Abstract
Objectives: Schizotypy relates to rejection sensitivity (anxiety reflecting an expectancy of social exclusion) and neuroticism (excessive evaluation of negative emotions). Positive schizotypy (e.g., perceptual aberrations and odd beliefs) and negative schizotypy (e.g., social and physical anhedonia) could relate to altered attention to rejection because of neuroticism. Methods: Forty-one healthy individuals were assessed on positive and negative schizotypy and neuroticism, and event-related potentials during rejecting, accepting and neutral scenes. Participants were categorised into high, moderate and low neuroticism groups. Using temporo-spatial principal components analyses, P200 (peak latency =290 ms) and P300 amplitudes (peak latency = 390 ms) were measured, reflecting mobilisation of attention and early attention, respectively. Results: Scalp-level and cortical source analysis revealed elevated fronto-parietal N300/P300 amplitude and P200-related dorsal anterior cingulate current density during rejection than acceptance/neutral scenes. Positive schizotypy related inversely to parietal P200 amplitude during rejection. Negative schizotypy related positively to P200 middle occipital current density. Negative schizotypy related positively to parietal P300, where the association was stronger in high and moderate, than low, neuroticism groups. Conclusions: Positive and negative schizotypy relate divergently to attention to rejection. Positive schizotypy attenuates, but negative schizotypy increases rejection-related mobilisation of attention. Negative schizotypy increases early attention to rejection partly due to elevated neuroticism.
Introduction
Social anxiety is the tendency to experience negative affect due to a fear of being embarrassed or humiliated in social situations (Kashdan, Citation2004; Lysaker et al. Citation2010; Kwapil et al. Citation2012). Rejection sensitivity (RS) is a type of social anxiety, where people expect to be excluded from interpersonal relationships, such as a close family member, friends and peers; in turn, RS increases expressions of vulnerability and aggression in these relationships (Langens and Schuler Citation2005; Lemay and Clark Citation2008; Blackhart et al. Citation2009; Sinclair et al. Citation2011).
RS exists among those at risk for psychosis, because of anxiety and avoidance of close relationships (Torgersen et al. Citation2002; Morrison et al. Citation2006; Kwapil et al. Citation2012; Salokangas et al. Citation2012). RS also relates to schizotypy at the normal end of the schizophrenia spectrum (Meehl Citation1962; Lenzenweger Citation1993; Premkumar et al. Citation2014). Studying this association within the normal population has predictive value, because (a) individuals scoring highly on schizotypy resemble schizophrenia patients on social isolation and non-responsiveness to others’ moods (Kwapil et al. Citation2008; Cohen et al. Citation2012; Llerena et al. Citation2012), and (b) further along the psychosis continuum, high positive schizotypy (a propensity for perceptual aberrations, magical ideation and referential thinking, e.g., belief in telepathy) and negative schizotypy (presence of social and physical anhedonia and constricted affect, e.g., having no close friends) predict schizophrenia spectrum disorder 10 years later (Kwapil et al. Citation2013). Thus, the RS-schizotypy association in the normal population may aid understanding of the stress diathesis model of psychosis, so that even mild variations in schizotypy and RS in the normal population could be studied as precursors of more severe at-risk mental states (Nuechterlein and Dawson Citation1984).
RS may relate more strongly to the social anxiety facet than other facets of schizotypy (Premkumar et al. Citation2014), because of excessive negative affect, e.g., fear of one’s own appearance, excessive emotional attention and reactivity to social threat (Moscovitch et al. Citation2010; Horton et al. Citation2014), and neuroticism. Neuroticism, being an excessive evaluation of negative emotions, relates strongly to both anxiety and schizotypy (Roelofs et al. Citation2008; Kotov et al. Citation2010; Macare et al. Citation2012). In turn, negative affect can have knock-on, but also independent, effects on positive and negative schizotypy (Vollema and van den Bosch, Citation1995; Brown et al. Citation2008; Horton et al. Citation2014), such that social anxiety relates more strongly to positive than negative schizotypy, while social avoidance relates more strongly to negative than positive schizotypy (Berry et al. Citation2007; Blanchard et al. Citation2011; Haralanova et al. Citation2012; Kwapil et al. Citation2013). Furthermore, reduced negative emotional attention relates to positive, but not negative schizotypy (Mohanty et al. Citation2008). In the context of RS, it is not clear whether neuroticism relates differentially to positive and negative schizotypy (Barrantes-Vidal et al. Citation2009).
Neural processing of social rejection in relation to schizotypy
The neural processing of social rejection may provide additional insight into the RS-schizotypy association. The dorsal anterior cingulate cortex (dACC) is involved in experiencing rejection as distressing (Rotge et al. Citation2015). Individuals with schizotypal traits have decreased dACC activity during rejecting scenes than individuals with low schizotypy, implying poorer emotional regulation in terms of distancing oneself in order to minimise distress (Premkumar et al. Citation2012). However, schizophrenia patients have increased dACC activity when experiencing rejection compared to healthy individuals, suggesting more salience towards rejection (Lee et al. Citation2014). Event-related potentials (ERPs), such as N100, P200 and P300, can inform whether rejection scenes are encoded efficiently as the level of schizotypy increases. When healthy individuals viewed scenes depicting rejecting interactions, positive schizotypy inversely related to dACC N100 current density, and to parietal P300 amplitude (at a trend level of significance) (Premkumar et al. Citation2014). These findings indicated reduced efficiency for feature detection and early attention during rejection scenes.
Reduced P300 is an endophenotype of the schizophrenia spectrum, including schizotypal personality disorder, where it denotes diminished attention to stimuli due to structural and functional abnormality in the P300 generators (Cermolacce et al. Citation2011; Guo et al. Citation2014). Schizophrenia patients have reduced P300 during unpleasant, relative to pleasant, pictures (Champagne et al. Citation2014). In healthy individuals and cannabis users, higher negative schizotypy scores relate to reduced P300 during emotional words (Skosnik et al. Citation2008), suggesting that the P300 deficit to emotional cues exists in the normal range of the psychosis continuum. Neuroticism may partly account for the schizotypy-P300 association during rejection scenes, because neuroticism increases anticipation of threat. Increased P300 during threatening facial expressions reflects hypervigilance for threat in socially anxious individuals (Moser et al. Citation2008). Higher scores on the anxiety domain within neuroticism relates to increased P300 amplitude during the auditory oddball task, reflecting distractibility towards infrequent stimuli (Fjell et al. Citation2005).
Generally, the P300 denotes early attention to infrequent stimuli in the auditory oddball task, but it also reflects motivation towards visual stimuli that are emotionally and personally salient (Carretié et al. Citation2013). Although the P300 response during social cues does not suggest domain specificity, its elevation during certain social interactions relative to others does suggest greater emotional motivation to these interactions. In an interpersonal context, the P300 denotes emotional motivation because P300 amplitude increases during personally salient cues, such as a close relative’s face, maternal love and self-relevant unpleasant words (Herbert et al. Citation2011; Lu et al. Citation2012; Dai et al. Citation2013). Precisely in the context of rejection, increased parietal P300 during rejection as ostracism from a game (Crowley et al. Citation2010) and scenes depicting rejecting interactions (Premkumar et al. Citation2014) indicates that rejection is better attended to than benign interactions. However, P300 was not elevated during rejection as negative peer feedback (Leitner et al. Citation2014; Van der Molen et al. Citation2014). This suggests that the P300 is specific to certain rejection scenarios.
An earlier component in the ERP timeline that can inform a preference for certain emotional cues is the P200/N200 complex. The P200/N200 complex robustly measures feature integration of visual stimuli and early mobilisation of attentional resources to emotional stimuli that have intrinsic value (Carretié et al. Citation2001). The P200/N200 complex is higher during negative than positive emotional stimuli (Rossignol et al. Citation2007; Feng et al. Citation2014). Schizophrenia patients have reduced P200 amplitude during angry faces, but increased P200 amplitude during angry vocal expressions (Horley et al. Citation2001; Pinheiro et al. Citation2013). In socially anxious individuals, elevated P200 to threatening faces reflected increased early attention and feature integration (Moser et al. Citation2008; Yuan et al. Citation2014), whereas reduced P200 to emotional body gestures reflected lesser attention allocation (Rossignol et al. Citation2013). In neglectful mothers, elevated P200 in response to faces of infants crying than laughing suggested selective attention to distress signals (Rodrigo et al. Citation2011).
Aims and objectives
The aims of the current study were to test whether (1) P200 and P300 activity during rejection scenes are higher than other social interactions, and (2) reduced P200/P300 activity relates to higher level of schizotypy, because the literature reviewed above shows that reduced P300 is an endophenotype of schizophrenia spectrum, and P200 amplitude during angry faces is reduced in schizophrenia patients. To follow-up on these aims, a further research question was whether neuroticism alters the association between positive/negative schizotypy and electrophysiological responses to rejection, because neuroticism relates to schizotypy, and social anxiety and neuroticism increase P200/P300 amplitude.
In our previous study of the relation between ERP amplitudes during rejection scenes and schizotypy in healthy individuals (Premkumar et al. Citation2014), three ERP components were determined manually by visually inspecting the grand average waveforms as is usual (e.g., Holmes et al. Citation2009), namely frontal N100/parieto-occipital P100, a frontal N300/parieto-occipital P300 and a frontal/parieto-occipital late slow wave (LSW). In the present study, we used a data-driven method, namely principal component analysis (PCA), to re-extract the P300 component free from the confounding influence of adjacent or latent components, and to freshly identify the P200 component from these previous data (Premkumar et al. Citation2014). PCA has been shown to be a powerful approach to separate temporally (temporal PCA) and spatially (spatial PCA) overlapping components (Chapman and McCrary, Citation1995; Carretié et al. Citation2013). For example, a combination of temporal and spatial PCA has been previously used to effectively isolate the neural responses to emotional pictures and affective words (Carretié et al. Citation2006; Foti et al. Citation2009; Hinojosa et al. Citation2014).
Materials and methods
Participants
The study was ethically approved by Nottingham Trent University’s School of Social Sciences Research Ethics Committee (No. 2012/55). Forty-one participants were recruited from a student population through the Psychology research credit scheme. They were right-handed and did not have a history of mental disorder, brain injury, neurological disorder, learning disabilities, loss of consciousness for more than five minutes, and/or a history of alcohol or drug abuse within the last 12 months, or taking any kind of mood-altering prescribed medication.
Psychometric assessments
Oxford and Liverpool inventory of feelings and experiences (O-LIFE)
The O-LIFE (Mason et al. Citation1995) is a 104-item schizotypy scale comprising four sub-scales, namely unusual experiences, cognitive disorganisation, introvertive anhedonia and impulsive nonconformity. Positive schizotypy is measured by the unusual experiences sub-scale (maximum score =30), where high scores indicate perceptual aberrations, hallucinatory experiences and magical thinking. Negative schizotypy is measured by the introvertive anhedonia sub-scale (maximum score =27), where high scores denote independence, solitude and lack of enjoyment from physical and social sources. The internal reliabilities (Cronbach’s alpha) were good for positive schizotypy (α =0.89 in Mason et al. Citation1995, and α =0.75 in the present sample) and negative schizotypy (α =0.82 in both Mason et al. Citation1995, and the present sample).
Big five inventory
Neuroticism was measured using the eight-item subscale of the Big Five Inventory (John et al. Citation1991), where neuroticism has strong construct validity with anxiety in healthy individuals and individuals with Axis I disorders (Kotov et al. Citation2010; Booth et al. Citation2013). Neuroticism measures anxiety and other subclinical symptoms of extensive worrying, such as psychosomatic complaints, unstable mood and sadness (Ettinger et al. Citation2005; Hong Citation2010). Participants were asked to read the opening phrase “I see myself as someone who …”, and then rate each item on a 5-point Likert scale ranging from “disagree strongly” to “agree strongly”. The neuroticism subscale (maximum score =40) has eight items, e.g., “worries a lot” and “gets nervous easily”. The reliability of the neuroticism subscale was good (α =0.85 in John and Srivastava Citation1999, and α =0.87 in the present sample).
EEG recording and experimental paradigm
EEG data were sampled at the rate of 2048 Hz and digitised in 24 bit using a BioSemi Active-II system that uses an internal loop as the reference (http://www.biosemi.com/faq/cms&drl.htm). A standard set of 64 Ag/AgCl electrodes was fitted using an electrode cap. Four additional electrodes were placed frontally (F9, F10, F11, F12) based on the 10-10 International system. A low-pass filter was applied in analogue-to-digital decimation filter, which has a 5th order sync response with a –3 dB point at 1/5th of the sampling rate. The high pass filter was applied in Brain Electrical Source Analysis (BESA, see ERP analysis). Electrooculographic (EOG) data were recorded supra- and infraorbitally (vertical EOG) as well as from the left vs. right orbital rim (horizontal EOG) during a computerised eye-movements task comprising a vertically and horizontally-moving central fixation.
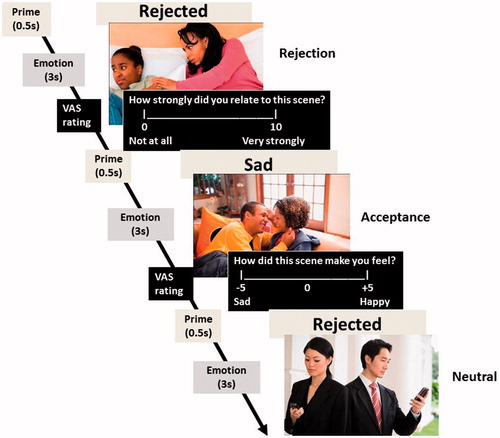
Participants performed a passive viewing affect processing task comprising scenes (presented for 3 s each) depicting rejecting, accepting and neutral social interactions (30 scenes per condition). The scenes were sourced either from the International Affective Pictures System (Lang, Bradley, & Cuthbert 1999) or purchased from a web-based company supplying stock photographic images for professional use (www.jupiterimages.co.uk). The task employed affective priming, because affective primes lead to “activation spreading” of a semantic context to a target stimulus and anticipation of the prime (Bartholow Citation2010; Lu et al. Citation2011). Rejection is a complex emotion (Power Citation2005; Çelik et al. Citation2013) that requires awareness of the circumstances that caused the emotion and therefore higher-order cognitive evaluation (Johnson-Laird and Oatley Citation1989). Therefore, at the centre of the screen a verbal prime (either the word “rejected” or “sad”) appeared for 500 ms before each scene so as to provide two emotional contexts in which to process the scenes. Thus, the task consisted of 180 trials based on 30 scenes per condition (rejection, acceptance and neutral); each scene was presented twice, once preceded by a “rejected” prime, and once by a “sad” prime. The task was divided into two blocks with all 90 scenes appearing once in each block, and the “rejected” and “sad” primes being split evenly between the two blocks. The scenes were randomly ordered in each block. After each scene, participants rated them for arousal and relevance (). Participants were asked to view the scenes, to visualise them in the context of the primes, and rate them for arousal and relevance. Participants were positioned 50 cm away from the computer screen while performing the task. The size of the pictures was 540 × 361 mm.
ERP analysis
Channels were re-referenced to average in BESA (version 5.37). Off-line high-pass (frequency =0.53 Hz, low cut-off slope =6db/oc) and low-pass filters (frequency =35 Hz, high cut-off slope =24bd/oc, zero phase) were applied to the data prior to averaging scalp-level waveforms within conditions. Eye-blink and horizontal eye-movement artefact corrections were performed using established methods (Picton et al. Citation2000; Scherg et al. Citation2002). EEG trials were epoched from –200 to 1999 ms, such that epochs were baseline-corrected to the first 200 ms. Trials that had artefacts exceeding 120 μV were removed automatically. The mean (SD) % of trials accepted for each condition were: rejection =96.42% (6.13), acceptance =95.47% (6.87) and neutral =96.50% (5.49).
Participant-level individual ERPs were resampled to 256 Hz and prepared for PCA using standard software (http://www.uam.es/carretie/soft/index.htm). Grand averages were obtained across the whole scalp after subtracting the baseline activity from each ERP (–200–0 ms). In , these grand averages correspond to midline frontal, midline parietal and right temporo-parietal electrode sites, where the experimental effects as described later were most prominent at the parietal P200 and fronto-parieto-occipital N300/P300. Temporal and spatial PCAs were performed on individual participants’ ERPs using the procedure adopted by Carretié et al. (Citation2013) and Dien (Citation2010, Citation2012). Individual ERPs were submitted to PCA through SPSS (Version 19). The scree plot was used to discern a nine-factor solution that was submitted to promax rotation (). The factor peak latencies of the nine principal components revealed two temporal factors (TFs) corresponding to the P200 (peak latency =290 ms; duration =240–390 ms) and N300/P300 (peak latency =390 ms; duration =190–570 ms). To further decompose the spatial distribution of the two TFs, spatial PCAs (sPCA) were performed on the temporal factor scores that are linearly related to amplitudes. The spatial PCA indicated two regions as explaining most of the variance of the P200, namely right parietal and midline occipital, and three regions as explaining most of the variance of the N300/P300, namely midline frontal, midline parietal and right temporo-parietal ().
Figure 2. Plot of the grand average waveform at midline frontal, midline parietal and right temporo-parietal sites from 200 ms pre-stimulus to 1-s post-stimulus during the affect processing task. The blue solid line depicts the waveform for rejection, red dotted line depicts the waveform for acceptance, and the green broken line depicts the waveform for neutral scenes.
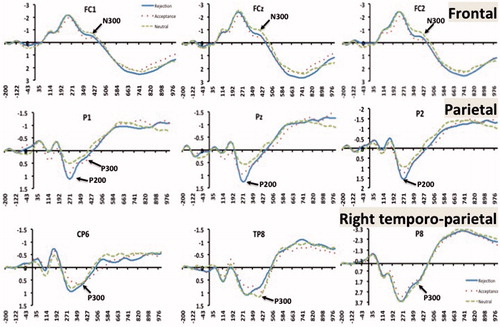
Figure 3. (a) Plot of the rescaled configuration matrix loadings of the nine temporal PCA factors from 200 ms pre-stimulus baseline to 1-s post-stimulus onset during the affect processing task, and (b) the scalp-level maps of the spatial scores of temporal factor 9 (P200) and 2 (N300/P300). Only positive values (depicted in red in Figure 3b) denote the intensity of spatial mapping, since the scalp maps are based on matrix loadings.
Cortical source analysis was performed on the P200 and N300/P300 temporal factor scores in Standardised/Exact Low-Resolution Brain Electromagnetic Tomography (sLORETA/eLORETA; http://www.uzh.ch/keyinst/loreta.htm, version 2008-11-04). LORETA solutions for the localisation of neural generators of ERP components show good overlap with the location of haemodynamic response provided by functional MRI (Whittingstall et al. Citation2010). Moreover, the use of temporal factor scores instead of raw voltages allowed us to obtain more accurate source-localisation solutions (Dien Citation2010; Carretié et al. Citation2004).
Statistical analysis
Difference between social interaction types in P200 and N300/P300 activity
Factor scores (linearly related to amplitudes, as indicated) were used to approximate P200 and N200/P300 amplitude. At the scalp-level, ANOVAs were performed on scores for each temporo-spatial factor with social interaction type (rejection, acceptance and neutral) as the within-subjects factor, followed by post hoc Bonferroni-corrected pairwise comparisons. At the cortical source level, voxel-wise whole cortical one-tailed non-parametric pairwise comparisons were performed between social interaction types using the non-parametric mapping (SnPM) tool, as implemented in the sLORETA/eLORETA software package.
Relation between schizotypy and P200 and N300/P300 activation during rejection scenes
Two stepwise linear regression analyses were performed, with the spatial factors of the P200 and N300/P300 components during rejection scenes as predictor variables and either positive or negative schizotypy as the criterion variable. Although acknowledging the overlap between schizotypy categories (Bentall et al. Citation1989; Vollema and van den Bosch Citation1995), we treated positive and negative schizotypy distinctly, because only one participant was high on both schizotypy subscales of those who scored above the 75th percentile on either the positive subscale (n=7) or negative subscale (n=8). Likewise, only four participants were low on both subscales of those who scored within the 25th percentile on either the positive subscale (n=9) or negative subscale (n=9).
At the cortical source level, the two schizotypy sub-scales were individually regressed onto P200 and N300/P300 current density during rejection interactions across the whole cortex. To test whether the relation between P200 or N300/P300 activation and schizotypy was partly explained by neuroticism, hierarchical regression analyses were performed with positive or negative schizotypy as the first-step predictor and neuroticism as the second-step predictor and the significant ERP correlate of schizotypy in the previous regression analysis as the criterion variable. To further tease apart the effect of neuroticism on the schizotypy-ERP activation association, the sample was divided into high (n=12), moderate (n=22) and low neuroticism groups (n=9), using the 70th and 30th percentiles as cut-off points for the high and low groups respectively, and between 30th and 70th percentiles as cut-off points for the moderate group. The correlation coefficients were then compared between neuroticism groups using Fisher’s r-to-z transformation.
Results
Sample characteristics
Participants were on average 21.07 ± 1.82 years old, and 26 (63%) of them were female. The missing schizotypy scores of three participants were replaced by the sample median. Participants scored on average 8.12 ± 4.5 out of 30 on positive schizotypy, 6.61 ± 4.76 out of 27 on negative schizotypy, and 23.95 ± 6.52 out of 40 on neuroticism. Men and women did not differ on positive schizotypy (t=0.707, P=0.484), negative schizotypy (t=0.284, P=0.778), or neuroticism (t=0.015, P=0.988). As reported earlier (Premkumar et al. Citation2014), scores on both schizotypy subscales were normal relative to the means of an adult community-based sample (Mason et al. Citation1995). Based on a one-sample t-test (t=1.654, P=0.11), level of neuroticism was also normal relative to an adult student sample (Peterson et al. Citation2006). Neuroticism correlated with positive and negative schizotypy (r=0.503 and r=0.579, respectively, P≤0.001).
Task effects in P200 and N300/P300 activation
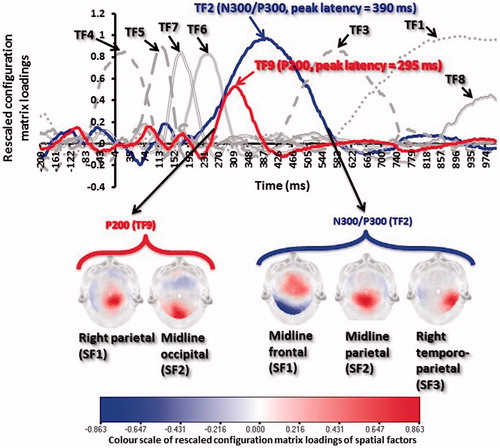
P200 amplitude (scalp level) did not differ between social interaction types [right parietal region: F(2, 80) = 1.840, P=0.166, partial η2 (eta-square) = 0.044, and midline occipital region: F(2, 80) = 1.422, P=0.243, partial η2=0.035]. P200 dACC cortical source current density was greater during rejecting than neutral interactions, t=1.919, P=0.04 ().
Figure 4. Cortical source current density difference map depicting greater dorsal anterior cingulate P200 activation during rejection than neutral scenes.
Midline frontal N300 amplitude (scalp level) differed between social interaction types [F(2, 80) = 6.643, P=0.004, partial η2 =0.142; see ], where pairwise comparisons revealed N300rejection> N300neutral, mean difference =0.23, t=3.346, P=0.005; and N300acceptance> N300neutral, mean difference =0.3, t=2.955, P=0.02. Midline parietal P300 amplitude (scalp level) also differed between social interaction types [F(2, 80) = 3.42, P=0.048, partial η2=0.079], where pairwise comparisons revealed P300rejection> P300neutral, mean difference =0.22, t=2.771, P=0.025. Right temporo-parietal P300 amplitude also differed between social interaction types [F(2, 80) = 10.364, P<0.001, partial η2=0.206], where pairwise comparisons revealed P300rejection> P300acceptance, mean difference =0.28, t=3.211, P=0.008; and P300neutral> P300acceptance, mean difference =0.43, t=3.941, P=0.001. P300 current source density (cortical level) did not differ between social interaction types (P>0.5).
Relation between P200/P300 activation during rejecting interactions and schizotypy
Decreased right parietal P200 amplitude (scalp level) predicted higher positive schizotypy, with right parietal P200 amplitude explaining 14% of the variance in positive schizotypy (). Greater midline parietal P300 amplitude predicted higher negative schizotypy, with midline parietal P300 amplitude explaining 11% of the variance in negative schizotypy. Within the source localisation model, higher negative schizotypy correlated with greater right middle occipital gyrus P200 current source density (r=0.581, P<0.001, 34% of the variance explained) ().
Figure 5. Correlation between negative schizotypy and right middle occipital gyrus P200 current density (MNI co-ordinates 30, –80, 15) during rejection scenes.
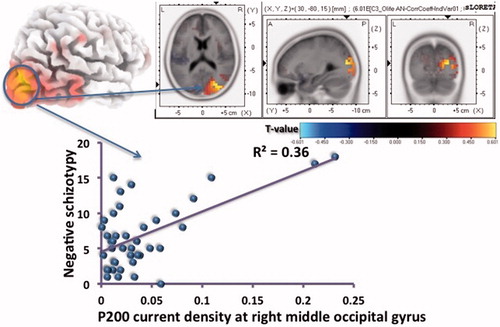
Table I. Regression of P200/P300 amplitude during rejection scenes on schizotypy.
Effect of neuroticism on the P200/P300 activation-schizotypy association
A hierarchical regression was performed, with positive schizotypy and neuroticism entered as separate predictors at step 1 and step 2 respectively, and right parietal P200 amplitude as the criterion variable (). The first model was significant (P=0.014, variance explained =14.5%). The second model was also significant (P=0.044). However, the non-significant change in variance explained (0.7%) indicated that neuroticism added little to the variance explained.
Table II. Hierarchical regression of schizotypy and neuroticism on P200/P300 activation during rejection scenes.
A hierarchical regression was performed with negative schizotypy and neuroticism as predictors and middle occipital gyrus P200 current density as the criterion variable (). The first model was significant (P<0.001, variance explained =33.8%). The second model was also significant (P<0.001). Although schizotypy no longer significantly predicted P200 current density, th non-significant change in variance explained (0.3%) indicated that neuroticism added little to explaining the variance in middle occipital gyrus P200 current density.
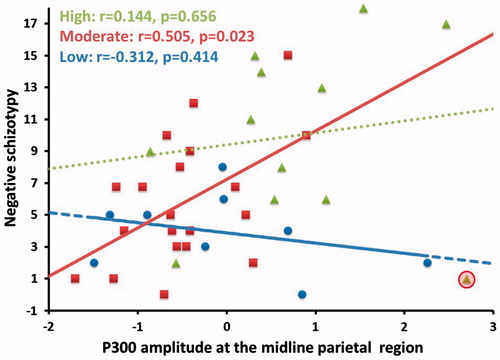
A hierarchical regression was performed with negative schizotypy and neuroticism as predictors and midline parietal P300 amplitude as the criterion variable (). The first model was significant, (P=0.03, variance explained =11.6%). The second model was also significant (P=0.023), where the change in variance explained approached statistical significance (P=0.09). With neuroticism in the model, schizotypy was no longer a significant predictor (P=0.381), indicating that neuroticism tended to share the variance explained (6.4%). Comparison of negative schizotypy-midline parietal P300 correlations between neuroticism groups revealed that the correlation was stronger in the moderate than low group (z=1.851, P=0.032), but there was no difference between the high and moderate groups (z=–1.0, P=0.159), the high and low groups (z=0.89, P=0.187) (). However, close inspection of the scatterplot revealed an outlier in the high neuroticism group. After excluding the outlier, the correlation was marginally stronger in the high (r=0.598, P=0.052) than low neuroticism group (z=1.875, P=0.061).
Figure 6. Scatterplot of midline parietal P300 amplitude during rejection scenes and negative schizotypy scores according to high, moderate and low neuroticism groups. The green dotted line represents the high neuroticism group, the red solid line represents the moderate neuroticism group, and the blue broken line represents the low neuroticism group. The circled data point indicates an outlier in the high neuroticism group.
Discussion
The current study aimed to examine neural activity during rejecting interactions relative to other social interactions in terms of P200/P300 activity. P200 current density was greater during rejecting than neutral interactions in the dACC. P300 amplitude was also greater during rejecting than accepting/neutral interactions. Positive schizotypy related to reduced right parietal P200 amplitude in this non-clinical, adult student sample, although neuroticism did not explain this association. Negative schizotypy related to greater midline parietal P300 amplitude, which was marginally explained by neuroticism, such that the association was stronger in high and moderate than low neuroticism groups. Negative schizotypy also related to greater middle occipital gyrus P200 activity, but was weakly explained by neuroticism.
Increased P200/P300 amplitude during rejecting interactions
Increased dACC P200 activity would indicate a preference for rejection cues in terms of feature integration and allocating attention to rejection. The dACC is an emotion regulation-related area that plays a pivotal role in experiencing social rejection as pain (Premkumar Citation2012; Eisenberger, Citation2012; Rotge et al. Citation2015). The dACC is engaged in evaluating the reward value of a rejected event (Blanchard and Hayden Citation2014). Greater dACC activity relates to experiencing more pain and distress over sustained exposure to rejection (Rotge et al. Citation2015). Our earlier findings that dACC P300 and late slow wave activity increased during rejection compared to acceptance/neutral scenes reflected early attention towards and sustained evaluation of rejection (Premkumar et al. Citation2014). The present study’s result suggests that dACC activity also plays a role in integrating features of rejection scenes and preparing for attention to rejection.
Increased N300/P300 amplitude in midline frontal and parietal scalp regions during rejection than neutral scenes supports previous evidence of increased parieto-occipital P300 amplitude during rejection relative to a control condition (Crowley et al. Citation2010). The findings reflect an attentional bias towards rejection and evaluating the personal salience of rejection, because evaluation of personal salience of emotions, such as rejection, occurs in the frontal and visual cortices (Herbert et al. Citation2011; Lu et al. Citation2012; Dai et al. Citation2013).
Relation of parieto-occipital P200/P300 activity during rejecting interactions to schizotypy
Parieto-occipital P200 and P300 amplitude related to schizotypy, which suggests that feature integration and attention orientation towards rejection in the visual cortex increase/decrease concurrently with a propensity for schizotypal traits. This may be so, because the P300 is a vulnerability marker for psychosis, where it is typically reduced in the auditory oddball task in schizophrenia patients, at-risk individuals and positive schizotypal individuals (Sumich et al. Citation2008; Mondragón-Maya et al. 2013). Additionally, schizotypal individuals may be intrinsically more sensitive to rejecting cues, because poorer emotional awareness in social situations is a vulnerability marker for psychosis (van Rijn et al. Citation2011). Positive schizotypy relates to having more anxious attachment, more prosodic expression in speech and more openness to experience, whereas negative schizotypy relates to having more avoidant attachment, less prosodic expression in speech, less openness to experience and more social anhedonia, i.e. engaging in fewer social activities, having fewer friends and finding less pleasure in social interactions (Berry et al. Citation2007; Kwapil et al. Citation2008; Cohen and Hong Citation2011). These interpersonal properties of positive and negative schizotypy may alter how those with high positive or negative schizotypy attend to rejection scenarios.
Rejecting attitudes expressed by others could perpetuate vigilance for such social cues. Hostility as rejection from a carer predicts disturbance and relapse in schizophrenia patients and more positive symptoms in at those at risk for psychosis (Bebbington and Kuipers Citation1994; Schlosser et al. Citation2010). Several studies allude to the reciprocal relation between schizotypal traits and rejection. Past experiences of difficult peer and family attachment alter attention to rejection, with some people becoming anxious when rejected, and others becoming avoidant or anhedonic (Berry et al. Citation2007). The fact that peers of individuals with high positive and negative schizotypy had rated them as less likeable, suggests that these traits attract rejecting interactions (Oltmanns et al. Citation2004). One explanation is that odd interpersonal behaviour, such as avoidance and hostility, can elicit anxiety, anger and lesser interest in others, in turn leading to social isolation in individuals with schizotypy (Zborowski and Garske Citation1993).
The inverse positive schizotypy-P200 association, but direct negative schizotypy-P200/P300 association suggests divergent patterns of the association between schizotypy types and preference for rejection cues. Separate neural processes underlie the positive and negative schizotypal dimensions (Vollema and van den Bosch Citation1995). Thus, greater positive symptom severity relates to reduced P300 amplitude during angry faces in patients with first-episode psychosis (Brennan et al. Citation2014), suggesting that positive symptoms attenuate attention to threat-related interactions. Higher positive, but not negative, schizotypy relates to diminished P300 during the auditory oddball task (Nuchpongsai et al. Citation1999; Sumich et al. Citation2008) and reduced occipital gyrus activation during self-referential processing (Debbané et al. Citation2014), implying attenuated attention to unexpected events and emotional awareness of others’ mental states. Reduced dACC N100 amplitude during rejection related to more positive schizotypy (Premkumar et al. Citation2014); thus poor N100-related feature detection may have a downstream disruptive influence on P200-related feature integration as the level of positive schizotypy increases. Lower striato-limbic activation during stressful problem-solving in negative than positive schizotypal individuals indicates lower reward-responsiveness towards or arousal by threat (Soliman et al. Citation2011).
The role of neuroticism in the association between negative schizotypy and P300 amplitude during rejection
Neuroticism partly explained the negative schizotypy-P300 middle parietal amplitude association, such that the association was strongest in those with moderate neuroticism. The negative schizotypy-P300 association in the high and moderate neuroticism groups may have been present due to behavioural inhibition. Behavioural inhibition is the tendency for social withdrawal, fear and reservation, and it increases social anxiety and attention bias towards threat, thus suggesting a heightened vigilance for threat (Lahat et al. Citation2011; Pérez-Edgar et al. Citation2011). Thus, those who have anxiety about being socially isolated may have an attention bias towards rejecting interactions.
The present study’s findings are somewhat contrary to a previous study’s finding that neuroticism moderated the positive schizotypy-psychopathology association, but not the negative schizotypy-psychopathology association (Barrantes-Vidal et al. Citation2009). This may be because the current sample had normal levels of neuroticism and schizotypy, and only one type of psychopathology, namely RS, was studied.
Limitations and future research
P200 parieto-occipital amplitude did not differ between social interaction types, because good information manipulation is needed to allocate attention to certain interactions such that higher P200 amplitude relates to higher intelligence (Lijffijt et al. Citation2009). Besides, social anxiety due to RS is one of many negative mood states found in schizotypy; thus, P200/P300 amplitude during rejection scenes could have been compared with that of other non-social negative emotions, such as fear and anger. Cognitive disorganisation and impulsive nonconformity subscales of the O-LIFE (Mason et al. Citation1995) are equally important in determining the ERP correlates of schizotypy. However, given that the aim was to distinguish between positive and negative schizotypy, controlling for cognitive disorganisation and impulsive nonconformity fell beyond the scope of the current paper. Findings about schizotypy and neuroticism would need to be confirmed in a larger group of individuals with schizotypy and neuroticism who are further along the psychosis continuum, since the current sample comprised Psychology students whose schizotypy and neuroticism levels fell within the normal range, which may have restricted effect sizes. Behavioural inhibition in neuroticism is one of the strongest predictors of social anxiety (Clauss and Blackford Citation2012); future research could specifically test whether behavioural inhibition moderates the schizotypy-RS association.
Conclusion
Positive schizotypy attenuates attention to rejection interactions, while negative schizotypy increases feature integration of and attention bias to rejecting interactions. Having a moderate level of neuroticism enhances the relation between negative schizotypy and increased early attention to rejection. These findings support evidence for the P300 as an index of the attention bias to rejection and a vulnerability marker of schizotypy. The findings have implications for how attention to rejection could be modified through threat-reduction techniques in order to minimise distress in individuals with schizotypal traits. Individuals at risk for psychosis, who have poorer communication styles with their carers and have carers expressing a high level of hostility as rejection, have more severe positive symptoms at 6-months follow-up than those with low expressed emotion carers (O’Brien et al. 2006; Schlosser et al. Citation2010). The present study’s findings could be used to emphasise the need for supportive interventions services for those at risk for psychosis, particularly those dealing with early episodes (Bird et al. Citation2010; Stafford et al. Citation2013; National Institute of Health and Clinical Excellence Citation2014).
Acknowledgements
The authors are grateful to Holly Cumbers and Alexandra Zervos for their help with the EEG data collection and analysis.
Statement of interest
None to declare.
References
- Bartholow BD. 2010. On the role of conflict and control in social cognition: Event-related brain potential investigations. Psychophysiology 47:201–212.
- Barrantes-Vidal N, Ros-Morente A, Kwapil TR. 2009. An examination of neuroticism as a moderating factor in the association of positive and negative schizotypy with psychopathology in a nonclinical sample. Schizophr Res 115:303–309.
- Bebbington P, Kuipers L. 1994. The predictive utility of expressed emotion in schizophrenia: An aggregate analysis. Psychol Med 24:707–718.
- Bentall RP, Claridge GS, Slade PD. 1989. The multidimensional nature of schizotypal traits: A factor analytic study with normal subjects. Br J Clin Psychol, 28:363–375.
- Berry K, Band R, Corcoran R, Barrowclough C, Wearden A. 2007. Attachment styles, earlier interpersonal relationships and schizotypy in a non-clinical sample. Psychol Psychother 80:563–576.
- Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. 2010. Early intervention services, cognitive-behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry 197:350–356.
- Blackhart GC, Nelson BC, Knowles ML, Baumeister RF. 2009. Rejection elicits emotional reactions but neither causes immediate distress nor lowers self-esteem: A meta-analytic review of 192 studies on social exclusion. Pers Soc Psychol Rev 13:269–309.
- Blanchard TC, Hayden BY. 2014. Neurons in dorsal anterior cingulate cortex signal postdecisional variables in a foraging task. J Neurosci 34:646–655.
- Blanchard JJ, Collins LM, Aghevli M, Leung WW, Cohen AS. 2011. Social anhedonia and schizotypy in a community sample: The Maryland longitudinal study of schizotypy. Schizophr Bull 37:587–602.
- Booth T, Murray AL, Marples K, Batey M. 2013. What role does neuroticism play in the association between negative job characteristics and anxiety and depression? Pers Individual Dif 55:422–427.
- Brennan AM, Harris AW, Williams LM. 2014. Neural processing of facial expressions of emotion in first onset psychosis. Psychiatry Res 219:477–485.
- Brown LH, Silvia PJ, Myin–Germeys I, Lewandowski KE, Kwapil TR. 2008. The relationship of social anxiety and social anhedonia to psychometrically identified schizotypy. J Soc Clin Psychol 27:127–149.
- Carretié L, Mercado F, Tapia M, Hinojosa JA. 2001. Emotion, attention, and the 'negativity bias', studied through event-related potentials. Int J Psychophysiol 41:75–85.
- Carretié L, Tapia M, Mercado F, Albert J, López-Martín S, de la Serna JM. 2004. Voltage-based versus factor score-based source localization analyses of electrophysiological brain activity: a comparison. Brain Topogr. 17:109–115.
- Carretié L, Hinojosa JA, Albert J, Mercado F. 2006. Neural response to sustained affective visual stimulation using an indirect task. Exp Brain Res 174:630–637.
- Carretié L, Kessel D, Carboni A, López-Martín S, Albert J, Tapia M, et al. 2013. Exogenous attention to facial vs non-facial emotional visual stimuli. Soc Cogn Affect Neurosci 8:764–773.
- Çelik P, Lammers J, van Beest I, Bekker MHJ, Vonk R. 2013. Not all rejections are alike; competence and warmth as a fundamental distinction in social rejection. J Exp Soc Psychol 49:635–642.
- Cermolacce M, Micoulaud JA, Naudin J, Vion-Dury J. 2011. Electrophysiology and schizophrenic vulnerability: the P300 component as endophenotype candidate? Encephale 37:353–360.
- Champagne J, Mendrek A, Germain M, Hot P, Lavoie ME. 2014. Event-related brain potentials to emotional images and gonadal steroid hormone levels in patients with schizophrenia and paired controls. Front Psychol 5:543
- Chapman RM, McCrary JW. 1995. EP component identification and measurement by principal components analysis. Brain Cogn 27:288–310.
- Clauss JA, Blackford JU. 2012. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry 51:1066–1075.e1.
- Cohen AS, Callaway DA, Najolia GM, Larsen JT, Strauss GP. 2012. On “risk” and reward: investigating state anhedonia in psychometrically defined schizotypy and schizophrenia. J Abnorm Psychol 121:407–415.
- Cohen AS, Hong SL 2011. Understanding constricted affect in schizotypy through computerized prosodic analysis. J Pers Disord 25:478–91.
- Crowley MJ, Wu J, Molfese PJ, Mayes LC. 2010. Social exclusion in middle childhood: Rejection events, slow-wave neural activity, and ostracism distress. Soc Neurosci 5:483–495.
- Dai J, Zhai H, Zhou A, Gong Y, Luo L. 2013. Asymmetric correlation between experienced parental attachment and event-related potentials evoked in response to parental faces. PLoS One 8:e68795
- Debbané M, Vrtička P, Lazouret M, Badoud D, Sander D, Eliez S. 2014. Self-reflection and positive schizotypy in the adolescent brain. Schizophr Res 152:65–72.
- Dien J. 2010. Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. . Psychophysiology 47:170–183.
- Dien J. 2012. Applying principal components analysis to event-related potentials: a tutorial. Dev Neuropsychol 37:497–517.
- Eisenberger NI. 2012. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 13:421–434.
- Ettinger U, Kumari V, Crawford TJ, Flak V, Sharma T, Davis RE, Corr PJ. 2005. Saccadic eye movements, schizotypy, and the role of neuroticism. Biol Psychol 68:61–78.
- Feng C, Li W, Tian T, Luo Y, Gu R, Zhou C, Luo YJ. 2014. Arousal modulates valence effects on both early and late stages of affective picture processing in a passive viewing task. Soc Neurosci 9:364–377.
- Fjell AM, Walhovd KB, Meling S, Johansen MB. 2005. Basic information processing of neurotics and stables: an experimental ERP approach to personality and distractibility. Scand J Psychol 46:493–502.
- Foti D, Hajcak G, Dien J. 2009. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology 46:521–530.
- Guo Q, Tang Y, Li H, Zhang T, Li J, Sheng J, et al. 2014. Both volumetry and functional connectivity of Heschl's gyrus are associated with auditory P300 in first episode schizophrenia. Schizophr Res 160:57–66.
- Haralanova E, Haralanov S, Beraldi A, Moller HJ, Hennig-Fast K. 2012. Subjective emotional over-arousal to neutral social scenes in paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci 262:59–68.
- Herbert C, Herbert BM, Ethofer T, Pauli P. 2011. His or mine? The time course of self-other discrimination in emotion processing. Soc Neurosci 6:277–288.
- Hinojosa JA, Albert J, López-Martín S, Carretié L. 2014. Temporospatial analysis of explicit and implicit processing of negative content during word comprehension. Brain Cogn 87:109–121.
- Holmes A, Bradley BP, Kragh-Nielsen M, Mogg K. 2009. Attentional selectivity for emotional faces: Evidence from human electrophysiology. Psychophysiology 46:62–68.
- Hong R. 2010. Neuroticism, Anxiety Sensitivity Thoughts, and Anxiety Symptomatology: Insights from an Experience-Sampling Approach. Cogn Ther Res 34:254–262.
- Horley K, Gonsalvez C, Williams L, Lazzaro I, Bahramali H, Gordon E. 2001. Event-related potentials to threat-related faces in schizophrenia. Int J Neurosci 107:113–130.
- Horton LE, Barrantes-Vidal N, Silvia PJ, Kwapil TR. 2014. Worries about Being Judged versus Being Harmed: Disentangling the Association of Social Anxiety and Paranoia with Schizotypy. PLoS One 9:
- John O, Donahue E, Kentle R. 1991. The Big Five Inventory—Versions 4a and 54. University of California, Berkeley, Institute of Personality and Social Research: Berkeley, CA.
- John OP, Srivastava S. 1999. The Big Five trait taxonomy: History, measurement, and theoretical perspectives. In: Pervin LA, John OP, editors. Handbook of personality: Theory and research (2nd ed.), New York: Guilford, pp 102–138.
- Johnson-Laird PN, Oatley K. 1989. The language of emotions: An analysis of a semantic field. Cognition and Emotion 3:81–123.
- Kashdan TB. 2004. The neglected relationship between social interaction anxiety and hedonic deficits: Differentiation from depressive symptoms. J Anxiety Disord 18:719–730.
- Kotov R, Gamez W, Schmidt F, Watson D. 2010. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psycho Bull 136:768–821
- Kwapil TR, Barrantes-Vidal N, Silvia PJ. 2008. The dimensional structure of the Wisconsin Schizotypy Scales: factor identification and construct validity. Schizophr Bull 34:444–457.
- Kwapil TR, Brown LH, Silvia PJ, Myin-Germeys I, Barrantes-Vidal N. 2012. The expression of positive and negative schizotypy in daily life: An experience sampling study. Psychol Med 42:2555–2566.
- Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. 2013. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans' ten-year longitudinal study. J Abnorm Psychol 122:807–815.
- Lahat A, Hong M, Fox NA. 2011. Behavioural inhibition: is it a risk factor for anxiety?. Int Rev Psychiatry 23:248–257.
- Langens TA, Schuler J. 2005. Written emotional expression and emotional well-being: The moderating role of fear of rejection. Pers Soc Psychol Bull 31:818–830.
- Lee H, Ku J, Kim J, Jang DP, Yoon KJ, Kim SI, Kim JJ. 2014. Aberrant neural responses to social rejection in patients with schizophrenia. Soc Neurosci 9:412–423.
- Lemay EP, Clark MS. 2008. “Walking on eggshells”: How expressing relationship insecurities perpetuates them. J Pers Soc Psychol 95:420–441.
- Lenzenweger MF. 1993. Explorations in schizotypy and the psychometric high-risk paradigm. In: Chapman LJ, Chapman JP, Fowler DC, editors. Progress in experimental personality and psychopathology research. New York: Springer; p. 66–116.
- Leitner JB, Hehman E, Jones JM, Forbes CE. 2014. Self-enhancement Influences Medial Frontal Cortex Alpha Power to Social Rejection Feedback. J Cogn Neurosci 26:2330–2341.
- Lijffijt M, Moeller FG, Boutros NN, Burroughs S, Lane SD, Steinberg JL, Swann AC. 2009. The Role of Age, Gender, Education, and Intelligence in P50, N100, and P200 Auditory Sensory Gating. J Psychophysiol 23:52–62.
- Llerena K, Park SG, Couture SM, Blanchard JJ. 2012. Social anhedonia and affiliation: examining behavior and subjective reactions within a social interaction. Psychiatry Res 200:679–686.
- Lu J, Li D, Xu J. 2012. An event-related potential study of maternal love in mothers. Brain Topogr 25:399–407.
- Lu Y, Zhang WN, Hu W, Luo YJ. 2011. Understanding the subliminal affective priming effect of facial stimuli: An ERP study. Neurosci Lett 502:182–185.
- Lysaker PH, Salvatore G, Grant ML, Procacci M, Olesek KL, Buck KD, et al. 2010. Deficits in theory of mind and social anxiety as independent paths to paranoid features in schizophrenia. Schizophr Res 124:81–85.
- Macare C, Bates TC, Heath AC, Martin NG, Ettinger U. 2012. Substantial genetic overlap between schizotypy and neuroticism: a twin study. Behav Genet 42:732–742.
- Mason O, Claridge G, Jackson M. 1995. New scales for the assessment of schizotypy. Pers Individ Dif 18:7–13.
- Meehl PE. 1962. Schizotaxia, schizotypy, schizophrenia. Am Psychol 17:827–838.
- Mohanty A, Heller W, Koven NS, Fisher JE, Herrington JD, Miller GA. 2008. Specificity of emotion-related effects on attentional processing in schizotypy. Schizophr Res 103:129–137.
- Mondragón-Maya A, Solís-Vivanco R, León-Ortiz P, Rodríguez-Agudelo Y, Yáñez-Téllez G, Bernal-Hernández J, et al. 2013. Reduced P3a amplitudes in antipsychotic naïve first-episode psychosis patients and individuals at clinical high-risk for psychosis. J Psychiatr Res 47:755–761.
- Morrison AP, French P, Lewis SW, Roberts M, Raja S, Neil ST, et al. 2006. Psychological factors in people at ultra-high risk of psychosis: Comparisons with non-patients and associations with symptoms. Psychol Med 36:1395–1404.
- Moscovitch DA, Suvak MK, Hofmann SG. 2010. Emotional response patterns during social threat in individuals with generalized social anxiety disorder and non-anxious controls. J Anxiety Disord 24:785–791.
- Moser JS, Huppert JD, Duval E, Simons RF. 2008. Face processing biases in social anxiety: an electrophysiological study. Biol Psychol 78:93–103.
- Nuechterlein KH, Dawson ME. 1984. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull 10:300–312.
- National Institute of Health and Clinical Excellence. 2014. Psychosis and schizophrenia in adults: treatment and management (Update) CG178. London: National Institute for Health and Clinical Excellence.
- Nuchpongsai P, Arakaki H, Langman P, Ogura C. 1999. N2 and P3b components of the event-related potential in students at risk for psychosis. Psychiatry Res 88:131–141.
- O’Brien MP, Gordon JL, Bearden CE, Lopez SR, Kopelowicz A, Cannon TD. 2006. Positive family environment predicts improvement in symptoms and social functioning among adolescents at imminent risk for onset of psychosis. Schizophr Res 81:269–275.
- Oltmanns TF, Friedman JNW, Fiedler ER, Turkheimer E. 2004. Perceptions of people with personality disorders based on thin slices of behavior. J Res Pers 38:216–229.
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, et al. 2011. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J Abnorm Child Psychol 39:885–895.
- Peterson CH, Casillas A, Robbins SB. 2006. The Student Readiness Inventory and the Big Five: Examining social desirability and college academic performance,. Pers Individ Dif 41:663–673.
- Picton TW, van Roon P, Armilio ML, Berg P, Ille N, Scherg M. 2000. The correction of ocular artifacts: A topographic perspective. Clin Neurophysiol 111:53–65.
- Pinheiro AP, Del Re E, Mezin J, Nestor PG, Rauber A, McCarley RW, et al. 2013. Sensory-based and higher-order operations contribute to abnormal emotional prosody processing in schizophrenia: an electrophysiological investigation. Psychol Med 43:603–618.
- Power MJ. 2005. The structure of emotion: An empirical comparison of six models. Cognition and Emotion 20:694–713.
- Premkumar P. 2012. Are you being rejected or excluded? Insights from neuroimaging studies using different rejection paradigms. Clin Psychopharmacol Neurosci 10:144–154.
- Premkumar P, Ettinger U, Inchley-Mort S, Sumich A, Williams SCR, Kuipers E, Kumari V. 2012. Neural processing of social rejection: The role of schizotypal personality traits. Hum Brain Mapp 33:695–706.
- Premkumar P, Onwumere J, Wilson D, Sumich A, Castro A, Kumari V, Kuipers E. 2014. Greater positive schizotypy relates to reduced N100 activity during rejection scenes. Neuropsychologia 61:280–290.
- Rodrigo MJ, León I, Quiñones I, Lage A, Byrne S, Bobes MA. 2011. Brain and personality bases of insensitivity to infant cues in neglectful mothers: an event-related potential study. Dev Psychopathol 23:163–176.
- Roelofs J, Huibers M, Peeters F, Arntz A. 2008. Effects of neuroticism on depression and anxiety: Rumination as a possible mediator. Pers Individ Dif 44:576–586.
- Rossignol M, Anselme C, Vermeulen N, Philippot P, Campanella S. 2007. Categorical perception of anger and disgust facial expression is affected by non-clinical social anxiety: an ERP study. Brain Res 1132:166–176.
- Rossignol M, Fisch SA, Maurage P, Joassin F, Philippot P. 2013. Reduced processing of facial and postural cues in social anxiety: insights from electrophysiology. PLoS One 8:e75234
- Rotge JY, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, et al. 2015. A meta-analysis of the anterior cingulate contribution to social pain. Soc Cogn Affect Neurosci 10:19–27.
- Salokangas RK, Patterson P, Heinimaa M, Svirskis T, From T, Vaskelainen L, et al. EPOS Group. 2012. Perceived negative attitude of others predicts transition to psychosis in patients at risk of psychosis. Eur Psychiatry 27:264–266.
- Scherg M, Ille N, Bornfleth H, Berg P. 2002. Advanced tools for digital EEG review: Virtual source montages, whole-head mapping, correlation, and phase analysis. J Clin Neurophysiol 19:91–112.
- Schlosser DA, Zinberg JL, Loewy RL, Casey-Cannon S, O'Brien MP, Bearden CE, et al. 2010. Predicting the longitudinal effects of the family environment on prodromal symptoms and functioning in patients at-risk for psychosis. Schizophr Res 118:69–75.
- Sinclair HC, Ladny RT, Lyndon AE. 2011. Adding insult to injury: Effects of interpersonal rejection types, rejection sensitivity, and self-regulation on obsessive relational intrusion. Aggress Behav 37:503–520.
- Skosnik PD, Park S, Dobbs L, Gardner WL. 2008. Affect processing and positive syndrome schizotypy in cannabis users. Psychiatry Res 157:279–282.
- Soliman A, O'Driscoll GA, Pruessner J, Joober R, Ditto B, Streicker E, et al. 2011. Limbic response to psychosocial stress in schizotypy: a functional magnetic resonance imaging study. Schizophr Res 131:184–191.
- Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. 2013. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 346:f185
- Sumich A, Kumari V, Gordon E, Tunstall N, Brammer M. 2008. Event-related potential correlates of paranormal ideation and unusual experiences. Cortex 44:1342–1352.
- Torgersen S, Edvardsen J, Oien PA, Onstad S, Skre I, Lygren S, Kringlen E. 2002. Schizotypal personality disorder inside and outside the schizophrenic spectrum. Schizophr Res 54:33–38.
- Van der Molen MJ, Poppelaars ES, Van Hartingsveldt CT, Harrewijn A, Gunther Moor B, Westenberg PM. 2014. Fear of negative evaluation modulates electrocortical and behavioral responses when anticipating social evaluative feedback. Front Hum Neurosci 7:936
- van Rijn S, Schothorst P, van 't Wout M, Sprong M, Ziermans T, van Engeland H, et al. 2011. Affective dysfunctions in adolescents at risk for psychosis: Emotion awareness and social functioning. Psychiatry Res 187:100–105.
- Vollema MG, van den Bosch RJ. 1995. The multidimensionality of schizotypy. Schizophr Bull 21:19–31.
- Velthorst E, Meijer C, G.R.O.U.P. Investigators. 2012. The association between social anhedonia, withdrawal and psychotic experiences in general and high-risk populations. Schizophr Res 138:290–294.
- Whittingstall K, Bartels A, Singh V, Kwon S, Logothetis NK. 2010. Integration of EEG source imaging and fMRI during continuous viewing of natural movies. Magn Reson Imaging 28:1135–1142.
- Yuan L, Zhou R, Hu S. 2014. Cognitive reappraisal of facial expressions: Electrophysiological evidence of social anxiety. Neurosci Lett. 577:45–50.
- Zborowski MJ, Garske JP. 1993. Interpersonal deviance and consequent social impact in hypothetically schizophrenia-prone men. J Abnorm Psychol 102:482–489.