Abstract
Background. The EXtracorporeal TReatments In Poisoning (EXTRIP) workgroup presents its systematic review and clinical recommendations on the use of extracorporeal treatment (ECTR) in valproic acid (VPA) poisoning. Methods. The lead authors reviewed all of the articles from a systematic literature search, extracted the data, summarized the key findings, and proposed structured voting statements following a predetermined format. A two-round modified Delphi method was chosen to reach a consensus on voting statements and the RAND/UCLA Appropriateness Method was used to quantify disagreement. Anonymous votes were compiled, returned, and discussed in person. A second vote was conducted to determine the final workgroup recommendations. Results. The latest literature search conducted in November 2014 retrieved a total of 79 articles for final qualitative analysis, including one observational study, one uncontrolled cohort study with aggregate analysis, 70 case reports and case series, and 7 pharmacokinetic studies, yielding a very low quality of evidence for all recommendations. Clinical data were reported for 82 overdose patients while pharmaco/toxicokinetic grading was performed in 55 patients. The workgroup concluded that VPA is moderately dialyzable (level of evidence = B) and made the following recommendations: ECTR is recommended in severe VPA poisoning (1D); recommendations for ECTR include a VPA concentration > 1300 mg/L (9000 μmol/L)(1D), the presence of cerebral edema (1D) or shock (1D); suggestions for ECTR include a VPA concentration > 900 mg/L (6250 μmol/L)(2D), coma or respiratory depression requiring mechanical ventilation (2D), acute hyperammonemia (2D), or pH ≤ 7.10 (2D). Cessation of ECTR is indicated when clinical improvement is apparent (1D) or the serum VPA concentration is between 50 and 100 mg/L (350–700 μmol/L)(2D). Intermittent hemodialysis is the preferred ECTR in VPA poisoning (1D). If hemodialysis is not available, then intermittent hemoperfusion (1D) or continuous renal replacement therapy (2D) is an acceptable alternative. Conclusions. VPA is moderately dialyzable in the setting of overdose. ECTR is indicated for VPA poisoning if at least one of the above criteria is present. Intermittent hemodialysis is the preferred ECTR modality in VPA poisoning.
Introduction
The EXtracorporeal TReatments In Poisoning (EXTRIP) workgroup is composed of international experts representing diverse specialties and professional societies () who were assembled to provide recommendations on the use of extracorporeal treatment (ECTR) in poisoning (www.extrip-workgroup.org). The rationale, background, objectives, methodology, and first recommendations were reported previously.Citation1–10 Here, the workgroup presents a systematic review and recommendations for ECTR in valproic acid (VPA) poisoning.
Table 1. Represented societies.
Pharmacology and toxicokinetics
VPA is widely used in the treatment of partial and generalized seizure disorders. Given its favorable safety profile and large therapeutic index, VPA is also commonly used for the management of bipolar disorder as well as for migraine prophylaxis. As a result, intentional and unintentional VPA overdoses are common. In 2013, the American Association of Poison Control Centers' National Poison Data System recorded a total of 7776 cases including VPA, of which 2923 were single exposures, including 65 cases of major toxicity and 2 deaths.Citation11
VPA has a small molecular mass of 144 Da. The time to reach peak plasma concentrations (Tmax) is 1–4 h during normal therapeutic dosing,Citation12 but may be prolonged to more than 7 h in overdose.Citation13 Divalproex sodium is a complex molecule that dissociates to VPA in the gastrointestinal tract with the potential for delayed peak plasma concentrations. VPA has a small volume of distribution (0.1–0.5 L/Kg) and exhibits saturable plasma protein binding; although at therapeutic concentrations (< 100 mg/L), VPA is 94% protein bound, protein binding decreases to as low as 15% as concentrations rise to greater than 1000 mg/L.Citation14 The corresponding increase in the active fraction of free (unbound) drug likely leads to greater clinical toxicity (). The drug is primarily metabolized in the liver by glucuronide conjugation and to a lesser extent by mitochondrial β-oxidation and cytosolic ω-oxidation. Cytosolic ω-oxidation of VPA may lead to the production of toxic metabolites such as 4-en-valproate.Citation15 Only a small proportion (< 3% of an administered dose) of VPA is excreted unchanged in the urine.Citation16 The endogenous plasma clearance of VPA is 5–10 mL/min,Citation12 and its elimination half-life is approximately 12 h at therapeutic concentrations, but increases to more than 30 h in overdose.Citation17,Citation18
Table 2. VPA: Physical and TK properties.
Valproic acid poisoning
Careful clinical assessment and objective evaluation of the severity of poisoning are necessary in considering whether patients with VPA poisoning may benefit from therapeutic interventions such as ECTR.
Serum VPA concentrations can be useful in establishing the severity of poisoning. Concentrations between 50 and 100 mg/L (350–700 μmol/L) are considered therapeutic. In overdose, central nervous system depression is the most common clinical manifestation of VPA poisoning. Ataxia, sedation, and lethargy commonly occur in mild poisoning with ingestions around 200 mg/kg.Citation19 At ingestions of 400 mg/kg or more, severe VPA poisoning is associated with coma and respiratory depression requiring mechanical ventilation, cerebral edema, hemodynamic instability, and shock that may lead to a fatal outcome.Citation19 Laboratory abnormalities reported during severe poisoning include hypernatremia, hypocalcemia, thrombocytopenia, evidence of impaired mitochondrial function (i.e., metabolic acidosis and hyperlactatemia), and hyperammonemia which is thought to play a role in the pathogenesis of cerebral edema.Citation15 In a prospective, multicenter case series, a serum VPA concentration of more than 450 mg/L (3125 μmol/L) was more likely to be associated with a moderate or major adverse outcome and a hospital stay of more than 48 h; a concentration of more than 850 mg/L (5900 μmol/L) was more likely to be associated with coma and metabolic acidosis.Citation13
Cases of VPA poisoning can often be managed with proactive care alone. Initial attention should be directed to the need for airway protection and cardiovascular stabilization. Patients presenting with a recent VPA ingestion may benefit from gastrointestinal decontamination with single-dose activated charcoal. The use of multiple-dose activated charcoal (MDAC) in the treatment of VPA poisoning is not currently recommended.Citation20
L-carnitine is proposed as an antidote for VPA poisoning. It is postulated that L-carnitine depletion may impair the mitochondrial transportation and β-oxidation of VPA, favor the production of toxic metabolites, and contribute to the development of hyperammonemia. L-carnitine supplementation may increase mitochondrial β-oxidation and thus limit cytosolic ω-oxidation and the production of toxic metabolites.Citation21 L-carnitine is commonly recommended in patients with VPA toxicity and hyperammonemic encephalopathy.Citation22 However, evidence supporting the use of L-carnitine as an antidote for VPA poisoning is limited.Citation23,Citation24
Acute VPA toxicity should be differentiated from valproate-induced hyperammonemic encephalopathy, which may exhibit clinical characteristics similar to mild VPA overdose but is characterized by elevated ammonia concentrations in the setting of VPA concentrations within or near the therapeutic range.Citation25
Although reports of ECTR for severe VPA poisoning are published, there is no current consensus on the indications for ECTR and on the most effective modality for VPA removal.Citation16 References suggest ECTR for severe VPA toxicity manifesting with seizures or refractory hypotension,Citation26 or with massive ingestions of 1 g/kg or more, rapid deterioration, hemodynamic instability, hepatic dysfunction, cerebral edema, and high serum concentrations of 850 mg/L (5450 μmol/L).Citation27,Citation28
Methods
A predetermined methodology, incorporating guidelines from the Appraisal of Guidelines for Research and Evaluation (AGREE)Citation29 and Grading of Recommendations Assessment, Development and Evaluation (GRADE),Citation30 was used and described in detail elsewhere.Citation2 The primary literature search was conducted on July 12th, 2012 in Medline, Embase, and Cochrane library (Review and Central).
The search strategy was as follows:
[(valpro*) AND (dialysis OR hemodialysis OR haemodialysis OR hemoperfusion OR haemoperfusion OR plasmapheresis OR plasma exchange OR exchange transfusion OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR extracorporeal therapy OR CRRT)]. As mentioned, cases of valproate-induced hyperammonemia with a VPA concentration within the therapeutic range (< 100 mg/L) were excluded from the clinical analysis (as ECTR is performed to remove excess ammonia rather than VPA) but could be included in the pharmacokinetic/toxicokinetic (PK/TK) analysis.
A manual search of conference proceedings of the European Association of Poison Centres and Clinical Toxicologists (EAPCCT) and the North American Congress of Clinical Toxicology (NACCT) annual meetings (2002–2014), and Google Scholar was performed, as well as the bibliography of each article obtained during the literature search.
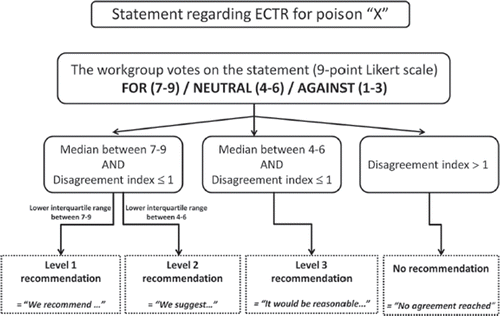
A subgroup of EXTRIP completed the literature search, reviewed each article, extracted data, and summarized findings. The subgroup and epidemiologist determined the level of evidence assigned to each clinical recommendation (). Dialyzability was determined based on criteria listed in . The potential benefit of the procedure was weighed against its cost, availability, alternative treatments, and its related complications. All of this information was submitted to the entire workgroup for consideration, along with structured voting statements based on a predetermined format.
Table 3. Strength of recommendation and level of evidence scale for clinical outcomes.
Table 4. Criteria for dialyzability.*
The strength of recommendations was evaluated by a two-round modified Delphi method for each proposed voting statement () and RAND/UCLA Appropriateness Method was used to quantify disagreement between participants.Citation31 Anonymous votes with comments were sent to the epidemiologist who then compiled and returned them to each participant. The workgroup met in person to exchange ideas and debate statements. A second vote was subsequently submitted and these results were used in developing the core EXTRIP recommendations. The literature search was updated on November 15, 2014 following the above-mentioned methodology; the new articles and summarized data were submitted to every participant who then updated their votes.
Results
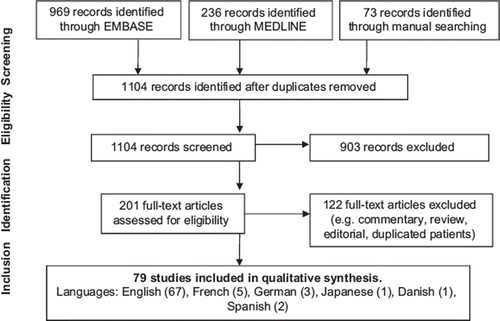
The search strategy performed on November 15, 2014 retrieved 1104 citations. After duplicates and articles without original data were removed 79 articles were accepted in the final analysis, including 1 observational study,Citation32 1 uncontrolled cohort with aggregate results,Citation13 70 case reports or case series,Citation14,Citation17,Citation18,Citation33–99 and 7 pharmacokinetic studies (i.e., when VPA concentration is therapeutic) ().Citation100–106 Patient-level data were available for clinical analysis in 82 patients (Supplementary Table 1 to be found at online http://informahealthcare.com/doi/abs/10.3109/15563650.2015.1035441) and for PK/TK grading in 55 patients. (Supplementary Table 2 to be found at online http://informahealthcare.com/doi/abs/10.3109/15563650.2015.1035441).
Clinical analysis
One observational study was identified which included patients admitted with a VPA concentration over 100 mg/L (694 μmol/L);Citation32 the group that received ECTR (n = 6) was compared with the one that did not (n = 26). Although all patients survived with complete neurological recovery, the ECTR group appeared to be more severely ill (i.e., significantly higher peak VPA concentration, treated more often with activated charcoal, and requiring intensive care admission, mechanical ventilation, and vasopressors more frequently) which suggests the presence of confounding-by-indication. The conclusion could be either inferred as a benefit of ECTR or an absence of effect, an interpretation which is further limited by a lack of power. Since the remainder of the clinical evidence is solely composed of case reports, the level of evidence can be considered very low.
Individual patient-level data were extracted from 82 patients (excluding 7 patients presented from an uncontrolled observational cohort where data could not be extracted) and presented in Supplementary Table 1 to be found at online http://informahealthcare.com/doi/abs/10.3109/15563650.2015.1035441. Aggregate data from all reported cases are presented in . Most reported poisonings involved regular-release VPA. The mean reported ingestion and VPA concentration were 39.8 g and 975 mg/L, respectively, both over the thresholds accepted to cause severe toxicity.Citation13,Citation19 The most commonly reported signs and symptoms were a decrease in consciousness followed by respiratory depression, hypotension, and metabolic acidosis. When reported, ammonia was almost always over the accepted normal range (> 30 μmol/L). Most patients required mechanical ventilation. Hemodialysis was by far the most commonly reported ECTR. The use of ECTR was associated with clinical improvement in the majority of cases with regard to mental status, respiratory depression, and hemodynamics; in some cases, especially when hemoperfusion or hemodialysis was used, this improvement was dramatic.Citation38,Citation44,Citation47,Citation56,Citation57,Citation61,Citation64,Citation71,Citation82,Citation83,Citation89,Citation90,Citation95,Citation97,Citation99 The use of continuous renal replacement therapy (CRRT) was often associated with slower clinical improvement over a period of days.Citation34,Citation48,Citation62 The timing of ECTR may also play an important role; clinical improvement was noted to occur faster when ECTR was used within 24 h of admission. Two fatalities were described, both in the cohort reported by Spiller et al.Citation13 VPA concentrations were over 1200 mg/L in both cases and hemodialysis was began more than 24 h after the onset of serious toxicity.
Table 5. Clinical data related to the 82 reported patients that received ECTR for VPA toxicity.*
Complications associated with ECTR
Although rare, both procedural hypotensionCitation32,Citation56,Citation82 and hemoperfusion-associated thrombocytopeniaCitation32,Citation33,Citation47,Citation53,Citation81,Citation85 were reported; however, it is difficult to determine if these were related to the ECTR or VPA toxicity itself. In one case, thrombocytopenia contributed to a hematoma from the insertion of a vascular access, which required platelet transfusion.Citation47 In another report, massive hemolysis and acute kidney injury (AKI) were reported during hemoperfusion when the prescribed blood flow exceeded the recommended flow.Citation85 Other complications specific to VPA may include withdrawal seizures in an epileptic patient if the VPA concentration falls below the therapeutic rangeCitation104 and possibly increasing intracranial pressure during ECTR in a patient with or at risk of cerebral edema.Citation107–110 There is also a theoretical concern of potential dialyzability of L-carnitine. No data were found to quantify the removal of L-carnitine that is administered during ECTR for poisoning; however, L-carnitine is reported to be extensively cleared during hemodialysis sessions when given in supplementation doses.Citation111–113
Dialyzability
The elimination of VPA is neither enhanced by urine alkalinization nor by MDAC in animals,Citation114 human volunteers,Citation115 or poisoned patients,Citation116 and is not currently supported by the latest position statements.Citation20 Because of its small molecular mass, low endogenous clearance, and small volume of distribution, VPA would appear to be readily removable by most ECTRs were it not for its high protein binding at therapeutic concentration. This is confirmed by a low sieving coefficientCitation117 and a relatively low extraction ratio through filters and columns at low VPA concentrations.Citation17 However, in situations such as uremia and overdose, the protein binding sites for VPA decreaseCitation103 or saturate, thereby increasing the fraction of unbound drug, rendering it more amenable to extracorporeal removal; despite using outdated dialysis parameters, approximately 20% of a therapeutic VPA dose was recovered in four uremic patients during a 4-hour hemodialysis, when protein binding was 70%.Citation102
In overdose, the extent of protein binding decreases further so the magnitude of the ECTR effect becomes greater, as is confirmed by the literature review; VPA clearance during dialysis is greater in overdose than at therapeutic dose (median: 87 vs. 22 mL/min, respectively). After intentional poisoning, three different reports quantified that over 10 g were recovered during a standard dialysis session.Citation18,Citation61,Citation67
Although the data are limited, intermittent convective techniques (online hemodiafiltration) appear equally efficient to diffusive techniques (); median VPA clearances for both intermittent hemodialysis and hemodiafiltration reach 90 mL/min with identical apparent half-lives.Citation44,Citation76 These are both superior to adsorptive-based hemoperfusion, which is often limited by extensive cartridge saturationCitation17,Citation53 and vastly superior to native endogenous clearance (5–10 mL/min).Citation12,Citation61,Citation76
Table 6. Kinetic aggregates of clearance and half-life for ECTR.
As expected by their lower blood flow and/or effluent flow, low-efficiency techniques such as CRRT therapies provide clearances (≅10–15 mL/min) that are considerably lower than high-flux dialysis. This is also the case for peritoneal dialysis. In every reported patient who received more than 1 type of ECTR, hemodialysis always resulted in a higher VPA clearance and/or a shorter apparent VPA half-life than other ECTRs.Citation17,Citation33,Citation36,Citation62,Citation67,Citation69,Citation95
While the addition of albumin in the dialysate may enhance clearance of VPA,Citation118 it remains unclear if it would show any superiority to traditional hemodialysis in overdose. Both liver support therapies and therapeutic plasma exchange are specifically suited for protein-bound poisons, so they would not be particularly useful in VPA overdose, during which most of the drug is not protein bound. Even at therapeutic concentration, removal of VPA was inconsequential during therapeutic plasma exchange.Citation101
In the majority of cases, the evidence for dialyzability is based on half-life comparison during and off ECTR. This evidence is further strengthened by articles that quantified removal via effluent or extruded column.Citation18,Citation58,Citation61,Citation64,Citation67,Citation96 According to the dialyzability criteria in , most of the cases that underwent an ECTR session (especially hemoperfusion or hemodialysis) would qualify as either “dialyzable” or “moderately dialyzable” (). The workgroup agreed with the conservative assessment that VPA was MODERATELY DIALYZABLE (Level of evidence = B). The dialyzability of VPA metabolites requires confirmation by further studies but appears to be substantial.Citation105
Table 7. Summary of the kinetic grading for individual patients.
Dialyzability of ammonia
Hyperammonemia often complicates VPA poisoning and may contribute to cerebral edema. Because ammonia distributes in total body water, hemodialysis appears to be the most efficient way to remove ammonia,Citation119,Citation120 and is superior to continuous techniques or peritoneal dialysis in this regard.Citation121,Citation122 The addition of convection to diffusion is promising to facilitate ammonia removal, but requires further study.Citation123 Because of its similar molecular size and distribution, ammonia clearance would likely approach that of urea.Citation124 Similar to urea, the optimization of blood flow, dialysate flow, and increasing the surface area of the filter will provide improved dialytic clearance,Citation125 as will increasing ultrafiltration flow during convection.Citation124
Recommendations: A summary of recommendations is presented in
Table 8. Executive summary of recommendations.
(1) General Statement: ECTR is recommended in severe VPA poisoning (1D)
Rationale: Although most patients presenting with a voluntary VPA overdose will have a relatively benign clinical course and a good outcome, some will develop life-threatening conditions that may be associated with prolonged coma, respiratory depression requiring mechanical ventilation, cerebral edema, hemodynamic instability and severe metabolic acidosis. Limited published clinical evidence exists supporting the clinical efficacy of L-carnitine as an antidote, and there is no benefit to either MDAC or urinary alkalinization.
The evidence supporting ECTR in VPA poisoning is composed of one observational study with major limitations and case reports and case series, with absent control groups and possible publication bias. The workgroup considered the following issues in evaluating the potential benefit of ECTR in VPA poisoning: both hemodialysis and hemoperfusion significantly enhance endogenous elimination of VPA and the use of high-efficiency ECTR is associated with rapid clinical improvement in the majority of published cases. ECTR may reduce the duration of coma and the requirement for mechanical ventilation, and prevent the development of cerebral edema. ECTR (especially hemodialysis) corrects acidemia and readily eliminates ammonia. The reported complications associated with hemodialysis are uncommon and the cost of performing ECTR may be balanced by a reduction in duration of coma and length of stay in an intensive care unit. Based on these arguments, the workgroup reached a consensus that the balance of risk versus benefit supports the use of ECTR in severe VPA poisoning and was strongly supported by the workgroup (27/28 participants voted between 7 and 9, median vote = 8).
(2) Indications for ECTR
ECTR is recommended for VPA poisoning if ANY of the following is present:
Serum VPA concentration > 1300 mg/L (9000 μmol/L) (1D)
Cerebral edema (1D) or shock (1D), attributed to VPA toxicity
ECTR is suggested for VPA poisoning if ANY of the following is present:
Serum VPA concentration > 900 mg/L (6250 μmol/L) (2D)
Coma or respiratory depression requiring mechanical ventilation (2D)
Acute hyperammonemia (2D)
pH is ≤ 7.10 (2D)
Rationale: Serum VPA concentrations may be helpful in evaluating the severity of poisoning. As concentration increases, protein binding becomes saturated, increasing the amount of free drug entering the central nervous system. A serum concentration of > 450 mg/L (3125 μmol/L) is associated with a moderate or major adverse outcome and > 850 mg/L (5900 μmol/L) is associated with coma and metabolic acidosis.Citation13 This cutoff is reflected by the conclusions reached by the workgroup, and ECTR is suggested with a serum concentration > 900 mg/L (6250 μmol/L) (2D) and recommended when > 1300 mg/L (9000 μmol/L) (1D).
Coma, respiratory depression requiring mechanical ventilation, hemodynamic instability, and metabolic acidosis are considered manifestations of severe VPA poisoning and may be associated with a higher risk of complications and morbidity. Severe VPA poisoning can lead to cerebral edema, which can be fatal. The etiology of cerebral edema related to VPA poisoning is controversial. It may be attributed to VPA, to one of its metabolites (such as 2-en-VPA and 4-en-VPA), or to hyperammonemia.Citation126 Given the potential consequences of cerebral edema, the workgroup recommended the use of ECTR in the presence of cerebral edema associated with VPA poisoning. Although shock is often quoted as a contraindication to ECTR, it was acknowledged that this was likely induced by VPA and its metabolites and might therefore be corrected by prompt initiation of extracorporeal removal. It was considered unlikely that hypotension would be exacerbated by ECTR if no net ultrafiltration was prescribed.
Acute hyperammonemia plays an important role in the pathogenesis of encephalopathy and brain edema that occurs in fulminant liver failure of many etiologies. Ammonia crosses the blood–brain barrier and increases extracellular concentrations of glutamate in the brain, which leads to N-methyl-D-aspartate (NMDA) receptor activation.Citation23 Hyperammonemia is efficiently corrected by hemodialysis, and rapid lowering of serum ammonia may help reverse encephalopathy.Citation125 VPA-associated hyperammonemia and encephalopathy can occur both chronically with therapeutic use and acutely in overdose, which may lead in the latter case to cerebral edema and a fatal outcome. The best therapeutic approach for acute hyperammonemia associated with severe VPA poisoning remains unknown. The workgroup concluded that ECTR is indicated with acute hyperammonemia in the context of severe VPA poisoning, although it declined to set a specific value for ECTR initiation. It is also acknowledged that, in this context, the entire spectrum of clinical manifestations will usually be considered. Since the focus of EXTRIP is on toxin removal, the workgroup did not specifically consider the question of the best treatment for patients with hyperammonemia in the setting of therapeutic VPA concentrations.
L-carnitine is commonly recommended initially for the treatment of VPA-associated hyperammonemia, but clinical evidence supporting this indication is limited and it is unclear if L-carnitine has any impact on the clinical manifestations of severe VPA poisoning.Citation22–24 The benefits of intravenous L-carnitine administration during HD are unknown, especially when considering that L-carnitine is dialyzable.Citation112
The reported amount of VPA ingested was not considered a reliable indicator by the workgroup since estimates of the ingested dose may be inaccurate and would not alone justify the potential risks associated with ECTR or the costs associated with transferring a patient to an ECTR center. Therefore, the decision to perform ECTR should not be based on history of ingestion alone but would warrant close monitoring when massive, as indications for ECTR may develop.
(3) Cessation of ECTR
ECTR should be continued until clinical improvement is apparent (1D) OR until the serum VPA concentration is between 50 and 100 mg/L (350–700 μmol/L) (2D).
Rationale: Cessation of ECTR should be based on appropriate correction of the manifestations of severe poisoning such as coma, acidemia, respiratory depression, and hemodynamic instability. This approach may not be applicable to patients with mixed ingestions of sedative drugs not removed by ECTR. Alternatively, ECTR should be continued until serum VPA concentration reaches the therapeutic range (between 50 and 100 mg/L (350 and 700 μmol/L)). This approach may prevent further toxicity, and minimize the risk of withdrawal seizures in a patient requiring VPA.Citation104 The concentration of VPA may increase (“rebound”) after high-efficiency ECTRs, a phenomenon most often caused by redistribution from deeper compartments into the plasma. Although this was often reported,Citation18,Citation32–34,Citation56,Citation61,Citation62,Citation64,Citation76 the extent of the rebound was invariably minimal and not associated with clinical deterioration. If concerning, rebound could be simply addressed with a second ECTR session.
(4) Choice of ECTR
Intermittent hemodialysis is the preferred ECTR in VPA poisoning (1D)
If hemodialysis is not available, both intermittent hemoperfusion (1D) and CRRT (2D) are acceptable alternatives.
Rationale: Several ECTRs have been performed in patients with severe VPA poisoning, in most cases either intermittent hemodialysis, hemoperfusion, CRRTs, or a combination of these. Although the analysis may be skewed by the inclusion of obsolete ECTR parameters (low blood flow and low-flux/low-efficiency membranes), the VPA clearance appears to be most favorable for intermittent hemodialysis, with a median ECTR clearance of 88 mL/min and reaching up to 140 mL/min with modern technology and optimization of operational parameters.Citation69,Citation127
The preference of hemodialysis over other ECTR modalities is based on its higher apparent removal of VPA ( and ), its capacity to correct acidemia (a common finding at presentation), and its superior clearance of ammonia.Citation119–122 Hemodialysis is also more available worldwide, which would likely limit the transfer time for its initiation. Hemoperfusion is more costly,Citation128 is limited by saturation of both resin and charcoal cartridges,Citation129 and is fraught with a higher incidence of complications,Citation130 some of which may be life-threatening.Citation85 Hypocalcemia or thrombocytopenia may also complicate hemoperfusion,Citation33,Citation47,Citation53,Citation81 which may be a concern as thrombocytopenia sometimes complicates VPA toxicity.Citation13,Citation131
The workgroup agreed that hemoperfusion may be a convincing alternative in the situation where hemodialysis would not be available. The efficiency of CRRT is noticeably inferior to high-efficiency intermittent dialysis but may be considered when technical or logistic reasons for performing it preclude its use.Citation132 CRRT has also theoretically less impact on patients with increased intracranial pressure and may therefore be a consideration with patients with documented cerebral edema.Citation133 Online hemodiafiltration appears promising but requires further study. Both therapeutic plasma exchange and liver support therapies are more costly, less available, and do not appear to offer any advantage over dialysis. As in most cases of poisoning, the benefit of peritoneal dialysis is insignificant and does not justify its cost and potential for complications.Citation134,Citation135 No data are currently available to evaluate the efficacy of exchange transfusion but, based on the low distribution volume of VPA, may be a consideration in neonates when other ECTRs may be technically complicated to perform.
Conclusion
Intentional overdose with VPA is a common toxicological problem. The majority of patients with VPA ingestions will have a relatively benign clinical course and a good outcome. The use of ECTR should be reserved for patients with severe VPA poisoning such as coma, respiratory depression requiring mechanical ventilation, pronounced acidemia, and high serum concentrations at which complications such as cerebral edema are expected to occur. Intermittent hemodialysis is the preferred ECTR in VPA poisoning. Hemoperfusion may represent an alternative to hemodialysis. CRRTs are associated with lower clearance rates and may be used when hemodialysis is not available. VPA is moderately dialyzable in overdose.
Supplementary material available online
Supplementary Tables 1 and 2.
ictx_a_1035441_sm9881.docx
Download MS Word (272.6 KB)ictx_a_1035441_sm9879.docx
Download MS Word (198 KB)Acknowledgments
We would like to acknowledge the tremendous work of our dedicated translators: Marcela Covica, Alexandra Angulo, Ania Gresziak, Samantha Challinor, Monique Cormier, Martine Blanchet, Gunel Alpman, Joshua Pepper, Lee Anderson, Andreas Betz, Tetsuya Yamada, Nathalie Eeckhout, Matthew Fisher, Ruth Morton, Denise Gemmellaro, Nadia Bracq, Olga Bogatova, Sana Ahmed, Christiane Frasca, Katalin Fenyvesi, Timothy Durgin, Helen Johnson, Martha Oswald, Ewa Brodziuk, David Young, Akiko Burns, Anna Lautzenheiser, Banumathy Sridharan, Charlotte Robert, Liliana Ionescu, Lucile Mckay, Vilma Etchart, Valentina Bartoli, Nathan Weatherdon, Marcia Neff, Margit Tischler, Sarah Michel, Simona Vairo, Mairi Arbuckle, Luc Ranger, Nerissa Lowe, Angelina White, Salih Topal, John Hartmann, Karine Mardini, Mahala Bartle Mathiassen, Anant Vipat, Gregory Shapiro, Hannele Marttila, and Kapka Lazorova. We also acknowledge the important contribution from our librarians and administrative aides: Marc Lamarre, David Soteros, Salih Topal, Henry Gaston, and Brenda Gallant.
Support and financial disclosure declaration
Funding: Funding for EXTRIP was obtained from industry in the form of unrestricted educational grants. These funds were used solely for expenses related to literature retrieval, translation of publications, and for reimbursement of conference calls and travel expenses for attendance at EXTRIP meetings. A list of EXTRIP sponsors can be found on www.extrip-workgroup.org. There was no industry input into meeting organization, scientific content, development, or publication of the recommendations. Furthermore, industry presence at meetings was not allowed, nor was industry awareness or comment on the recommendations sought or accepted.
Declaration of interests
The authors declare that they have no conflict of interest financial or otherwise related to this work. Complete financial disclosure for each EXTRIP member can be found on www.extrip-workgroup.org.
References
- Ghannoum M, Nolin TD, Lavergne V, Hoffman RS; EXTRIP workgroup. Blood purification in toxicology: nephrology's ugly duckling. Adv Chronic Kidney Dis 2011; 18:160–166.
- Lavergne V, Nolin TD, Hoffman RS, Robert D, Gosselin S, Goldfarb DS, et al. The EXTRIP (Extracorporeal Treatments In Poisoning) workgroup: guideline methodology. Clin Toxicol 2012; 50:403–413.
- Ghannoum M, Nolin TD, Goldfarb DS, Roberts DM, Mactier R, Mowry JB, et al. Extracorporeal treatment for thallium poisoning: recommendations from the EXTRIP Workgroup. Clin J Am Soc Nephrol 2012; 7:1682–1690.
- Mactier R, Laliberte M, Mardini J, Ghannoum M, Lavergne V, Gosselin S, et al. Extracorporeal treatment for barbiturate poisoning: recommendations from the EXTRIP Workgroup. Am J Kidney Dis 2014; 64:347–358.
- Gosselin S, Juurlink DN, Kielstein JT, Ghannoum M, Lavergne V, Nolin TD, et al. Extracorporeal treatment for acetaminophen poisoning: recommendations from the EXTRIP workgroup. Clin Toxicol (Phila) 2014; 52:856–867.
- Lavergne V, Ouellet G, Bouchard J, Galvao T, Kielstein JT, Roberts DM, et al. Guidelines for reporting case studies on extracorporeal treatments in poisonings: methodology. Semin Dial 2014; 27:407–414.
- Yates C, Galvao T, Sowinski KM, Mardini K, Botnaru T, Gosselin S, et al. Extracorporeal treatment for tricyclic antidepressant poisoning: Recommendations from the EXTRIP workgroup. Semin Dial 2014; 27:381–389.
- Roberts DM, Yates C, Megarbane B, Winchester JF, Maclaren R, Gosselin S, et al. Recommendations for the role of extracorporeal treatments in the management of acute methanol poisoning: a systematic review and consensus statement. Crit Care Med 2015; 43:461–472.
- Ghannoum M, Yates C, Galvao TF, Sowinski KM, Vo TH, Coogan A, et al. Extracorporeal treatment for carbamazepine poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila) 2014; 52:993–1004.
- Decker BS, Goldfarb DS, Dargan PI, Friesen M, Gosselin S, Hoffman RS, et al. Extracorporeal treatment for lithium poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin J Am Soc Nephrol 2015; pii: CJN.10021014. [Epub ahead of print].
- Mowry JB, Spyker DA, Cantilena LR, Jr., McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Klotz U, Antonin KH. Pharmacokinetics and bioavailability of sodium valproate. Clin Pharmacol Ther 1977; 21:736–743.
- Spiller HA, Krenzelok EP, Klein-Schwartz W, Winter ML, Weber JA, Sollee DR, et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol 2000; 38:755–760.
- van den Broek MP, Sikma MA, Ververs TF, Meulenbelt J. Severe valproic acid intoxication: case study on the unbound fraction and the applicability of extracorporeal elimination. Eur J Emerg Med 2009; 16:330–332.
- Sztajnkrycer MD. Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol 2002; 40:789–801.
- Thanacoody RH. Extracorporeal elimination in acute valproic acid poisoning. Clin Toxicol (Phila) 2009; 47:609–616.
- Franssen EJ, van Essen GG, Portman AT, de Jong J, Go G, Stegeman CA, Uges DR. Valproic acid toxicokinetics: Serial hemodialysis and hemoperfusion. Ther Drug Monit 1999; 21:289–292.
- Johnson LZ, Martinez I, Fernandez MC, Davis CP, Kasinath BS. Successful treatment of valproic acid overdose with hemodialysis. Am J Kidney Dis 1999; 33:786–789.
- Isbister GK, Balit CR, Whyte IM, Dawson A. Valproate overdose: A comparative cohort study of self poisonings. Br J Clin Pharmacol 2003; 55:398–404.
- Vale J, Krenzelok, EP., Barceloux VD. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol 1999; 37:731–751.
- Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila) 2009; 47:101–111.
- Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother 2010; 44:1287–1293.
- Lheureux PE, Penaloza A, Zahir S, Gris M. Science review: carnitine in the treatment of valproic acid-induced toxicity - what is the evidence? Crit Care 2005; 9:431–440.
- Mock CM, Schwetschenau KH. Levocarnitine for valproic-acid-induced hyperammonemic encephalopathy. Am J Health-Syst Pharm 2012; 69:35–39.
- Lewis C, Deshpande A, Tesar GE, Dale R. Valproate-induced hyperammonemic encephalopathy: A brief review. Curr Med Res Opin 2012; 28:1039–1042.
- Rivers C. Valproic acid poisoning. UpToDate, Post TW (Ed). 2013.
- Valproate De Sodium. Toxinz, National Poisons Centre, New Zealand. 2015.
- Doyon S. Anticonvulsants. In: Hoffman RS, Howland MA, Lewin NA, Nelson L, Goldfrank LR, Flomenbaum N, editors. Goldfrank's toxicologic emergencies. 10th edition/ed. New York: McGraw Hill; 2015. p. 645–656.
- Appraisal of guidelines for research & evaluation. AGREE instrument. London: The AGREE Collaboration; 2001.
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328:1490.
- Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, USA: RAND; 2011. Available from: http://www.rand.org/.
- Singh SM, McCormick BB, Mustata S, Thompson M, Prasad GV. Extracorporeal management of valproic acid overdose: a large regional experience. J Nephrol 2004; 17:43–49.
- Al Aly Z, Yalamanchili P, Gonzalez E. Extracorporeal management of valproic acid toxicity: a case report and review of the literature. Semin Dial 2005; 18:62–66.
- Alluin A, Jezequel J, Gauthier N, Desmaretz JL, Canevet C. Acute valproic acid intoxication: interest of a treatment by extracoporeal elimination combined with L-carnitine. Ann Fr Anesth Reanim 2011; 30:752–754.
- Auinger K, Muller V, Rudiger A, Maggiorini M. Valproic acid intoxication imitating brain death. Am J Emerg Med 2009; 27:1177 e5–6.
- Bergmann I, Bannister K. Successful treatment of a severe sodium valproate intoxication with haemodialysis. Nephrology 2010; 15:85.
- Blayac D, Roch A, Michelet P, De Francheschi E, Auffray JP. [Deep lactic acidosis after valproate self-poisoning]. Ann Fr Anesth Reanim 2004; 23:1007–1110.
- Bohm M, Meencke HJ. Coma following valproate intoxication. Aktuelle Neurologie 2002; 29:516–519.
- Brubacher JR, Dahghani P, McKnight D. Delayed toxicity following ingestion of enteric-coated divalproex sodium (Epival). J Emerg Med 1999; 17:463–467.
- Chou HF, Yang RC, Chen CY, Jong YJ. Valproate-induced hyperammonemic encephalopathy. Pediatr Neonatol 2008; 49:201–204.
- Colak A, Memis D, Guzel A, Cerci H, Gurkaynak B. Valproic acid intoxication with suicide attempt in a pediatric patient. Pediatr Int 2011; 53:781–783.
- Davison AS, Milan AM, Roberts NB. The consequences of valproate overdose. Clin Chem 2011; 57:1233–1237.
- Deras P, Gignon L, Toumi M, Louart G, Muller L, Boyer JC, et al. Lactic acidosis after voluntary intoxication with valproic acid. Ann Fr Anesth Reanim 2010; 29:63–64.
- Dharnidharka VR, Fennell RS 3rd, Richard GA. Extracorporeal removal of toxic valproic acid levels in children. Pediatr Nephrol 2002; 17:312–315.
- Dichtwald S, Dahan E, Adi N, Moses A, Sorkine P. Molecular adsorbent recycling system therapy in the treatment of acute valproic acid intoxication. Isr Med Assoc J 2010; 12:307–308.
- Eyer F, Felgenhauer N, Gempel K, Steimer W, Gerbitz KD, Zilker T. Acute valproate poisoning: pharmacokinetics, alteration in fatty acid metabolism, and changes during therapy. J Clin Psychopharmacol 2005; 25:376–380.
- Fernandez MC, Walter FG, Kloster JC, Do SM, Brady LA, Villarin A, et al. Hemodialysis and hemoperfusion for treatment of valproic acid and gabapentin poisoning. Vet Hum Toxicol 1996; 38:438–443.
- Field J, Daly FS. Continuous veno-venous haemodiafiltration in sodium valproate overdose complicated by cerebral oedema: a case report. Crit Care Resusc 2002; 4:173–176.
- Frifelt JJ, Wanscher MC, Wienholtz G, Horwitz N, Brockhattingen A. Acute valproate poisoning. Ugeskr Laeger 1987; 149:3255–3257.
- George SM. Therapeutic plasma exchange for valproic acid poisoning - an innovative approach. Am J Kidney Dis 2009; 53:A37.
- Goncalves JA, Santos C, Montalban JM, Filipe RA, Chorao R, Freixo J, et al. Continuous venovenous haemodiafiltration as a solution for acute intoxication with sodium valproate. Nefrologia 2010; 30:134–135.
- Gracia R, Shepherd G, Rivera W, Keyes DC. Prolonged QTc with a massive valproic acid overdose. Clin Toxicol (Phila) 2004; 42:557.
- Graudins A, Aaron CK. Delayed peak serum valproic acid in massive divalproex overdose—treatment with charcoal hemoperfusion. J Toxicol Clin Toxicol 1996; 34:335–341.
- Guillaume CP, Stolk L, Dejagere TF, Kooman JP. Successful use of hemodialysis in acute valproic acid intoxication. J Toxicol Clin Toxicol 2004; 42:335–336.
- He S, Rifkin SI, Durr JA. Hemoperfusion (HP) vs hemodialysis (HD) in a case of combined overdose (OD) with acetaminophen and valproic acid (VPA): implications of our findings. Am J Kidney Dis 2010; 55:A64.
- Hicks LK, McFarlane PA. Valproic acid overdose and haemodialysis. Nephrol Dial Transplant 2001; 16:1483–1486.
- Hydzik P, Wilimowska J, Groszek B. Liver albumin dialysis (MARS) in severe valproic acid poisoning. Clin Toxicol 2009; 47:482.
- Jacobs FM, Pinot G, Prat D, Pilorge C, Brivet F. Valproic acid overdose and continuous venovenous hemodiafiltration. NDT plus 2008; 1:60–61.
- Jezequel J, Gauthier N, Alluin A, Desmaretz JL, Guenault N, Desaintfuscien E, et al. Severe valproic acid poisoning: Place of antidotal treatment and extracorporeal removal techniques. Reanimation 2010; 19:587–92.
- Jung J, Eo E, Ahn KO. A case of hemoperfusion and L-carnitine management in valproic acid overdose. Am J Emerg Med 2008; 26:388 e3–4.
- Kane SL, Constantiner M, Staubus AE, Meinecke CD, Sedor JR. High-flux hemodialysis without hemoperfusion is effective in acute valproic acid overdose. Ann Pharmacother 2000; 34:1146–1151.
- Kay TD, Playford HR, Johnson DW. Hemodialysis versus continuous veno-venous hemodiafiltration in the management of severe valproate overdose. Clin Nephrol 2003; 59:56–58.
- Khan E, Huggan P, Celi L, MacGinley R, Schollum J, Walker R. Sustained low-efficiency dialysis with filtration (SLEDD-f) in the management of acute sodium valproate intoxication. Hemodial Int 2008; 12:211–214.
- Kielstein JT, Woywodt A, Schumann G, Haller H, Fliser D. Efficiency of high-flux hemodialysis in the treatment of valproic acid intoxication. J Toxicol Clin Toxicol 2003; 41:873–876.
- Kostic M, Pan C, Leikin J, Gummin D. Critical valproate toxicity reversed with hemodialysis in a toddler. Clin Toxicol 2010; 48:606.
- Kroll P, Nand C. Hemodialysis in the treatment of valproic acid overdose. J Clin Psychiatry 2002; 63:78–79.
- Lavigne T, Meziani F, Kremer H, Castelain V, P. B, Jaeger A. Severe valproic acid poisoning treated with l-carnitine and hemodialysis. Lack of correlation between symptoms, serum valproic acid concentrations and ammonemia. Clin Toxicol (Phila). 2004; 42:560.
- Letonja M, Petrovic D, Brvar M, Bunc M. Cardiotoxicity in acute valproate poisoning. Clin Toxicol 2009; 47:454–455.
- Licari E, Calzavacca P, Warrillow SJ, Bellomo R. Life-threatening sodium valproate overdose: a comparison of two approaches to treatment. Crit Care Med 2009; 37:3161–3164.
- Luzhnikov EA, Sukhodolova GN, Ostapenko YN, Kovalenko LA, Kovalchuk AS, Dolginov DM. A case of successful therapy in acute valproic acid poisoning in a child using hemodiafiltration. Clin Toxicol 2010; 48:248.
- Matsumoto J, Ogawa H, Maeyama R, Okudaira K, Shinka T, Kuhara T, et al. Successful treatment by direct hemoperfusion of coma possibly resulting from mitochondrial dysfunction in acute valproate intoxication. Epilepsia 1997; 38:950–953.
- Meek MF, Broekroelofs J, Yska JP, Egbers PH, Boerma EC, van der Voort PH. Valproic acid intoxication: Sense and non-sense of haemodialysis. Neth J Med 2004; 62:333–336.
- Mestrovic J, Filipovic T, Polic B, Stricevic L, Omazic A, Kuzmanic-Samija R, Markić J. Life-threatening valproate overdose successfully treated with haemodialysis. Arh Hig Rada Toksikol 2008; 59:295–298.
- Meyer S, Kuhlmann MK, Peters FT, Limbach HG, Lindinger A. Severe valproic acid intoxication is associated with atrial tachycardia: secondary detoxication by hemoperfusion. Klin Padiatr 2005; 217:82–85.
- Min YG, Tse ML. Image in toxicology: pseudo-subarachnoid hemorrhage in a case of severe valproic acid poisoning. Clin Toxicol 2011; 49:699–700.
- Minari M, Maggiore U, Tagliavini D, Rotelli C, Cabassi A, David S, Fiaccadori E. Severe acute valproic acid intoxication successfully treated with hemodiafiltration without hemoperfusion. Ann Emerg Med 2002; 39:204–205.
- Minville V, Roche Tissot C, Samii K. [Haemodialysis, L-carnitine therapy and valproic acid overdose]. Ann Fr Anesth Reanim 2004; 23:357–360.
- Mortensen PB, Hansen HE, Pedersen B, Hartmann-Andersen F, Husted SE. Acute valproate intoxication: biochemical investigations and hemodialysis treatment. Int J Clin Pharmacol Ther Toxicol 1983; 21:64–68.
- Nasa P, Sehrawat D, Kansal S, Chawla R. Effectiveness of hemodialysis in a case of severe valproate overdose. Indian J Crit Care Med 2011; 15:120–122.
- Novotny WE, Meggs W, Hymes L, Ford M. Mild valproic acid poisoning and development of near fatal cerebral edema. Clin Toxicol 2014; 52:329–330.
- Peces R, Fernandez EJ, Sanchez RJ, Peces C, Montero A, Selgas R. [Hemoperfusion in the treatment of acute valproic acid intoxication]. Nefrologia 2007; 27:370–373.
- Pertoldi F, D’Orlando L, Galliazzo S, Mercante WP. Haemodialysis in the management of severe valproic acid overdose. Clin Intensive Care 1997; 8:244–246.
- Pons S, Gonzva J, Prunet B, Gaillard T, Brisou P, Vest P, Emile L. Acute overdose of enteric-coated valproic acid and olanzapine: unusual presentation and delayed toxicity. Clin Toxicol 2012; 50:268.
- Ray S, Skellett S. Valproate toxicity in a child: two novel observations. Clin Toxicol (Phila) 2013; 51:60.
- Rahman MH, Haqqie SS, McGoldrick MD. Acute hemolysis with acute renal failure in a patient with valproic acid poisoning treated with charcoal hemoperfusion. Hemodial Int 2006; 10:256–259.
- Roodhooft AM, Van Dam K, Haentjens D, Verpooten GA, Van Acker KJ. Acute sodium valproate intoxication: occurrence of renal failure and treatment with haemoperfusion-haemodialysis. Eur J Pediatr 1990; 149:363–364.
- Siebenlist D, Klobe R, Gattenlohner W. Plasmaperfusion in the treatment of a severe sodium valproate intoxication. Intensivmedizin und Notfallmedizin 1991; 28:365–367.
- Stankova E, Mechkarska B, Gesheva M. Acute poisoning with valproic acid - a case report. Acta Medica Bulgarica 2007; 34:32–40.
- Sliwkiewicz K, Winnicka R, Rzepecki J, Kolacinski Z, Krakowiak A. Acute intoxication with valproic acid, treated with hemodialysis/hemoperfusion in series - case report. Clin Toxicol 2010; 48:248.
- Tank JE, Palmer BF. Simultaneous “in series” hemodialysis and hemoperfusion in the management of valproic acid overdose. Am J Kidney Dis 1993; 22:341–344.
- Temel V, Arikan M, Temel G. High-flux hemodialysis and levocarnitine in the treatment of severe valproic Acid intoxication. Case Rep Emerg Med 2013; 2013:526469.
- Tsai MF, Chen CY, Chiu KC. Valproate-induced hyperammonemic encephalopathy treated by hemodialysis. Ren Fail 2008; 30:822–824.
- Tominaga A. Case of valproic acid overdose treated by hemodialysis. Chudoku Kenkyu 2014; 27:45–46.
- van der Merwe AC, Albrecht CF, Brink MS, Coetzee AR. Sodium valproate poisoning. A case report. S Afr Med J 1985; 67:735–736.
- van der Wouden EA, Dekkers A, Kruis HM, van Geijlswijk IM, Tjan DH, Feith GW. Extracorporeal elimination in acute valproate intoxication. BMJ Case Rep 2009; 2009: pii: bcr06.2008.0337. doi: 10.1136/bcr.06.2008.0337. Epub 2009 Feb 2.
- van Kuelen JG, van Wijk JA, Touw DJ, van der Deure J, Markhorst DG, Gemke RJ. Effectiveness of haemofiltration in valproic acid intoxication. Acta Paediatr 2001; 90:958–959.
- Williams S, Clark R. Hemodialysis of a valproic acid poisoning. J Toxicol Clin Toxicol 1995; 33:491.
- Von Bardeleben RS, Sturer A, Weilemann LS. Valproic acid intoxications in 96 documented cases 1995–1998: the role of hemoperfusion in therapy. Clin Toxicol (Phila) 1998; 36:471–472.
- Wolze O, Summa JD, Platt D, Degel F, Eicher H. A report of a severe sodium valproate poisoning case and its treatment by hemoperfusion and forced diuresis. Intensivmedizin und Notfallmedizin 1988; 25:281–284.
- Bastiaans DE, van Uden IW, Ruiterkamp RA, de Jong BA. Removal of valproic acid by plasmapheresis in a patient treated for multiple sclerosis. Ther Drug Monit 2013; 35:1–3.
- Lai CW, Leppik IE, Jenkins DC, Sood P. Epilepsy, myasthenia gravis, and effect of plasmapheresis on antiepileptic drug concentrations. Arch Neurol 1990; 47:66–68.
- Marbury TC, Lee CS, Bruni J. Hemodialysis of valproic acid in uremic patients. Dial Transplant 1980; 9:961–964.
- Orr JM, Farrell K, Abbott FS, Ferguson S, Godolphin WJ. The effects of peritoneal dialysis on the single dose and steady state pharmacokinetics of valproic acid in a uremic epileptic child. Eur J Clin Pharmacol 1983; 24:387–390.
- Gubensek J, Buturovic-Ponikvar J, Ponikvar R, Cebular B. Hemodiafiltration and high-flux hemodialysis significantly reduce serum valproate levels inducing epileptic seizures: case report. Blood Purif 2008; 26:379–380.
- Kochen W, Schneider A, Ritz A. Abnormal metabolism of valproic acid in fatal hepatic failure. Eur J Pediatr 1983; 141:30–35.
- Kandrotas RJ, Love JM, Gal P, Oles KS. The effect of hemodialysis and hemoperfusion on serum valproic acid concentration. Neurology 1990; 40:1456–1458.
- Bertrand YM, Hermant A, Mahieu P, Roels J. Intracranial pressure changes in patients with head trauma during haemodialysis. Intensive Care Med 1983; 9:321–323.
- Shi ZW, Wang ZG. Acute cerebral and pulmonary edema induced by hemodialysis. Chin Med J (Engl) 2008; 121:1003–1009.
- Krane NK. Intracranial pressure measurement in a patient undergoing hemodialysis and peritoneal dialysis. Am J Kidney Dis 1989; 13:336–339.
- Lin CM, Lin JW, Tsai JT, Ko CP, Hung KS, Hung CC, et al. Intracranial pressure fluctuation during hemodialysis in renal failure patients with intracranial hemorrhage. Acta Neurochir Suppl 2008; 101:141–144.
- Bain MA, Faull R, Milne RW, Evans AM. Oral L-carnitine: metabolite formation and hemodialysis. Curr Drug Metab 2006; 7:811–816.
- Fornasini G, Upton RN, Evans AM. A pharmacokinetic model for L-carnitine in patients receiving haemodialysis. Br J Clin Pharmacol 2007; 64:335–345.
- Vernez L, Dickenmann M, Steiger J, Wenk M, Krahenbuhl S. Effect of L-carnitine on the kinetics of carnitine, acylcarnitines and butyrobetaine in long-term haemodialysis. Nephrol Dial Transplant 2006; 21:450–458.
- Chyka PA, Holley JE, Mandrell TD, Sugathan P. Correlation of drug pharmacokinetics and effectiveness of multiple-dose activated charcoal therapy. Ann Emerg Med 1995; 25:356–362.
- al-Shareef A, Buss DC, Shetty HG, Ali N, Routledge PA. The effect of repeated-dose activated charcoal on the pharmacokinetics of sodium valproate in healthy volunteers. Br J Clin Pharmacol 1997; 43:109–111.
- Farrar HC, Herold DA, Reed MD. Acute valproic acid intoxication: enhanced drug clearance with oral-activated charcoal. Crit Care Med 1993; 21:299–301.
- Kronfol NO, Lau AH, Colon-Rivera J, Libertin CL. Effect of CAVH membrane types on drug-sieving coefficients and clearances. ASAIO Trans 1986; 32:85–87.
- Churchwell MD, Pasko DA. Enhanced valproic acid dialytic clearance with an albumin-based dialysate in continuous venovenous hemodialysis. Blood Purif 2005; s23:154–155.
- Siegel NJ, Brown RS. Peritoneal clearance of ammonia and creatinine in a neonate. J Pediatr 1973; 82:1044–1046.
- Lai YC, Huang HP, Tsai IJ, Tsau YK. High-volume continuous venovenous hemofiltration as an effective therapy for acute management of inborn errors of metabolism in young children. Blood Purif 2007; 25:303–308.
- Wiegand C, Thompson T, Bock GH, Mathis RK, Kjellstrand CM, Mauer SM. The management of life-threatening hyperammonemia: a comparison of several therapeutic modalities. J Pediatr 1980; 96:142–144.
- Rutledge SL, Havens PL, Haymond MW, McLean RH, Kan JS, Brusilow SW. Neonatal hemodialysis: effective therapy for the encephalopathy of inborn errors of metabolism. J Pediatr 1990; 116:125–128.
- Chen CY, Chen YC, Fang JT, Huang CC. Continuous arteriovenous hemodiafiltration in the acute treatment of hyperammonaemia due to ornithine transcarbamylase deficiency. Ren Fail 2000; 22:823–836.
- Slack AJ, Auzinger G, Willars C, Dew T, Musto R, Corsilli D, et al. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int 2014; 34:42–48.
- Cordoba J, Blei AT, Mujais S. Determinants of ammonia clearance by hemodialysis. Artif Organs 1996; 20:800–803.
- Dupuis RE, Lichtman SN, Pollack GM. Acute valproic acid overdose. Clinical course and pharmacokinetic disposition of valproic acid and metabolites. Drug Saf 1990; 5:65–71.
- Bouchard J, Roberts DM, Roy L, Ouellet G, Decker BS, Mueller BA, et al. Principles and operational parameters to optimize poison removal with extracorporeal treatments. Semin Dial 2014; 27:371–380.
- Maslov OG, Brusin KM, Kochmashev VF, Sentsov VG. Comparative evaluation of methods of extracorporeal detoxification in acute carbamazepine poisoning. Klinicheskaia Toksikologiia 2011; 12: 1169–1179.
- Ghannoum M, Bouchard J, Nolin TD, Ouellet G, Roberts DM. Hemoperfusion for the treatment of poisoning: technology, determinants of poison clearance, and application in clinical practice. Semin Dial 2014; 27:350–361.
- Shannon MW. Comparative efficacy of hemodialysis and hemoperfusion in severe theophylline intoxication. Acad Emerg Med 1997; 4:674–678.
- Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol 2000; 22:62–65.
- Ghannoum M, Roberts DM, Hoffman RS, Ouellet G, Roy L, Decker BS, Bouchard J. A stepwise approach for the management of poisoning with extracorporeal treatments. Semin Dial 2014; 27:362–370.
- Richardson D, Bellamy M. Intracranial hypertension in acute liver failure. Nephrol Dial Transplant 2002; 17:23–27.
- Ghannoum M, Gosselin S. Enhanced poison elimination in critical care. Adv Chronic Kidney Dis 2013; 20:94–101.
- Ouellet G, Bouchard J, Ghannoum M, Decker BS. Available extracorporeal treatments for poisoning: overview and limitations. Semin Dial 2014; 27:342–349.