Abstract
Objectives. To review current evidence of efficacy of Ginkgo biloba extract EGb 761® in dementia with behavioural and psychological symptoms (BPSD). Methods. Randomized, placebo-controlled trials assessing the effects of EGb 761® in dementia patients with BPSD were included if the diagnosis was made in accordance with internationally accepted criteria, the treatment period was at least 22 weeks, outcome measures covered BPSD and at least two of the following domains of assessment, i.e. cognition, activities of daily living and clinical global assessment, and methodological quality was adequate. An analysis of covariance (ANCOVA) model was used to calculate the pooled effect estimates and to compare effects of EGb 761® and placebo; furthermore, combined risk differences of response rates were calculated. Results. Four published trials were identified, involving altogether 1,628 outpatients with mild to moderate dementia. Least-square mean differences for change from baseline in cognition, BPSD (including caregiver distress rating), activities of daily living, clinical global impression, and quality of life favoured EGb 761® (P < 0.001 for all comparisons). Conclusions. The pooled analyses provide evidence of efficacy of EGb 761® at a daily dose of 240 mg in the treatment of out-patients suffering from Alzheimer's, vascular or mixed dementia with BPSD.
Introduction
Population aging and the resulting increased prevalence of both Alzheimer's disease (AD) and vascular dementia (VaD) has significant implications worldwide. It is estimated that the number of people living with dementia worldwide (35.6 million in 2010) will increase to 65.7 million by 2030 and 115.4 million by 2050 (CitationAlzheimer's Disease International 2010). There is no cure for dementia, and evidence for the effectiveness of preventive measures is sparse.
Although behavioural and psychological symptoms of dementia (BPSD), also referred to as neuropsychiatric symptoms, have been found in 80% to nearly 100% of patients with dementia (CitationSteinberg et al. 2008; Citationvan der Mussele et al. 2013), patients with such symptoms have been excluded from many anti-dementia drug trials, which limits the generalizability of the trial results to the actual patient population (CitationSchneider et al. 1997a). It was speculated earlier that the presence and severity of BPSD, namely depression, might influence cognitive abilities and, therefore, treatment-related improvements in BPSD (or depression) might indirectly improve cognition. There was concern therefore that a drug that actually affects BPSD might be falsely considered as an anti-dementia drug (CitationLeber 1990). However, CitationPowlishta et al. (2004) have demonstrated that cognitive impairment even in very mild AD is driven by the underlying disease and its severity is independent of concomitant depression.
Vascular risk factors such as hypertension and stroke are associated with a higher frequency of BPSD (CitationTreiber et al. 2008). The presence of BPSD is associated with a faster progression to severe dementia (CitationRabins et al. 2013) and has a negative impact on the patients’ quality of life (CitationBanerjee et al. 2009; CitationKarttunen et al. 2011). Clinical trials of EGb 761® and an earlier systematic review found BPSD to be effect modifiers in the treatment of dementia (CitationSchneider et al. 2005; CitationIhl et al. 2010; CitationJanssen et al. 2010). The biological mechanisms underlying this effect are not fully understood, but they may be related to vascular effects of EGb 761®. Consequently, some of the recent trials of Ginkgo biloba extract EGb 761® specifically enrolled patients with BPSD.
EGb 761® is one of the anti-dementia drugs with proven benefits (CitationJanssen et al. 2010; CitationWeinmann et al. 2010) that is recommended by international guidelines for the symptomatic treatment of dementia (CitationIhl et al. 2011b). An earlier Cochrane Review (Birks and Grimley Evans 2007) came to a less favourable conclusion, but that review included studies of different Ginkgo products in patients with a variety of conditions (subjective complaints, mild cognitive deficits, full-blown dementia), and the three latest trials in dementia with BPSD were not available yet when it was conducted. EGb 761®* has a multi-factorial pharmacodynamic profile. It preserves and improves mitochondrial function and energy metabolism, promotes hippocampal neurogenesis and neuroplasticity and enhances cerebral blood flow by decreasing blood viscosity (CitationMüller et al. 2012; CitationLang et al. 2013). With data from two further randomized, placebo-controlled trials that were not available at the time of Janssen et al.'s (2010) review, there is now a solid database for a systematic review and pooled data analysis specifically addressing the question of efficacy and safety of EGb 761® in patients with dementia who have clinically significant BPSD. With nearly identical inclusion and exclusion criteria in these clinical trials, the data could be pooled. Since the majority of patients with dementia exhibit BPSD (CitationPetrovic et al. 2007; CitationSteinberg et al. 2008; CitationDi Iulio et al. 2010; CitationVan der Mussele et al. 2013), they represent the core target group for anti-dementia drug treatment in general, as well as for the treatment of BPSD, in particular.
Methods
Data sources
We used a sensitive search strategy including the following electronic databases: PubMed, including MedLine (from beginning to December 2013), EMBASE (from January 2006 to December 2013) and PASCAL (from beginning to December 2013). The following search terms were used (with * characterizing a wildcard, and the items AND and OR being used as Boolean functions): (ginkg* OR gingk*) AND clinical trial[pt] for PubMed including MedLine, ((ginkg* OR gingk*) NOT medline[sb]) AND (clinical* OR trial OR randomized) for PubMed excluding Medline, (GINKGO OR GINGKO) AND (HUMAN/CT OR HOMME/CTFR) for PASCAL, and (ginkgo or gingko) AND CT = (CLINICAL TRIAL; CLINICAL STUDY; DOUBLE BLIND PROCEDURE) AND py > 2005 for EMBASE. Furthermore, the reference sections of systematic reviews were screened for primary publications. In addition, the manufacturer of EGb 761®, Dr. Willmar Schwabe GmbH & Co. KG was asked about studies that were not identified by the search or had not been published when the search was performed.
Selection and critical appraisal of clinical trials
Randomized, placebo-controlled, double-blind clinical trials assessing the effects of an oral dosage form of EGb 761® in patients with the diagnosis of AD, VaD or mixed dementia (i.e. with features of both AD and cerebrovascular disease) of at least 22 weeks’ duration were selected, if (a) the patients enrolled were diagnosed with dementia in accordance with internationally accepted diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders III-R and IV [DSM-III-R, DSM-IV (CitationAPA 1987, Citation1994)], International Classification of Diseases 10 [ICD-10 (CitationWHO 1992)], National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association [NINCDS-ADRDA (CitationMcKhann et al. 1984)], or the National Institute for Neurological Disorders and Stroke and Association Internationale pour la Recherche et l’Enseignement en Neuroscience [NINDS/AIREN (CitationRomán et al. 1993)]); (b) had clinically significant BPSD as defined by minimal scores on the Neuropsychiatric Inventory (NPI, CitationCummings 1997) or other appropriate rating scales; (c) outcome measures were defined for BPSD and at least two of the three typical domains of assessment: cognition, activities of daily living (ADL), and clinical global assessment; and (d) methodological quality was adequate. The methodological quality of the trials was considered adequate, if (a) randomization, allocation concealment and blinding, (b) sample size estimation, (c) numbers and disposition of patients who discontinued the trial prematurely, and (d) statistical analyses were reported and judged adequate.
Statistical analysis
This pooled data analysis is based on individual patient data. The sponsor, Dr. Willmar Schwabe GmbH & Co. KG provided the pertinent data from all selected studies. For each of the four clinical trials, the effects of EGb 761® with respect to the domains cognition, BPSD, activities of daily living, and quality of life were compared to placebo using an analysis of covariance (ANCOVA) model. The ANCOVA model includes terms for treatment group and baseline value of respective outcome variable as covariate. Analysis of the clinical global assessment does not include baseline values since they are not available for all clinical trials. In this case, an analysis of variance (ANOVA) model with a term for treatment group was used. If different outcomes were used in different trials, outcomes (change scores and baseline values, if included in the model) were standardised within each clinical trial by dividing scores by the between patient standard deviation to create a dimensionless measure (CitationHiggins et al. 2001). In the case of missing values for the outcome variables, the last observation during randomised treatment was carried forward (LOCF). Treatment effects in single trials are presented as differences of (standardised) least square means (or differences of standardised means for clinical global assessment) with 95% confidence intervals, respectively.
The design of all clinical trials was very similar with regard to inclusion and exclusion criteria, and duration of treatment. The effects of EGb 761® with respect to the domains cognition, BPSD, activities of daily living, and quality of life were compared to placebo using ANCOVA. The ANCOVA models included terms for clinical trial, treatment group and baseline value of the outcome variable as covariate. Clinical global assessment was analysed using an ANOVA model with terms for clinical trial and treatment group. Overall effect size measured by rating scales is expressed as standardised least square mean differences or least square mean differences if appropriate for the comparison (same outcome in every clinical trial) with 95% confidence intervals. In order to compare overall effect sizes with results of other analyses standardised mean differences (Cohen's D) are reported in addition to the least square mean differences. According to CitationCohen (1988), standardised effect sizes of 0.2 / 0.5 / 0.8 were classified as small / moderate / large effects, respectively.
Response rates for EGb 761® in all domains were compared to placebo in this analysis, as far as the same outcome measures were available for the selected clinical trials. Overall effects for response rates calculated using a fixed effects model and the Mantel-Haenszel method, are expressed as risk differences with 95% confidence intervals. In addition, numbers needed to treat (NNTs) with 95% confidence intervals were calculated using the overall risk differences.
Statistical significance is assumed with P < 0.05. All analyses were based on the entire patient sample and different types of dementia. SAS® package (release 9.2) running on a personal computer was used for the analyses of continuous variables and the calculation of NNTs with 95% confidence intervals. The meta-analyses of response rates were performed using Review Manager, version 5 (The Cochrane Collaboration, Oxford, England).
Results
Clinical trial characteristics
Four trials identified by database searches (CitationNapryeyenko and Borzenko 2007; CitationIhl et al. 2011a; CitationHerrschaft et al. 2012; CitationNikolova et al. 2013) met our inclusion criteria. Six trials that were included in the review by CitationWeinmann et al. (2010) were excluded from the present meta-analysis, because the patients enrolled were not required to have BPSD and BPSD, if present, were not quantified. All eligible trials used the defined extract EGb 761® at daily doses of 240 mg. There was no clinical trial with another ginkgo product that met our inclusion criteria. One trial was performed in Bulgaria (CitationNikolova et al. 2013), two in Ukraine (CitationIhl et al. 2011a; CitationNapryeyenko and Borzenko 2007) and one in three countries (Republic of Belarus, Republic of Moldova, and Russian Federation) (CitationHerrschaft et al. 2012).
All trials included outpatients selected by the following inclusion criteria:
| (a) | mild to moderate dementia; tests used in the four trials comprised the TE4D screening test for dementia (CitationMahoney et al. 2005) and the SKT short cognitive performance test (CitationErzigkeit 1992; CitationKim et al. 1993). The total score in the TE4D was always ≤ 35 and the total score in the SKT was between 9 and 23. These values correspond roughly to the range from 14 to 25 on the Mini-Mental State Examination or 17 to 35 on the cognitive subscale of the Alzheimer's Disease Assessment Scale (CitationIhl et al. 1999); | ||||
| (b) | either probable AD according to NINCDS/ADRDA, or probable vascular dementia according to NINDS/AIREN, or mixed dementia (possible Alzheimer's disease with cerebrovascular disease according to the respective sub-criteria of NINCDS/ADRDA and NINDS/AIREN); all trials included used these criteria; | ||||
| (c) | clinically significant BPSD (NPI total score > 5), but no severe or major depression (total score on the 17-item Hamilton Rating Scale for Depression < 20 (CitationHamilton 1960)); and age ≥ 50. The duration of treatment varied between 22 (CitationNikolova et al. 2013; CitationNapryeyenko and Borzenko 2007) and 24 weeks (CitationIhl et al. 2011a; CitationHerrschaft et al. 2012). | ||||
Sample sizes and demographic characteristics of patient populations are presented in . In total, 1628 patients were randomised, 814 patients to each treatment group. Drop-out rates were low and similar in both treatment groups in all trials. Out of the 1628 randomised patients 1598 (EGb 761®: 796; placebo: 802) were evaluable with respect to efficacy (Full Analysis Set, FAS). The FAS included (a) all randomised patients who received at least one dose of the study drug and had at least one efficacy assessment after baseline, and (b) all randomised patients who had discontinued prematurely due to an adverse event potentially related to the drug under investigation. Demographic characteristics were well balanced across treatment groups in each trial. In the trial of CitationNikolova et al. (2013), about 10% fewer women were included and the patients were about 4 years older compared to the other trials.
Table I. Characteristics of trial populations.
Baseline scores of all outcome measures are presented in . They were well balanced across treatment groups in each trial. The means of the SKT total score varied between 15 (CitationNikolova et al. 2013; CitationHerrschaft et al. 2012) and 17 points (CitationIhl et al. 2011a). The highest NPI total score and NPI distress score were observed in CitationNapryeyenko and Borzenko (2007) with mean values of about 21 and 13 points, respectively. In the other trials the mean NPI total score and the mean NPI distress score were about 17 and 10 points, respectively. The mean of the GBS-ADL (ADL subscale of the Gottfries-Bråne-Steen (GBS, CitationBråne et al. 2001)) was higher in CitationNapryeyenko and Borzenko's (2007) than in Nikolova et al.'s (2013) study. The same holds true for the mean of the GBS total score. Mean scores of the ADL-IS were similar in the treatment groups as well as across trials (CitationIhl et al. 2011a; CitationHerrschaft et al. 2012).
Table II. Baseline scores of clinical outcome measures [mean/(SD)].
Clinical outcomes
Mean changes (with standard deviations) from baseline to end of treatment are presented for clinical outcomes in five domains of assessment: cognition, BPSD, ADL, global rating and quality of life (QoL). For all outcome measures except the QoL scale, negative mean change scores indicate improvement and negative estimates of treatment effects indicate a larger effect of EGb 761® compared to placebo. For the QoL scale, positive changes of the total score show an improvement, positive estimates of treatment effects favour EGb 761®.
Cognition
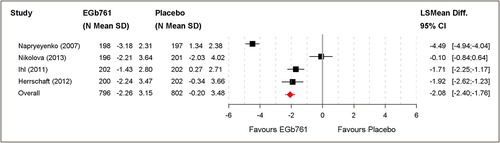
In all trials, the SKT short cognitive performance test was used for cognitive assessment. In three trials, cognition improvement was significantly superior in patients treated with EGb 761® than with placebo. Pooled analysis resulted in an overall mean change score of SKT of –2.26 ± 3.15 for EGb 761® and of –0.20 ± 3.48 for placebo treatment. This corresponds to a standardised mean difference of –0.62 (Cohen's D). The least square mean difference was –2.08 (95% CI [–2.40; –1.76]; P < 0.001). This is roughly equivalent to a mean difference of –2.7 on the ADAS-cog (CitationIhl et al. 1999). A significant difference was confirmed in favour of EGb 761® ().
Behavioural and psychological symptoms (BPSD)
All trials used the NPI total score and the NPI caregiver distress score for the assessment of BPSD and BPSD-related caregiver distress, respectively. Mean change of NPI total scores in the four clinical trials lumped together (1598 patients: 796; 802) were reduced by at least 3 points in all EGb 761®-treated groups (). In three trials patients’ mean change in NPI total and NPI caregiver distress scores improved significantly with EGb 761® compared to placebo.
Figure 2. Changes of NPI total scores [A] and caregiver distress scores [B] based on the full analysis set (FAS).
![Figure 2. Changes of NPI total scores [A] and caregiver distress scores [B] based on the full analysis set (FAS).](/cms/asset/b03bbc47-96c5-42e4-b9a3-25a9fa04449c/iwbp_a_1066513_f0002_oc.jpg)
Pooled analysis resulted in an overall mean change of NPI total score of –4.52 ± 6.56 with EGb 761® and –0.71 ± 6.94 with placebo. This corresponds to a standardised mean difference of –0.56 (Cohen's D). The least square mean difference was –3.85 (95% CI [–4.50; –3.21]; P < 0.001) (). Mean change NPI caregiver distress score pooled analysis resulted in –2.64 ± 4.44 with EGb 761® and –0.33 ± 3.98 with placebo. This corresponds to a standardised mean difference of –0.55 (Cohen's D). The least square mean difference was –2.30 (95% CI [–2.68; –1.91]; P < 0.001) (). A significant difference was confirmed in favour of EGb 761® for both NPI evaluations.
Activities of Daily Living (ADL)
Two trials (CitationNikolova et al. 2013; CitationNapryeyenko and Borzenko 2007) used the ADL subscale of the Gottfries-Bråne-Steen scale (GBS, CitationBråne et al. 2001) and two trials (CitationIhl et al. 2011a; CitationHerrschaft et al. 2012) used the Activities-of-Daily-Living International Scale (ADL-IS, CitationReisberg et al. 2001) to evaluate ADL.
Data from four clinical trials (1598 patients: 796; 802) are presented for activities of daily living. In all trials, activities of daily living improved more in patients actively treated than in those receiving placebo.
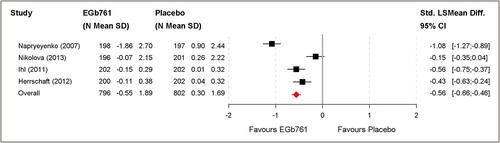
Pooled analysis of all four trials resulted in a moderate standardised least square mean difference of –0.56 (95% CI [–0.66; –0.46]; P < 0.001). A significant difference was confirmed in favour of EGb 761® ().
Clinical global impression of change from baseline
Two trials (CitationNapryeyenko and Borzenko 2007; CitationNikolova et al. 2013) used the GBS total score and two trials (CitationIhl et al. 2011a; CitationHerrschaft et al. 2012) used the Alzheimer's Disease Cooperative Study Clinical Global Impression of Change (ADCS-CGIC, CitationSchneider et al. 1997b) for the clinical global rating of change from baseline.
In all trials, the global impression of change from baseline was rated better for patients treated with EGb 761® compared with placebo. In three trials (CitationNapryeyenko and Borzenko 2007; CitationIhl et al. 2011a; CitationHerrschaft et al. 2012) the differences between the treatment groups were statistically significant ().
Figure 4. Clinical global assessment (change scores of GBS-total score, ADCS-CGIC) based on the full analysis set (FAS).
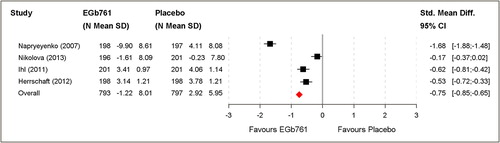
Pooled analysis resulted in a moderate to large standardised MEAN-difference of –0.75 (95% CI [–0.85; –0.65]; P < 0.001). A significant difference was confirmed in favour of EGb 761® ().
Quality of Life
Health-related QoL was evaluated in two clinical trials (806 patients: 402; 404) (CitationIhl et al. 2011a; CitationHerrschaft et al. 2012) using DEMQOL-PROXY total mean score (CitationSmith et al. 2005). In both trials, quality of life mean improvement was significantly superior in patients with EGb 761® than with placebo.
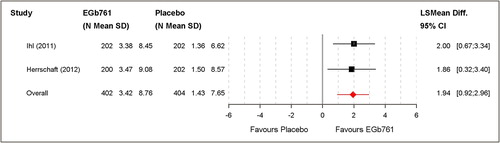
Pooled analysis resulted in an overall mean change of 3.42 ± 8.76 for EGb 761® and 1.43 ± 7.65 for placebo treatment. This corresponds to a standardised mean difference of 0.24 (Cohen's D). The least square mean difference was 1.94 (95% CI [0.92; 2.96]; P < 0.001). A significant difference was confirmed in favour of EGb 761® ().
Subgroup analyses
Subgroup analyses revealed statistically significant superiority of EGb 761® for all outcome measures in all three diagnostic subgroups (probable AD, probable vascular dementia, mixed dementia), except QoL in the very small VaD subgroup ().
Table III. Aggregated outcomes for diagnostic subgroups; mean changes from baseline with standard deviations; LS mean differences (SKT, NPI, DEMQOL-Proxy), standardised LS mean differences (GBS-ADL, ADL-IS) or standardised mean differences (GBS total score, ADCS-CGIC) and 95% confidence intervals.
Responder analysis
Response criteria were chosen on the basis of obvious clinical relevance and published expert consensus. In the cognitive domain, an improvement by 4 points on the ADAS-cog is generally regarded as clinically relevant (CitationRogers et al. 1998). According to regression analyses performed by CitationIhl et al. (1999), a 3-point change in the SKT corresponds to a 3.9-point change in the ADAS-cog and can thus be considered as a clinically relevant change. According to an expert consensus, an improvement by 4 points in NPI total score is considered clinically relevant (CitationMega et al. 1999). Regarding activities of daily living, any improvement that is perceived by a caregiver or other observer after 5–6 months is clinically relevant, at least in patients with a naturally progressive type of dementia, such as AD. Similarly, any improvement after 5–6 months in a patient's condition that is perceived by a clinician not involved in a patient's treatment and not knowing the results of tests and rating scales can be considered as clinically relevant. Regarding the structured global rating scale (GBS total score), there is no generally accepted relevance criterion. Therefore, improvement by approximately 25% (8 points) was chosen as a response criterion as this leap corresponds to a change either from moderate to mild impairment or from mild to no impairment in four of twelve cognitive functions,.
The results of the responder analyses are shown in . Statistically significant superiority of EGb 761® compared to placebo could be demonstrated for all response criteria. The NNTs ranged from 4 to 5.
Table IV. Summary of results of the responder analysis.
Safety and tolerability
Across the four trials, 821 adverse events in 479 patients taking EGb 761® and 998 adverse events in 488 patients receiving placebo were reported. Eighteen serious adverse events were reported by 18 patients treated with EGb 761® and 22 serious adverse events were documented for 20 patients taking placebo (). There was no clustering of any type of event in the EGb 761® treated patients.
Table V. Numbers of serious adverse events under EGb 761® and placebo treatment, respectively.
Discussion
The present pooled analysis provides evidence of the efficacy of Ginkgo biloba extract EGb 761® at a daily dose of 240 mg in the treatment of dementia patients with clinically relevant BPSD. Active drug treatment was statistically and clinically significantly superior to placebo in improving patients’ cognitive performance, BPSD, functional abilities and overall condition. As a consequence, the distress perceived by caregivers due to the patients’ BPSD was alleviated. Rates of adverse events and serious adverse events did not differ between EGb 761® and placebo.
Significant superiority of EGb 761® was seen in three of the four trials and in the pooled analysis, while in one trial (CitationNikolova et al. 2013) drug– placebo differences, although favouring active treatment, were small and not statistically significant. Patients in this trial seemed to have milder overall pathology and ADL impairment, although their cognitive abilities assessed by the SKT and the severity of their BPSD were similar to those of the patients enrolled in the other trials. There were, however, considerable improvements in placebo-treated patients which increased during the study period and, according to the authors, are likely to reflect continued improvements in overall care for dementia patients at the clinical sites while the study was running.
The pooled analyses were performed using standard methods. Since the analysed clinical trials were very similar with respect to study design, patient selection and duration of treatment, an ANCOVA was used to compare the effects of EGb 761® and placebo. Other reviews have been conducted in the evaluation of drugs in neurology and psychiatry, including anti-dementia drugs, using similar methods (CitationStacey et al. 2004; CitationGauthier et al. 2008; CitationBeesdo et al. 2009).
All studies included patients with AD, VaD or dementia with mixed AD/VaD pathology. The clinical researchers give reasons for this, pointing out that EGb 761® interferes with both AD and vascular pathologies (CitationLang et al. 2013) and that, irrespective of the attributed clinical diagnosis, mixed pathology has been found in neuropathology studies to be more prevalent than AD or VaD alone (CitationMatthews et al. 2009; CitationSchneider et al. 2007). Nevertheless, clinical diagnoses in accordance with internationally accepted criteria (NINCDS/ADRDA, NINDS/AIREN) were established prospectively at patient screening in all studies, which allowed meta-analyses for the different diagnostic subgroups. For all three subgroups significant superiority of EGb 761® over placebo was found in all but one outcome measure as there was no significant effect on QoL in the VaD subgroup. It can only be speculated whether this was due to a lack of statistical power in this very small subsample, or perhaps to different determinants of QoL, e.g. stroke-related neurological deficits, in VaD.
Whereas earlier trials (CitationLe Bars et al. 2000, CitationKanowski and Hoerr 2003) suggested slightly stronger treatment effects of EGb 761® in patients with AD, this notion is not supported by the present analyses of trials in patients with clinically significant BPSD. DSM-III-R diagnostic criteria (CitationAPA 1987) for multiple-infarct dementia, that were applied in the earlier trials, and the NINDS-AIREN criteria for probable VaD (CitationRomán et al. 1993) applied in the later trials may have selected somewhat different types of VaD patients.
The effect sizes for cognition, ADL, BPSD (NPI total and caregiver distress scores) and clinical global impression were overall moderate, for QoL a small effect size was observed. CitationWeinmann et al. (2010), who included in their meta-analysis studies in patients without clinically significant BPSD, reported a similar effect size for cognition (Cohen's D of –0.58), but a clearly smaller effect size for ADL (Cohen's D of –0.32). The reason for the difference may be that both cognitive impairment and BPSD presumably contribute to ADL impairment and improvements in both domains may lead to a larger improvement in ADL.
The mean differences between treatment groups in terms of test and rating scale scores are usually smaller than the improvements considered as clinically relevant, unless all patients achieve a clinically meaningful improvement. Response rates and NNTs were therefore calculated to assess the clinical relevance of the statistically significant effects. NNTs below 10 may indicate a meaningful difference between treatments (CitationCitrome 2014). However, the severity of a disease as well as the safety and costs of a treatment should be taken into consideration. NNTs between 6 and 9 have commonly been reported for placebo-controlled trials of psychotropic drugs (CitationCitrome 2014). For acetylcholinesterase inhibitors at recommended doses in mild to moderate dementia NNTs between 4 and 13 for cognitive response and between 5 and 12 for global improvement have been reported based on similar response criteria (CitationLivingston and Katona 2000; CitationLanctôt et al. 2003). NNTs of 4 and 5 as found for EGb 761® may therefore be regarded as clinically meaningful. Caution is advisable, however, when comparing NNTs for different drugs if data are not derived from head-to-head trials.
Of note, only patients with BPSD scoring 6 or higher on the 12-item NPI were enrolled in these trials. A total score of 6 or higher has been interpreted as indicating “moderate to severe disturbance” (CitationLyketsos et al. 2000), which may well be considered as clinically relevant. With BPSD prevalence rates of up to 87% in dementia patients in a community-based study (CitationSteinberg et al. 2008) and 97% in memory clinic dementia patients (CitationVan der Mussele et al. 2013), the study results seem to apply to a considerable part of patients with dementia.
The positive effects of EGb 761® on BPSD and BPSD-related caregiver distress, in particular, deserve consideration. BPSD often render caring for a patient with dementia more difficult and distressing than the cognitive impairment. Although EGb 761® significantly decreased BPSD-related caregiver distress, the studies considered in this pooled analysis did not assess possible postponement of nursing home placement (CitationGilley et al. 2004; Citationde Vugt et al. 2005).
The average NPI scores of the patients in the included trials were within the range typically reported from outpatient trials, i.e. between 10 and 24 (CitationRodda et al. 2009; CitationLockhart et al. 2011), and higher than in community-based cohorts (CitationLyketsos et al. 2000; CitationTatsch et al. 2006). Whether our findings can be extrapolated to patients with very severe BPSD remains uncertain. Analyses reported by CitationIhl et al. (2010) suggest that treatment benefits increase with increasing severity of BPSD, at least up to NPI total scores around 35. There are, however, no trials of EGb 761® in in-patients with severe BPSD.
Overall, the present pooled analysis demonstrates that Ginkgo biloba extract EGb 761® is both safe and moderately effective in the treatment of out-patients suffering from dementia with mild to moderate behavioural and psychological symptoms.
Acknowledgements
None
Statement of Interest
Armin von Gunten participated at a roundtable and symposium, and received an honorarium, organised by Dr. Willmar Schwabe GmbH & Co. KG. Karl Überla provided methodological advice and received an honorarium from Dr. Willmar Schwabe GmbH & Co. KG; Sandra Schlaefke is an employee of Dr. Willmar Schwabe GmbH & Co. KG receiving a fixed salary.
References
- Alzheimer's Disease International. 2010. World Alzheimer Report 2010. The Global Economic Impact of Dementia. London: Alzheimer's Disease International.
- APA American Psychiatric Association. 1987. Diagnostic and statistical manual of mental disorders. 3rd ed. Revised. Washington, DC: American Psychiatric Association.
- APA American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association.
- Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, del Valle M. 2009. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry 24:15–24.
- Beesdo K, Hartford J, Russell J, Spann M, Ball S, Wittchen HU. 2009. The short- and long-term effect of duloxetine on painful physical symptoms in patients with generalized anxiety disorder: results from three clinical trials. J Anxiety Disord 23:1064–1071.
- Birks J, Grimley Evans J. 2009. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009:1. Art. No.: CD003120. DOI: 10.1002/14651858.CD003120.pub3.
- Bråne G, Gottfries CG, Winblad B. 2001. The Gottfries- Bråne-Steen Scale: Validity, reliability and application in anti-dementia drug trials. Dement Geriatr Cogn Disord 12:1–14.
- Citrome L. 2014. Quantifying clinical relevance. Innov Clin Neurosci 11:26–30.
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. 567 p.
- Cummings JL. 1997. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48(Suppl 6): S10–16.
- de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Verhey FRJ. 2005. A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. Int Psychogeriatr 17:1–13.
- Di Iulio F, Palmer K, Blundo C, Casini AR, Gianni W, Caltagirone C, et al. 2010. Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer's disease and mild cognitive impairment subtypes. Int Psychogeriatr 22:629–640.
- Erzigkeit H. 1992. SKT Manual. A short cognitive performance test for assessing memory and attention. Weinheim: Beltz Test.
- Gauthier S, Loft H, Cummings JL. 2008. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer's disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry 23:537–545.
- Gilley DW, Bienias JL, Wilson RS, Bennett DA, Beck TL, Evans DA. 2004. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer's disease. Psychol Med 34:1129–1135.
- Hamilton M. 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62.
- Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. 2012. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 46:716–723.
- Higgins JPT, Whitehead A, Turner RM, Omar RZ, Thompson SG. 2001. Meta-analysis of continuous outcome data from individual patients. Stat Med 20:2219–2241.
- Ihl R, Grass-Kapanke B, Jänner M, Weyer G. 1999. Neuropsychometric tests in cross sectional and longitudinal studies – a regression analysis of ADAS-Cog, SKT and MMSE. Pharmacopsychiatry 32:248–254.
- Ihl R, Tribanek M, Bachinskaya N. 2010. Baseline neuropsychiatric symptoms are effect modifiers in Ginkgo biloba extract (EGb 761®) treatment of dementia with neuropsychiatric features. Retrospective data analyses of a randomized controlled trial. J Neurol Sci 299:184–187.
- Ihl R, Bachinskaya N, Korczyn A, Vakhapova V, Tribanek M, Hoerr R, et al. 2011a. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features. A randomized controlled trial. Int J Geriatr Psychiatry 26:1186–1194.
- Ihl R, Frölich L, Winblad B, Schneider L, Burns A, Möller HJ, et al. 2011b. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer's disease and other dementias. World J Biol Psychiatry 12:2–32.
- Janssen IM, Sturtz S, Skipka G, Zentner A, Garrido MV, Busse R. 2010. Ginkgo biloba in Alzheimer's disease: a systematic review. Wien Med Wochenschr 160:539–546.
- Kanowski S, Hoerr R. 2003. Ginkgo biloba extract EGb 761® in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry 36:297–303.
- Karttunen K, Karppi P, Hiltunen A, Vanhanen M, Välimäki T, Martikainen J, et al. 2011. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer's disease. Int J Geriatr Psychiatry 26:473–482.
- Kim YS, Nibbelink DW, Overall JE. 1993. Factor structure and scoring of the SKT test battery. J Clin Psychol 49:61–71.
- Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR. 2003. Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: a meta-analysis. Can Med Assoc J 169:557–564.
- Lang F, Hoerr R, Noeldner M, Koch E. 2013. Ginkgo biloba extract EGb 761®: From an ancient Asian plant to a modern European herbal medicinal product. In: Wagner H, Ulrich-Merzenich G, editors. Evidence and rational based research on Chinese drugs. Wien: Springer. p 431–470.
- Le Bars PL, Kieser M, Itil KZ. 2000. A 26-week analysis of a double-blind, placebo-controlled trial of the Ginkgo biloba extract EGb 761® in dementia. Dement Geriatr Cogn Disord 11:230–237.
- Leber P. 1990 (US Food and Drug Administration). Guidelines for the clinical evaluation of antidementia drugs. First Draft 8 November 1990. Rockville (MD): Food and Drug Administration.
- Livingston G, Katona C. 2000. How useful are cholinesterase inhibitors in the treatment of Alzheimer's disease? A number needed to treat analysis. Int J Geriatr Psychiatry 15: 203–207.
- Lockhart IA, Orme ME, Mitchell SA. 2011. The efficacy of licensed-indication use of donepezil and memantine monotherapies for treating behavioural and psychological symptoms of dementia in patients with Alzheimer's disease: Systematic review and meta-analysis. Dement Geriatr Cogn Disord Extra 1:212–227.
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS. 2000. Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatry 157:708–714.
- Mahoney R, Johnston K, Katona C, Maxmin K, Livingston G. 2005. The TE4D-Cog: a new test for detecting early dementia in English-speaking populations. Int J Geriatr Psychiatry 20:1172–1179.
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. 2009. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med 6:e1000180.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944.
- Mega MS, Masterman DM, O'Connor SM, Barclay TR, Cummings JL. 1999. The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol 56:1388–1393.
- Müller WE, Heiser J, Leuner K. 2012. Effects of the standardized Ginkgo biloba extract EGb 761® on neuroplasticity. Int Psychogeratr 24(Suppl 1):S21–24.
- Napryeyenko O, Borzenko I, GINDEM-NP Study Group. 2007. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung 57:4–11.
- Nikolova G, Yancheva S, Raychev I, Hoerr R, for the PLAGIN Study Group. 2013. Ginkgo biloba extract in dementia: A 22-week randomised, placebo-controlled, double-blind trial. Bulgarian Neurology 14:139–143.
- Petrovic M, Hurt C, Collins D, Burns A, Camus V, Liperoti R, et al. 2007. Clustering of behavioural and psychological symptoms in dementia (BPSD): A European Alzheimer's Disease Consortium (EADC) study. Acta Clin Belg 62:426–432.
- Powlishta KK, Storandt M, Mandernach TA, Hogan E, Grant EA, Morris JC. 2004. Absence of effect of depression on cognitive performance in early-stage Alzheimer Disease. Arch Neurol 61:1265–1268.
- Rabins PV, Schwartz S, Black BS, Corcoran C, Fauth E, Mielke M, et al. 2013. Predictors of progression to severe Alzheimer's disease in an incidence sample. Alzheimers Dement 9: 204–207.
- Reisberg B, Finkel S, Overall J, Schmidt-Gollas N, Kanowski S, Lehfeld H, et al. 2001. The Alzheimer's Disease Activities of Daily Living International Scale (ADL-IS). Int Psychogeriatr 13:163–181.
- Rodda J, Morgan S, Walker Z. 2009. Are cholinesterase inhibitors effective in the management of the behavioural and psychological symptoms of dementia in Alzheimer's disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int Psychogeriatr 21:813–824.
- Rogers Sl, Farlow MR, Doody RS, Mohs R, Friedhoff LT and the Donepezil Study Group. 1998. A 24 week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology 50:136–145.
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. 1993. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43:250–260.
- Schneider LS, Olin JT, Lyness SA, Chui HC. 1997a. Eligibility of Alzheimer's disease clinic patients for clinical trials. J Am Geriatr Soc 45:923–928.
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. 1997b. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. Alzheimer Dis Assoc Disord 11(Suppl 2): S22–32.
- Schneider LS, DeKosky ST, Farlow MR, Tariot PN, Hoerr R, Kieser M. 2005. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer's type. Curr Alzheimer Res 2:541–551.
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. 2007. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69:2197–2204.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. 2005. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 9(10).
- Stacey B, Parsons B, Huang S, Peyser S, Dukes E. 2004. Gabapentin and improved health status in elderly patients with postherpetic neuralgia: A pooled analysis of three clinical studies. Pharmacy and Therapeutics 29:646–651.
- Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al. 2008. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 23:170–177.
- Tatsch MF, Bottino CM, Azevedo D, Hototian SR, Moscoso MA, Folquitto JC, et al. 2006. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: Prevalence and relationship with dementia severity. Am J Geriatr Psychiatry 14:438–445.
- Treiber KA, Lyketsos CG, Corcoran C, Steinberg M, Norton M, Green RC, et al. 2008. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer's disease: the Cache County Study. Int Psychogeriatr 20:538–553.
- Van der Mussele S, Le Bastard N, Vermeiren Y, Saerens J, Somers N, Mariën P, et al. 2013. Behavioral symptoms in mild cognitive impairment as compared with Alzheimer's disease and healthy older adults. Int J Geriatr Psychiatry 28:265–275.
- Weinmann S, Roll S, Schwarzbach C, Vauth C, Willich SN. 2010. Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr 10:14–26.
- WHO World Health Organization. 1992. International Statistical Classification of Diseases and Related Health Problems. 10th revision. Geneva: World Health Organization.