for cocaine and marijuana. Acetaminophen and ethanol were not detected. Head CT normal. WBC elevated.
Clinical Course: The patient became hypotensive, was started on vasopressors, and transferred by air to a tertiary care facility. En route, he became agitated and combative with a further drop in his BP necessitating intubation. At the second HCF ECG showed sinus tachycardia with possible right bundle branch block. He remained intubated and sedated. Follow-up ammonia 362 mmol/L, HCO3 19, and Cr 1.8. BP deteriorated and during central line placement the patient had cardiac arrest with successful resuscitation. Multiple cardiac arrests over the next 12 hrs occurred which responded to CPR, defibrillation, and ACLS medications. He developed bradydysrhythmias and hyperkalemia with K 7.0 with anuria. CRRT was initiated. He continued to decline with prolonged resuscitation and expired approximately 24 hrs after initial presentation.
Autopsy Findings: Severe hepatic microvesicular steatosis, no significant inflamation involves the portal triads. Focal sinusoidal hemorrhage not associated with inflamation was present, moderate cerebral edema, and early bronchopneumonia. Negative postmortem viral and bacterial cultures. Cause of death listed as Reye's syndrome following a presumed viral gastroenteritis and not an illness triggered by cocaine. Writer's note: cocaine has been associated with acute liver failure with a clinical picture including pathological changes similar to that observed here (Silva, 1991, Balaguer, 2005) supporting “probably responsable”.
Case 2040. THC homolog inhalation, unknown: undoubtedly responsible.
Scenario/Substances: A 23 y/o male was the driver of a car involved in a motor vehicle collision. He was found by police at the scene in an agitated and incoherent state. He fled the scene on foot, assaulted police, was captured, and restrained. As EMS arrived and as he was transferred, he became unresponsive. Restraints were removed and CPR was started. Subsequent history from a relative was the patient had been using K2, a synthetic stimulant, for 2 days.
Physical Exam: Intubated on ventilator. BP 169/80, HR 54, RR 15, T normal, O2 sat 99%.
Laboratory Data: pH 7.2, K 2.8, BUN 19, Cr 3.1, CK 32,000, repeat CK 146,000, UDS positive for THC only,
Clinical Course: The patient was given fentanyl and benzodiazepines for sedation, sodium bicarbonate, and placed on post arrest hypothermia protocol. Patient remained unresponsive after therapeutic hypothermia was stopped and was hypertensive, which required nitroglycerin and nicardipine. Repeat labs on Day 2: BUN 171, Cr 6.9 mg, CVVHD was started. On Day 3 the patient was determined to have no brain activity and he expired.
Autopsy Findings: Multiple small abrasions were noted on face and extremities. Hypoxic-ischemic encephalopathy with severe edema and herniation of the cerebellar tonsils was noted. Microscopic: renal tubular necrosis, focal pericardial and interstitial cardiac hemorrhages, cardiac contraction band necrosis. Pre-mortem blood: positive for JHW-210. Cause of death: Agitated delirium associated with synthetic marijuana use following police restraint and arrest procedure. Manner of death: could not be determined.
Case 2063. Acute amphetamine (hallucinogenic), morphine ingestion: undoubtedly responsible.
Scenario/Substances: A 24 y/o male and his female friend used special K or “Molly” (synthetic stimulants) at a party, 30 min later he had a tonic–clonic seizure. EMS was called approximately 1 hr later and found him seizing, febrile, and sweating.
Past Medical History: Opiate abuse.
Clinical Course: Upon arrival at the ED he was unresponsive, diaphoretic, with ongoing seizures. BP 60/27, HR 144, T 41.1°C. He had perioral cyanosis. He received 4 mg of lorazepam, but was still seizing, following intubation he was given a neuromuscular blocker, had gastric lavage, and was given activated charcoal. The patient was given ice packs and sent for CT scan, upon return to the ED BP 82/45, HR 192. Active cooling which was begun with cooling blankets, ice packs to the groin and axilla, cold irrigation to his bladder and stomach, and a cool airflow. Acetaminophen and dantrolene were administered, as well as calcium, insulin, and glucose. Neurological assessment showed intact brainstem reflexes. Bedside EEG was suspicious for epileptiform activity. He was transferred to the ICU. DIC was suspected with INR 28, he was given FFP, cryoprecipitate, vitamin K, and platelets. He developed acute renal failure and shock liver with persistent hypoglycemia. No brain activity was detected; life support was withdrawn and the patient expired.
Autopsy Findings: Cerebral edema with cerebellar tonsillar herniation, severe diffuse pulmonary edema with bilateral effusions, congenital absence of right kidney, chronic passive congestion of the liver, multiple infarctions of papillary musculature of left ventricle. Toxicology testing showed morphine total 229 ng/mL and free morphine 30.6 ng/mL. Testing for stimulants was incomplete.
Case 2085. Acute amphetamine (hallucinogenic) unknown: undoubtedly responsible.
Scenario/Substances: A 26 y/o female was found unresponsive after using synthetic stimulants. EMS found the patient in asystole. CPR was initiated and direct cardioconversion was performed twice in the field.
Past Medical History: Alcohol and heroin abuse.
Laboratory Data: pH 7.15 / pCO2 57, WBC 20.3, K 2.9, glucose 353, CK 204, CKMB 9.2, troponin 0.23, UDS positive for benzodiazepines, cocaine, and opiates.
Clinical Course: In ED BP 168/122, HR 135, RR 14, T 34°C, O2 sat, 68%. Diffuse, coarse breath sounds with bilateral crackles.The patient was admitted to the ICU, sedated, and intubated with sinus tachycardia at 135. Clonic seizure-like activity was noted, continuous EEG monitoring was instituted and midazolam infusion up to 32 mg/hour was given as well as IV fluids and norepinephrine to support BP. Therapeutic hypothermia was instituted to maintain 33 C. After warming, the patient displayed refractory status epilepticus and a pentobarbital coma was initiated resulting in burst suppression. When the pentobarbital was subsequently discontinued status epilepticus returned. Based on the prognosis, the family opted for institution of comfort measures and the patient expired on Day 12.
Autopsy Findings: Not available.
Case 2149. Acute methamphetamine exposure: undoubtedly responsible.
Scenario/Substances: A 30 y/o male was stopped by the police on a routine traffic stop. Drug sniffing dogs detected a scent but could not locate any drugs. He was then taken to a hospital where a UDS was positive for methamphetamine, THC, and opiates. He was then released to home. Three hours later he called his brother, stating that he was dying and complaining of chest pain, shortness of breath, funny heartbeats, and he was pounding on his chest. He then had a seizure. EMS was called and took him to the ED. It was later reported by friends that he had ingested 2.5 grams of crystal meth.
Past Medical History: Substance abuse (methamphetamines, marijuana), hepatitis C, peptic ulcer disease with massive GI bleed.
Clinical Course: In the ED he was seizing, systolic BP in the 90s, T 40.6°C. He was intubated and given 2 L IV fluids. Lumbar puncture showed Glu 57, protein 53 mg/dL. Head CT was unremarkable. He was transferred to a tertiary care hospital where his blood glucose was found to be 20 and he was given D50W 25 grams. HR 120s, BP normotensive, T 38.9°C, anuric. He had a complicated course over the next 5 days including the development and treatment of rhabdomyolysis (CK to 98,643), sepsis, acute renal failure, ARDS, DIC, and hepatic failure (AST 4482, ALT 3163, bilirubin 16.1). On Day 6 the patient had a brain hemorrhage, herniated, and expired.
Autopsy Findings: Autopsy not performed. Coroner's cause of death: multi-system organ failure and acute intraparenchymal hemorrhage due to polydrug intoxication. Contributing factors were hepatitis C and polysubstance abuse.
Case 2240. Cocaine ingestion, inhalation/nasal: undoubtedly responsible.
Scenario/Substances: A 37 y/o male was taken into police custody after exhibiting erratic and crude behavior. Bystanders reported he had used drugs earlier in the day (took some pills, smoked something). He became unresponsive in police custody and was taken to the ED. He received 25 g dextrose and atropine for bradycardia (HR in the 40s) prior to ED arrival.
Past Medical History: Hypertension, lymphoma, cocaine abuse.
Laboratory Data: ABG-pH 6.5 / pCO2 49.5 / HCO3 6
Serum acetaminophen, ethanol, and salicylate were not detected. AST 127, ALT 103, alk phos 119, ammonia > 1, 000, lactate 38.4.
Clinical Course: The patient was intubated using etomidate and rocuronium on arrival in the ED. His initial HR was in the 150s (after atropine), systolic BP in the 120s, afebrile, O2 sat normal, pupils small and reactive. After 100 mEq of sodium bicarbonate ABG-pH 7.1 / pCO2 29 / HCO3 10. ECG: sinus rhythm rate in the 150s, QRS 94, QTc 444. Head CT unremarkable. N-acetylcysteine and fomepizole therapy were initiated in the ED, and he was admitted to the ICU. Follow-up laboratory data: ABG-pH 7.23 / PCO2 15.5, anion gap 30.2, ammonia 278, magnesium 7.2. He became hypotensive, unresponsive to 3 vasopressors. Based on the prognosis, the family opted for institution of comfort measures and he expired ˜20 hrs after ED arrival.
Autopsy Findings: Midline cerebellar hemorrhagic infarct with focal 4th ventricle hemorrhage and cerebellar subarachnoid acute hemorrhage. There was no evidence of cerebral contusion, skull fracture, vascular trauma, cerebral aneurysms, arteriovenous malformations, or subdural hemorrhage. Mild cardiomegaly and microscopic changes consistent with hypertension. Hospital blood cocaine 0.30 mg/L, benzoylecgonine 0.5 mg/L. Ethanol, methanol, acetone, isopropanol, opiates, methamphetamine, MDMA, phencyclidine, and methadone were not detected. The cause of death was ruled accidental due to acute hemorrhagic cerebellar infarct, due to cocaine toxicity, in addition to multisytem organ failure, cerebral hypoxia, and hypertension.
Case 2506. Acute-on-chronic methamphetamine inhalation: probably responsible.
Scenario/Substances: A 58 y/o male with history of substance abuse presented to the ED ˜1 hr after smoking methamphetamine.
Past Medical History: Previous CVA and MI, methamphetamine abuse, reportedly non-compliant with medications.
Physical Exam: Male with tonic–clonic seizure and rigidity of his left greater than right side, hyperthermia, and unresponsiveness.
Laboratory Data: ABG-pH 7.47 / pCO2 37 / PO2 90, Hct 33.8, platelets 281, CK 1478, UDS positive for methamphetamine.
Clinical Course: On ED arrival patient was seizing and was intubated for airway protection. Within the next several hours he developed hyperthermia (43.3°C) and rigidity of both upper and lower extremities (left > right). A propofol infusion, benzodiazepines, and a cooling blanket lowered his body temperature to normal. Head CT showed cerebral edema without focal abnormalities. While in the ICU he continued to have persistent thermal dysregulation requiring cooling blankets to maintain a normal temperature although he maintained a BP of 130/60 and a HR in the 50s without vasopressors. On Day 2 he exhibited nonreactive pupils, decerebrate posturing of the upper extremities and decorticate posturing of the lower extremities. Based on the prognosis, comfort measures were instituted and he expired on Day 4.
Autopsy Findings: Autopsy results not available.
Case 2542. Hallucinogenic amphetamine, cocaine, marijuana, gabapentin exposure: undoubtedly responsible.
Scenario/Substances: Police brought a 27 y/o male to the ED in handcuffs after he tried to break into a home without any pants on. The patient was out of control and agitated en route.
Physical Exam: HR 185, RR 32. The patient was toxic appearing, screaming, spitting, and frothing at mouth (rusty colored sputum), unclothed from the waist down. Skin appeared cyanotic, pupils were dilated.
Laboratory Data: ECG showed a wide complex tachycardia ABG-pH 6.94 / pCO2 44 / pO2 242 / HCO3 9 / BE 24.9, lactate 20, K 7.8, Cl 91, HCO3 13, BUN 23, Cr 3.93, phosphate 9.3, Mg 3.7, Ca 13.2. bilirubin 1.8 (total) 0.4 (direct), albumin 5.8, alk phos 57, ALT 44, AST 80, amylase 145, lipase 74, troponin 1.23, CK 2876, PT 16.6, INR 1.3, PTT 33, WBC 14.5, Hct 44, Hgb 15.1, platelets 321.
Clinical Course: On arrival at the receiving ED, the patient was screaming “I smoked crystal meth, I admit it!” and that his genitalia were “on fire”. His agitation continued despite two doses of haloperidol, two doses of midazolam and ketamine, and six security guards were at the bedside. He then became cyanotic, and vomited coffee ground material. He received etomidate and rocuronium and was intubated in rapid sequence and was placed on a ventilator. HR 180, systolic BP 70 (palpation), stool was guaiac positive, and ECG showed wide complex tachycardia. Cardioversion was unsuccessful X 2, the rhythm deteriorated to VF and he was defibrillated several times. Bladder temperature 42.4°C at this time and ice packs were applied to his groin and axillae and he was given a dose of dantrolene. He received standard ACLS and 5 ampules of sodium bicarbonate, 2 ampules of calcium chloride, and a total of 6 mg of epinephrine. Resuscitation for 45 min but was unsuccessful.
Autopsy Findings: The cause of death was ruled excited delirium due to acute intoxication by cocaine. Pertinent findings included cardiomegaly, post-mortem blood was positive (qualitative) for pyrovalerone, benzoylecognine, methylecognine (negative for cocaine), caffeine, nicotine, cotinine, lidocaine, and etomidate. Blood delta-9-THC 4.1 ng/mL, 11-OH-delta-9-THC 1.1 ng/mL, 11-nor-9-carboxy-delta-9-THC 53 ng/mL, gabapentin 3.1 mcg/mL. Post-mortem blood testing was negative for ethanol, levamisole, amphetamine, methamphetamine, mephedrone, methylenedioxypyrovalerone, and methylone. Post-mortem urine was positive (qualitative) for cocaine, levamisole, caffeine, nicotine, cotinine, lidocaine metabolite, gabapentin, and pyrovalerone. In her ruling, the coroner blamed cocaine and ignored the presence of bath salts in blood and urine. The reviewers agree cocaine contributed, but feel the pyrovalerone played a stronger role in the victim's toxidrome and death.
Abbreviations and Normal ranges for Abstracts
Disclaimer—all laboratories are different and provide their own normal ranges. Units and normal ranges are provided here for general guidance only. These values were taken from Harrison's (10), Goldfrank (11) or Dart.(12)
Serum electrolyte summary table
serum electrolytes have units of mEq/L = mmol/L
˜ = approximately
ABG-pH / pCO2 / pO2 / HCO3 /BE
ABG = arterial blood gases
ABG-pCO2 = partial pressure of carbon dioxide [38–42]
ABG-pH = hydrogen ion concentration [7.38–7.42]
ABG-pO2 = partial pressure of oxygen [90–100]
Base Excess = [− 2 to + 2 mEq/L]
ACLS = advanced cardiac life support, protocol for the provision of cardiac resuscitation
ADHD = attention deficit hyperactivity disorder
AF = atrial fibrillation
AICD = automatic implanted cardiodefibrillator
Alk phos = alkaline phosphatase [13–100] U/L
ALT = Alanine aminotransferase [7–41] U/L = (SGPT)
AMA = against medical advice
Ammonia = [25–80] mcg/dL = [15–47] mcmol/L
amp = ampoule
APLS = advanced pediatric life support, protocol for the provision of cardiac resuscitation
ARDS = acute respiratory distress syndrome
AST = Aspartate aminotransferase [12–38] U/L = (SGOT)
AVblock = atrio–ventricular block
BAL = British anti-Lewisite
BE = base excess, mmol/L
Bicarbonate = [22–26] mEq/L
Bilirubin = total [0.3–1.3] mg/dL, direct [0.1, 0.4] mg/dL, indirect [0.2, 0.9] mg/dL
BLQ = below the limit of quantitation
BMI = body–mass index
BP = Blood Pressure, systolic / diastolic, (Torr)
BPH = benign prostatic hypertrophy
BUN = see Urea nitrogen
C = degrees Centigrade
Ca = calcium, [8.7–10.2] mg/dL
CABG = coronary artery bypass graft
CAD = coronary artery disease
CIWA = Clinical Institute Withdrawal Assessment for Alcohol
CK = creatinine kinase (CPK), total: [39–238] U/L females, [51–294] U/L males
CKMB = MB fraction of CK [0.0–5.5 mcg/L = 0.0–5.5 ng/mL] Fraction of total CK activity [0–0.04 = 0–4.0%]
Cl = chloride [102–109] mEq/L
CNS = central nervous system
COHb = carboxyhemoglobin
COPD = chronic obstructive pulmonary disease
CPR = cardio pulmonary resuscitation
Cr = creatinine [0.5–0.9] mg/dL females, [0.6–1.2] males,
CRRT = continuous renal replacement therapy
CSF = cerebrospinal fluid
CT = computed tomography (CAT scan)
CVA = cerebrovascular accident
CVVHD = continuous venovenous hemodiafiltration
CxR = chest radiograph, chest xray
D10W = 10% dextrose in water
D50W = 50% dextrose in water
D5NS = 5% dextrose in normal saline
D5W = 5% dextrose in water
Day = when capitalized, Day = hospital day,that is, days since admission
DIC = disseminated intravascular coagulation
Dx = diagnosis
ECG = electrocardiogram (EKG), leads = I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
ECMO = extracorporeal membrane oxygenation
ED = emergency department, in these abstracts refers to the initial healthcare facility
EDDP = principal methadone metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine
EEG = electroencephalogram
EF = ejection fraction
ELISA = enzyme-linked immunosorbent assay
EMS = emergency medical services, paramedics, the first responders
ER = extended release (sustained release)
FFP = fresh frozen plasma
FiO2 = fraction of inspired oxygen
g = grams
g/dL = grams per deciliter
GCS = Glasgow Coma Score, ranges from 3 to 15
GERD = gastroesophageal reflux disease
GI = gastrointestinal
Glu = glucose, fasting [75–110] mg/dL
HCF = healthcare facility
HCG = human chorionic gonadotropin test for pregnancy
HCO3 = bicarbonate
HCP = health care provider
Hct = hematocrit [35.4–44.4] females, [38.8–46.4]% males
Hgb = hemoglobin [12.0–15.8] g/dL females, [13.3–16.2] g/dL males
HIV = human immunodefficiency virus
Hour = when capitalized, Hour = hours since admission
HR = Heart rate, beats per min
hrs = hours
ICP = intracranial pressure
ICU = intensive care unit
IgE = immunoglobulin E
IM = intramuscular
INR = international normalized ratio (PT to control) [0.8–1.2]
IU/L = international units per Liter
IV = intravenous
K = potassium, [3.5–5] mEq/L
kg = kilogram
L = Liter
Lactate = lactic acid [4.5–14.4] mg/dL arterial, [4.5–19.8] mg/dL venous
LBBB = left bundle branch block on ECG
Leukocyte = white blood count [3.54–9.06] 103/mm3 count
m/o = months old
MAP = mean arterial pressure
mcg/dL = micrograms per deciliter
mcg/L = micrograms per Liter
mcg/min = micrograms per minute
mcg/mL = micrograms per milliliter
mcmol/L = micromoles per liter
MDA = 3,4-methylenedioxyamphetamine
MDMA = methylenedioxymethamphetamine (ecstasy)
ME = medical examiner
mEq = milliequivalents
mEq/L = milliequivalents per Liter
Mg = magnesium [1.5–2.3] mg/dL
mg = milligrams
mg/dL = milligrams per deciliter
mg/kg = milligrams per kilogram
mg/L = milligrams per Liter
min = minutes
ml = milliliter
mmol/L = millmoles per Liter
mosm/kg = milliosmoles per kilogram
mosm/L = milliosmoles per Liter
MRI = Magnetic Resonance Imaging
ms = milliseconds
Narrative Headers:
Past Medical History: available relevant past medical history
Physical Exam: initial physical exam if available
Laboratory Data: initial results, give units except for units given in abbreviations
Clinical Course: concise narrative of HCF and beyond with outcome
Autopsy Findings: = medical examiner and/or autopsy results
NG = nasogastric
ng/mL = nanograms per milliliter
not detected = analyte below the level of quantitation, negative
NPO = nil per os, nothing by mouth
NS = normal saline
O2 sat = oxygen percent saturation [94–100]% at sea level
OR = operating room
Osm = osmole
PALS = pediatric advanced life support
PC = poison center ( = PCC, or Poison Control Center)
PCC = prothrombin complex concentrate
PCP = primary care provider
PEA = pulseless electrical activity
PEEP = positive end expiratory pressure
PICU = pediatric intensive care unit
Platelets = platelet count [150–400] × 109/L
PO = per os (“by mouth” in Latin)
Potassium = [3.5–5] mEq/L
ppm = parts per million
PR = P-R interval [120–200] ms on the ECG
prn = as needed
PT = prothrombin time, INR is preferred, but PT may be used if INR is not available
PTA = Prior to admission
PTT = partial thromboplastin time [26.3–39.4] sec
QRS = ECG QRS complex duration [60–100] ms
QT = Q to T interval on the ECG waveform, varies with HR
QTc = QT interval corrected for HR, usually QTcB = QT / RR½ (Bazett correction) 1–15 y/o [< 440] ms, adult male [< 430] ms, adult female [< 450] ms
RBBB = right bundle branch block on ECG
RBC = red blood cell(s)
RR = respiratory rate, breaths per minute
s/p = status post
sec = seconds
SL = sublingual
SVT = supraventricular tachycardia
Synthetic Stimulant = one or more of the products (6-APB, bath salts, plant food, Bliss, Ivory Wave, Purple Wave, Vanilla Sky, et al.) or chemicals (3,4 methylenedioxypyrovalerone [MDPV], 6-(2-aminopropyl)benzofuran [6-APB], butylone, desoxypipradrol [2-DPMP], ethylone, flephedrone, naphyrone, mephedrone, methylenedioxypyrovalerone, methylone, methcathinone, etc.)
T (oral) = Temperature (oral) [36.4, 37.2]°C or
T (rectal) = Temperature (rectal) [36.4, 37.2]°C or
T (tympanic) = Temperature (tympanic) [36.4, 37.2]°C
t-bili = total bilirubin
THC = tetrahydrocannabinol
THC Homolog = one or more of the products (Blaze, Dawn, herbal incense, K2, Red X, spice, et al.) or chemicals (cannabicyclohexanol, CP-47,497, JWH-018, JWH-073, JWH-200, et al.)
TPN = total parenteral nutrition
Tprot = total protein
Troponin I = normal range [0–0.08] ng/mL, Cut-off for MI > 0.04 ng/mL
U/dL = units per deciliter
U/L = units per liter
U/mL = units per milliliter
UA = urinalysis
UDS = urine drug screen
Urea nitrogen = [6–17] mg/dL (BUN)
VBG = venous blood gasses
VF = Ventricular fibrillation
VT = Ventricular tachycardia
WBC = white blood count, see leukocyte count
WNL = within normal limits
y/o = years old
