Abstract
The aquatic ecotoxicity testing of nanoparticles is complicated by unstable exposure conditions resulting from various transformation processes of nanoparticles in aqueous suspensions. In this study, we investigated the influence of exposure timing on the algal test response to silver nanoparticles (AgNPs), by reducing the incubation time and by aging the AgNPs in algal medium prior to testing. The freshwater green algae Pseudokirchneriella subcapitata were exposed to AgNO3, NM-300 K (a representative AgNP) and citrate stabilized AgNPs from two different manufacturers (AgNP1 and AgNP2) in a standard algal growth inhibition test (ISO 8692:2004) for 48 h and a short-term (2 h) 14C-assimilation test. For AgNO3, similar responses were obtained in the two tests, whereas freshly prepared suspensions of citrate stabilized AgNPs were less toxic in the 2-h tests compared to the 48-h tests. The 2-h test was found applicable for dissolved silver, but yielded non-monotonous concentration–response relationships and poor reproducibility for freshly prepared AgNP suspensions. However, when aging AgNPs in algal medium 24 h prior to testing, clear concentration–response patterns emerged and reproducibility increased. Prolonged aging to 48 h increased toxicity in the 2-h tests whereas aging beyond 48 h reduced toxicity. Our results demonstrate that the outcome of algal toxicity testing of AgNPs is highly influenced not only by the test duration, but also by the time passed from the moment AgNPs are added to the test medium. This time-dependency should be considered when nanomaterial dispersion protocols for ecotoxicity testing are developed.
Introduction
Silver nanoparticles (AgNPs) are the most commonly used engineered nanomaterials in consumer products (DTU Environment, Citation2013; Rejeski, Citation2009), utilized widely due to their antimicrobial properties (SCENIHR, Citation2014; Wijnhoven et al., Citation2009). Concern has been raised for the potential hazardous environmental effects of AgNPs, as silver in ionic form is known to be very toxic to aquatic life (Hansen & Baun, Citation2012; Ratte, Citation1999). In recent years, the toxic effects of AgNPs have become one of the most studied areas within the field of nanoecotoxicology. Several studies attribute the observed toxicity of AgNPs to the release of ionic silver (Kim et al., Citation2011; Miao et al., Citation2009; Newton et al., Citation2013; Turner et al., Citation2012; Zhao & Wang, Citation2012), whereas others identify possible nanoparticle-specific effects adding to the toxicity and/or observe toxicity unexplained solely by the measured ionic silver fraction (Miao et al., Citation2010; Navarro et al., Citation2008; Oukarroum et al., Citation2012; Stensberg et al., Citation2014).
Ecotoxicity tests are, however, complicated by the fact that NPs suspended in media are highly heterogeneous and dynamic systems (Pettitt & Lead, Citation2013) in which a series of time-dependent transformation processes are ongoing during incubation. This affects the concentration–response relationships and ultimately the validity and reproducibility of aquatic toxicity tests with NPs (Hartmann et al., Citation2010, Citation2013).
Thorough characterization of NP size, shape, aggregation, dissolution, etc., in ecotoxicity assays has therefore been recommended (Fabrega et al., Citation2011; Handy et al., Citation2012a; Pettitt & Lead, Citation2013), but complete NP characterization during exposure in ecotoxicity assays is, however, a very cumbersome and complicated task (Fabrega et al., Citation2011).
The highly dynamic nature of aqueous NP suspensions is further emphasized in algal tests. As the algal density increases exponentially, so does the amount of exudates, which may interact with the NPs and alter their behavior and effects (Handy et al., Citation2012b). The rather complex test media needed to sustain the rapid growth, even for a short-term incubation of 48–96 h add to the complexity of the test system. Furthermore, the presence of NPs in algal tests may interfere with the quantification of the algal biomass (Handy et al., Citation2012a; Hartmann et al., Citation2013). Despite the difficulties, algal tests provide fast, cheap and sensitive test results for an environmentally relevant organism (Arensberg et al., Citation1995) and are as such mandatory in all hazard assessments schemes used for both chemical classification and regulation.
Variable exposure concentrations in aquatic toxicity tests during incubation is not a novel problem related only to NPs, but is a common and well-known problem for other chemicals, for which it may be difficult to maintain at least 80% of the nominal test concentration during the exposure period required for guideline tests (OECD, Citation2000). To maintain a constant exposure concentration when testing difficult substances, Rosenkrantz (Citation2013) proposed and applied a short-term algal test using 14C-assimilation from 14C-labeled bicarbonate. For NPs, the use of short-term assays has also been proposed for ecotoxicity testing (Hartmann, Citation2011) and this 14C-assimilation short-term test may provide a beneficial setting for the testing of NPs as it may limit the influence of time-dependent NP transformation processes.
To date, the use of freshly prepared stock suspensions is a common approach, recommended for aquatic ecotoxicity testing of NPs (Reidy et al., Citation2013). The opposite approach of preparing NP suspensions and leaving them to age for a certain period prior to aquatic toxicity testing is very scarcely described in the literature. The term “aging” is often used in relation to different weathering processes of NPs in the environment, such as sunlight exposure (D’Agata et al., Citation2014) and bioaccessibility in soil (Coutris et al., Citation2012). Here we use the term aging as the sum of NP transformation processes occurring upon transfer to algal medium and during incubation.
With the dynamic behavior of NPs in aquatic suspensions being time-dependent, we expect that timing is essential to obtain stable exposure levels of NPs in aquatic toxicity testing and hypothesize that: (1) a shorter test duration may mitigate a major part of the variability in algal toxicity of AgNPs; (2) the use of freshly prepared NP suspension for aquatic toxicity testing may contribute to the dynamic behavior of NPs, causing changes in exposure levels to occur from the onset of preparing suspensions in medium and throughout the testing period.
The aim of this study is to investigate the influence of aging and exposure timing on the algal test response to silver nanoparticles (AgNPs). Through a series of controlled experiments, we therefore test the influence of a shortened test duration and aging of the NP suspension in medium prior to testing as means to obtain increased stability during incubation and limit the confounding factors. The purpose of this study is not to fully quantify and identify the silver and NP speciation and its influence on toxicity. Rather, we aim at utilizing the algal toxicity response as an indicator in itself, for the NP stability and applicability of the two algal tests.
Methods
Materials and preparation of NP suspensions
Test materials included 30 nm spherical citrate stabilized AgNPs obtained from two different suppliers (AgNP1 and AgNP2) along with the OECD representative 15 nm spherical AgNPs (NM-300 K). Silver nitrate (purity ≥99.0%, Sigma-Aldrich, Steinheim, Germany) was included to provide a reference for dissolved silver toxicity.
AgNP1 was provided by Dr. Jon Veinot (University of Alberta, Canada) as an aqueous suspension with a measured concentration of 20 mg Ag L−1. Briefly, this was prepared from 0.05 g AgNO3 dissolved in 500 mL distilled water, mixed with 1.1 g citric acid for 10 min followed by addition of two drops of 4 M NaOH solution and slow heating for a few hours until the final yellow solution was formed. AgNP2 was purchased from Cline Scientific (Göteborg, Sweden) as an aqueous suspension of 20 mg Ag L−1 in Milli-Q water containing <0.005% tannic acid and <0.05% trisodium citrate dihydrate, with a reported size of 29.9 ± 4.5 nm according to transmission electron microscopy (TEM). NM-300 K was provided by Joint Research Centre of the European Commission as a part of the European MARINA FP7 project as a dispersion in stabilizing agents, consisting of 4% w/w each of polyoxyethylene glycerol trioleate and polyoxyethylene (20) sorbitan monolaurat (Tween 20), having a reported size of 15 nm (90% <20 nm) and a silver content of 10.16% by weight (Klein et al., Citation2011).
All stock solutions (AgNO3) and suspensions (AgNPs) were prepared fresh before toxicity testing, by diluting with ISO 8692 medium (ISO, 2004), consisting of inorganic macronutrients (MgCl2, CaCl2, MgSO4, NH4Cl) and micronutrients (KH2PO4, FeCl3, Na2EDTA, H3BO3, MnCl2, ZnCl2, CoCl2, CuCl2, Na2MoO4, NaHCO3). AgNP1 stock suspensions for the 48-h algal test, were prepared with Milli-Q water, in compliance with the procedures decided within the MARINA FP7 project. The flasks with stock suspensions of AgNP1 and NM-300 K were placed in an ultrasonic bath (Bransonic© 3510 with an output of 130 W, 42 kHz± 6%) and exposed to ultra-sonication for 20 min.
Nanoparticle characterization and chemical analysis
AgNP suspensions in ISO algal medium were characterized for particle size distribution, hydrodynamic diameter and zeta potential by Dynamic Light Scattering (DLS) using a Malvern ZetaSizer Nano ZS (Malvern Instruments, Worcestershire, UK). Measurements were conducted on 1 mL sample in standard disposable cuvettes (or capillary cells for zeta potential determinations) at 25 °C with a scattering angle of 173°.
For TEM, a drop of AgNP suspension was placed on a carbon coated copper grid and left overnight to dry out under a plastic lid. The size and morphology of AgNPs were observed by a FEI Company Tecnai G2 T20, which also allowed for elemental analysis using Energy Dispersive X-ray spectroscopy.
Total silver concentrations in AgNP1 suspensions and AgNO3 solutions were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES) during the 7 days aging experiment (see section “Algal tests with aged NP suspensions”). Samples were acidified by addition of concentrated HNO3 and left for 24 h (AgNO3) or digested in a microwave oven (Anton Paar Multiwave 3000 solv) at 200 °C for 30 min (AgNP1). All samples were taken as duplicates and measurements were replicated three times.
Cultivation of algae
The unicellular freshwater green alga Pseudokirchneriella subcapitata used in this study is a laboratory culture obtained from the Norwegian Institute for Water Research, Oslo, Norway (NIVA) and grown in ISO 8692 medium (ISO, 2004). The algae were cultivated in 20 mL glass vials fitted with a screw cap lid with a hole to allow for CO2 diffusion from the atmosphere. The vials were placed at a shaking table (300 rpm) with continuous illumination from below by fluorescent tubes (30 W/33; Philips, Amsterdam, The Netherlands) with an intensity of 100 ± 20 µmol m−2 s−1 and at a temperature of 20 ± 2 °C.
Algal growth rate inhibition tests (48-h incubation)
Tests were conducted according to the ISO 8692 algal growth inhibition test protocol (ISO, 2004), with 48-h incubation according to Arensberg et al. (Citation1995). A range of test concentrations was prepared from stock solutions/suspensions and ISO 8692 algal medium, which were then inoculated with appropriate amounts of algal culture to obtain an initial density of 104 cells mL−1. Each test included six controls containing medium and algae only and five or six test exposure concentrations in triplicates in the following ranges: 0.80–31 µg Ag L−1 (AgNO3) and 6.3–200 µg Ag L−1 (AgNP2). For AgNP1 and NM-300 K, 15 concentrations were included in single replicates with ranges of 0.78–1000 and 0.20–250 µg Ag L−1, respectively. The tests were incubated at conditions identical to the cultivation process and ISO 8692 validity criteria were met for all tests, with control growth rates of minimum 1.0 day−1 and a maximum change in pH of 1.0 unit during the 48-h incubation. Samples of 0.4 mL were taken at times 0, 24 and 48 h and the algal growth rates were calculated on the basis of total algal pigments in each sample quantified by acetone extraction, as described by Mayer et al. (Citation1997). The fluorescence of the samples was subsequently measured on a Cary Eclipse Fluorescence Spectrophotometer (Agilent, Santa Clara, CA) using an excitation wavelength of 430 nm and emission wavelength of 670 nm.
Algal 14C-assimilation tests (2-h incubation)
The short-term algal toxicity was determined by a newly developed 2-h test, using 14C-incorporation during photosynthesis as toxicity endpoint. This 14C-assimilation test is a modification of the methods described by Kusk & Nyholm (Citation1992) and Petersen & Kusk (Citation2000). Seven to eight test concentrations were prepared in triplicates in the ranges: 1.3–76 µg Ag L−1 (for AgNO3), 10–1016 µg Ag L−1 (for NM-300 K) 10–1000 µg Ag L−1 (for NM-300 K and AgNP1) and 12–200 µg Ag L−1 (for AgNP2). The test concentrations were prepared in volumetric flasks using ISO 8692 algal medium and inoculated with an exponentially growing algal culture to obtain an initial density of 105 cells mL−1. For each test concentration, 5 mL were transferred to 20-mL glass scintillation vials and the test was initiated by immediately adding 50 µL of H14CO3-solution to all vials (specific activity: 20 µCi mL−1; obtained from DHI, Hoersholm, Denmark). The vials were then closed with airtight screw caps and placed on a shaker table at conditions identical to those of the cultivation process. After 2-h incubation, tests were terminated by adding 0.2 mL 10% HCl to each vial (yielding pH <2). Vials were then left open overnight in a fume hood to allow for evaporation of 14CO2 excess, i.e. the 14C that was not incorporated into algal biomass during the 2-h incubation. After evaporation, 10 mL scintillation liquid (Optiphase “Hisafe” 3, Perkin Elmer, Waltham, MA) was added to each vial. After a thorough mixing they were left in the dark for at least 8 h before liquid scintillation counting (TRI-CARB 1600 TR Liquid Scintillation Analyzer, Packard, Meriden, CT). All tests included six replicates of controls with algae and medium only as well as three replicates of a control with medium and H14CO3-solution. The latter was included to confirm that all added H14CO3 was converted into 14CO2 and removed in the evaporation step. A maximum change in pH of 0.5 units was measured during the 2-h incubation.
Algal tests with aged NP suspensions
For initial experiments with not freshly prepared suspensions, stock suspensions of AgNP1 and AgNP2 were prepared in medium as described above (“Materials and preparation of NP suspensions”). These were stored for 24 h in the dark at 20 °C prior to testing in the 2-h 14C-assimilation test (in the following referred to as “aged”). Seven to eight test concentrations were prepared from the aged stock suspensions in the concentration ranges: 10–1000 (AgNP1) and 20–920 µg Ag L−1 (AgNP2). In a second test run the 24-h aging procedure was repeated, but with stock suspensions placed on a shaker table at 200 rpm.
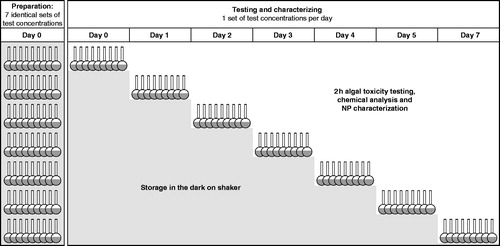
To follow the influence of aging over a period of 7 days, a stock suspension of AgNP1 was prepared in ISO 8692 algal medium and used immediately for the preparation of seven identical sets of eight test concentrations in the range 10–1000 µg Ag L−1 (see for an overview). The highest concentration of each set was prepared in duplicates, to provide suspensions for chemical analyses and NP characterization during aging by the use of TEM, ICP-OES and DLS. Each set of test concentrations also included duplicate vials with 6 µg Ag L−1 added as AgNO3, to serve as a reference for toxicity of dissolved silver during aging and for ICP-OES analysis. On the same day as test concentrations were prepared (day 0), a 2-h algal toxicity test including chemical analysis and NP characterization was carried out with one set of test concentrations. The six remaining sets of test concentrations were put on a shaker in the dark at 20 °C. After 1, 2, 3, 4, 5, and 7 days, sets of test concentrations were used consecutively for 2-h toxicity testing, chemical analysis and AgNP characterization.
Figure 1. Overview of the experimental setup for testing the influence of aging (0–7 days) on the characteristics and 2-h algal toxicity of citrate stabilized AgNPs (AgNP1). Seven identical sets of test concentrations covering the range 10–1000 µg Ag L−1 were prepared in ISO 8692 algal medium on day 0 and aged in the dark on a shaker (300 rpm) before testing and characterization was conducted after 0, 1, 2, 3, 4, 5 or 7 days.
Statistical analysis and chemical speciation modeling
The statistical program LOG457 was used to estimate growth rate and carbon assimilation inhibition in the 48- and 2-h experiments, respectively. A logarithmic-normal distribution of data was assumed and concentration–response curves were fitted by nonlinear regression analysis of growth rates (48-h tests) or carbon assimilation (2-h tests) versus concentration (Christensen et al., Citation2009). Finally, effect concentrations (e.g. EC50) were estimated along with their corresponding 95% confidence intervals. The concentration–response data, fitted curves and estimated EC-values are all based on the nominal concentrations.
The silver speciation of AgNO3 suspended in ISO 8692 algal medium was estimated by the chemical speciation modeling program Visual MINTEQ 3.0 (Gustafsson, Citation2010), assuming an atmospheric CO2 pressure (CO2 partial pressure of 0.00038 atm.) and with a fixed pH of 8.
Results
Characterization of AgNPs in medium suspensions
The size and morphology of AgNPs were investigated by DLS and TEM of 1 mgAg L−1 suspensions in ISO algal medium. The average hydrodynamic diameter (Z-average) was 106, 49 and 38 nm for NM-300 K, AgNP1 and AgNP2, respectively. Two size peaks (intensity based) were measured for NM-300 K and AgNP1 by DLS and different particle sizes were supported by TEM images. According to TEM images, the morphology of the AgNPs was close to spherical, although rods, triangles and cubical structures were also observed for AgNP1 (Figure S3, day 0). An overview of physicochemical properties of the tested AgNPs is given in the Supplementary material (Table S3).
Algal toxicity testing with 2- and 48-h incubation
The 2-h 14C-assimilation test and the standard ISO 48-h test produced comparable results for AgNO3, as shown by similarity of the EC-values and corresponding 95% confidence intervals shown in . In contrast, the observed EC-values for tests carried out with freshly prepared AgNP suspensions varied between the 2- and 48-h tests. The EC50 for NM-300 K was approximately three times lower in the 2-h test compared to the 48-h test (). The opposite was the case for AgNP1 and AgNP2, having EC50 values approximately two times higher in the 2-h test (). Regardless of the test applied, the most toxic silver form was AgNO3 and the least toxic AgNP1. Interestingly, AgNP1 and AgNP2 of similar nominal size and stabilizing agent (citrate) did not yield comparable EC-values, neither in the 2-h nor the 48-h test, as the AgNP2 was about four to five times more toxic in both tests. In both the 2- and 48-h tests, addition of cysteine in eqiumolar (or slight excess) to the added silver in the stock suspensions of AgNO3 and AgNP1 did not eliminate toxicity, although toxicity was reduced significantly; the EC50 values were increased by a minimum factor of 40 for AgNO3, 20 for NM-300 K and two for AgNP1 ().
Table 1. Effective concentrations (EC10- and EC50-values) and 95% confidence intervals in µg Ag L−1 from 2-h 14C-assimilation and 48-h growth inhibition algal tests with AgNO3 and AgNPs (freshly prepared).
The influence of AgNP aging on algal toxicity testing
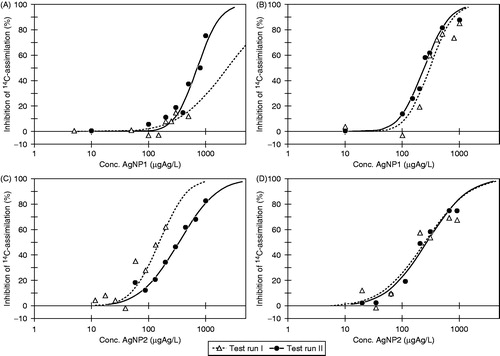
As illustrated in , it was not possible to obtain comparable concentration–response data in two individual test runs with neither AgNP1 nor AgNP2 in the 2-h algal toxicity test when using freshly prepared AgNP suspensions. The estimated EC50-values obtained in the two individual test runs showed this difference as well (Table S1), being 2400 [800;7200]95% µg Ag L−1 (test run 1) versus 710 [620;830]95% µg Ag L−1 (test run 2) for AgNP1 and 150 [130;190]95% µg Ag L−1 (test run 1) versus 340 [300;380]95% µg Ag L−1 (test run 2) for AgNP2. In contrast, two test runs with AgNO3 produced very similar concentration–response data (Figure S1) and EC50-values of 4.3 [2.3;8.0]95% µg Ag L−1 and 6.0 [5.1;7.1]95% µg Ag L−1 for test runs 1 and 2, respectively. However, when the stock suspensions were prepared 24 h prior to testing (24 h aging), the resulting concentration–response data from individual test runs showed much greater similarity with respect to concentration–response curves () as well as EC-values. Thus, comparable EC50-values were obtained of 300 [260;350]95% µg Ag L−1 versus 240 [230;260]95% µg Ag L−1 for AgNP1 and 250 [190;330]95% µg Ag L−1 versus 270 [230;320]95% µg Ag L−1 for AgNP2 (Table S1).
Figure 2. Concentration–response data and fitted curves by Log457 from 2-h 14C-assimilation algal tests with AgNP1 (A and B) and AgNP2 (C and D) being freshly prepared (A and C) or aged for 24 h in ISO 8692 algal medium in the dark (B and D). For each scenario (A–D) two individual test runs were conducted.
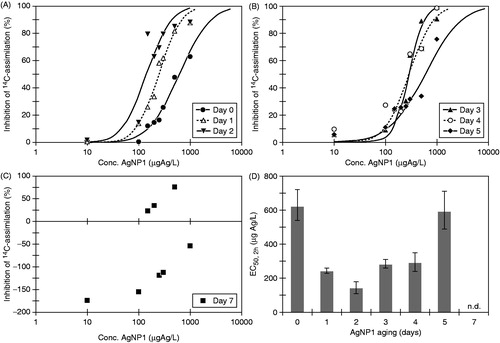
The influence of aging was further investigated by extending the aging period up to 7 days for AgNP1 (). The measured algal toxicity increased with aging period during the first 48 h, whereas further aging up to 7 days reduced toxicity ( and Table S2). Clear concentration–response relationships were observed for suspensions aged 0–2 days () and though toxicity decreased for suspensions aged for 3–5 days, monotonous concentration–response relationships were still evident (). After 7 days of aging, no concentration–response relationship could be obtained (). A somewhat similar pattern was observed for concomitant testing of AgNO3 in a concentration of 6 µg Ag L−1 with toxicity increasing slightly for suspensions aged 0–3 days and decreasing when aged 4–7 days (Figure S2). Our results show that the outcome of algal toxicity testing of AgNPs depends on the time passed from the moment AgNPs are added to the test medium. Further, the results illustrate that the exposure experienced by the organisms and hence the response will change within the time window of standard algal tests (48–96 h).
Figure 3. Concentration–response data and fitted curves by Log457 from 2-h algal 14C-assimilation algal tests with AgNP1 after aging in ISO 8692 algal medium (A) for 0–2 days, (B) for 3–5 days, (C) for 7 days. The EC50-values with 95% confidence intervals are plotted for suspensions aged for 05 days (D).
Characterization of AgNPs during aging
During the 7 days of aging, the zeta potential of the AgNP1 suspension (measured at 1 mgAg L−1) increased from approximately −29 to −17 mV, with the most pronounced shift occurring after 3–4 days of aging (). The average hydrodynamic size remained at approximately 50 nm until 3–4 days of aging where a marked increase was observed (). Thus, the DLS measurements indicate a change in the stability and aggregation state of the AgNP1 suspension occurring after 3–4 days of aging in algal medium. In addition, simple visual inspection of the AgNP1 suspensions revealed aggregation with aging, as large stringy aggregates were observed at the bottom of the flasks after 5 and 7 days of aging. These large visible aggregates (which were excluded when sampling for TEM and DLS measurements) did not disaggregate when the suspensions were vigorously shaken by hand.
Table 2. Size and zeta potential measured by DLS for citrate stabilized AgNPs (AgNP1) suspended in ISO algal medium (1 mg Ag L−1) after 0–7 days of aging in the dark on a shaker (300 rpm).
The recovery of total silver in suspensions aged for 0–7 days was in the range 52–136% for AgNO3 and 43–121% for AgNP1. The recovery varied randomly with aging period for both AgNO3 and AgNP1. No correlation between recovered amounts of silver, the toxicity observed, and aging period could be found.
From the TEM imaging, no apparent change in particle size, morphology or aggregation state with aging could be detected, as both single particles and aggregates/agglomerates were observed regardless of aging period (Figure S3). Also, distinct structures were observed that may be identified as AgNP1 interaction products with medium components, including cubic NaCl crystals around single AgNPs (Figure S3, day 7) and larger more complex structures interacting with the AgNPs (Figure S3, days 0 and 3). However, these structures along with the aggregates are likely artifacts from the drying process on the TEM grids or the exposure to vacuum in the electron microscope chamber.
Discussion
The influence of exposure duration in algal toxicity testing of dissolved silver and AgNPs
For AgNO3, comparable results were obtained in the 2- and 48-h algal tests, confirming that the toxicity of dissolved silver to P. subcapitata occurs very rapidly and that the 2-h 14C-assimilation test is applicable of quantifying the toxicity of dissolved silver. The estimated EC50-values from the two different tests were very similar, although different endpoints are applied. The 48-h test provides inhibition of growth rate in proliferating algae, whereas the 2-h test yields inhibition in the carbon-uptake of algal cells but is not a measure for population growth. The uptake of silver ions in the green alga Chlamydomonas reinhardtii has previously been reported as a very fast process (Navarro et al., Citation2008; Piccapietra et al., Citation2012) with an internal silver concentration reaching steady state after 1-h exposure to 500 nM AgNO3 corresponding to 54 µg Ag L−1 (Piccapietra et al., Citation2012). Another study found uptake rates of silver to be higher in P. subcapitata than C. reinhardtii, although this difference in uptake did not result in greater toxicity (Hiriart-Baer et al., Citation2006).
In contrast to the results obtained with dissolved silver, the tests with AgNPs yielded very different results in the two types of algal tests applied, when tested as freshly prepared suspensions. Where NM-300 K gave lower EC-values in the 2-h test compared to the 48-h test, the opposite was the case for AgNP1 and AgNP2. While different dissolution due to NP sizes could play a role in this, also NP morphology and stabilizing or capping agents may be of importance (Misra et al., Citation2012). If the toxicity of AgNPs depends on ionic release, the toxicity in the 2- and 48-h tests would be either similar or increase with exposure time, depending on the ionic release rate. This was clearly not the case and since the addition of cysteine in equimolar concentrations to silver did not eliminate toxicity, dissolution cannot be the only process contributing to the algal toxicity observed.
Influence of AgNP aging on the algal test response
The shortened exposure period in the 2-h algal test provided less time for processes such as aggregation, sedimentation and dissolution to occur and interfere with the bio-accessible exposure concentration. Therefore response data were expected to be more reproducible as a result of more stable exposure conditions. Nevertheless, two consecutive test runs with freshly prepared AgNP1 or AgNP2 suspensions did not produce comparable concentration–response data. However, when combining the shorter exposure period (2 h) with 24 h aging of AgNP1 and AgNP2 suspensions in medium prior to testing, comparable and reproducible concentration–response relationships were obtained for the tested AgNPs. Our results imply that timing issues are very important when conducting ecotoxicity tests with AgNPs, not only in relation to the exposure duration, but also in the preparation of AgNP suspensions for testing. This impact of timing or aging may in part be related to the kinetics of AgNP dissolution. The release of silver ions from AgNPs in various media has been studied by different modeling and/or measuring approaches, all describing a similar kinetic pattern of a very fast initial ion release, followed by a stagnation or equilibrium phase, often occurring within few days, but in some cases within longer periods (Cunningham et al., Citation2013; Lee et al., Citation2012; Liu & Hurt, Citation2010; Kittler et al., Citation2010; Zhang et al., Citation2011). The ion release kinetics of AgNPs may attribute to explain why a shortened exposure did not provide a more stable exposure concentration. The freshly prepared AgNP suspensions were likely unstable due to a very fast initial ion release, whereas the suspensions aged for 24 h possibly were less dynamic.
Consequently, it may be questioned whether the widely recognized approach of using freshly prepared NP suspensions for aquatic ecotoxicity testing is the best practice for AgNPs. Using freshly prepared AgNP suspensions for testing, disregards the dissolution kinetics and likely causes substantial, uncontrollable dissolution and change the exposure concentration markedly during a standard 48–96-h test. In contrast, our results suggest that aging of NP suspensions may minimize this issue, possibly by paying respect to the dissolution patterns described in the literature. Also, aggregation is induced upon transfer into aquatic media. Roemer et al. (Citation2011) reported an increasing aggregation upon dilution of citrate stabilized AgNPs in OECD daphnia media that stabilized after 5 h. However, in our study no substantial aggregation was observed by TEM, DLS or visual inspection after 24 h aging. The toxicity of AgNP1 increased upon 24 h aging, which is in good agreement with the dissolution patterns described in the literature as well as the apparent lack of aggregation and sedimentation after 24 h as assessed by DLS and visual inspection of suspensions.
The 2 h toxicity testing with AgNP1 suspensions aged for 1–7 days, revealed greatest toxicity after 48 h aging. Hereafter, the toxicity decreases with aging and no concentration–response data was obtained after 7 days of aging. These changes in toxicity correlates well with the measured DLS data, as aggregation occurred around 3–4 days of aging. This aggregation may be induced by temporal changes in the speciation of medium components, as the media composition is shown to influence AgNP aggregation (Tejamaya et al., Citation2012). Also, degradation of the stabilizing agent may have occurred, which is also reported to induce NP aggregation (Hartmann et al., Citation2013).
The 2-h algal toxicity with aging provides a measure for the bioavailable AgNP fraction over the course of 7 days. According to the variable toxicity observed within the first 96 h of aging, this bioavailable fraction is highly unstable, implying that the concentration axis for AgNPs in a standard 96-h algal test is far from constant during the incubation. Thus, from an organism point-of-view it is unlikely that a constant exposure concentration is experienced throughout the incubation period. The 2-h test provides a tool for quantifying toxicity at any given moments along the time-axis that begins when AgNPs are introduced into algal medium.
All in all, the changes in AgNP toxicity with aging are possibly due to a fast release of ions in the initial 48 h, followed by interactions of those ions and AgNPs with medium components, causing reduced bioavailability and toxicity of the AgNPs. Kittler et al. (Citation2010) investigated the dissolution and toxicity to human mesenchymal cells of PVP- and citrate stabilized AgNPs during storage (aging) of stock suspensions prepared with water. In agreement with our study, toxicity increased with aging, although the aging period is substantially longer. Three days of aging caused 70% reduction in viability, whereas complete cell death occurred upon 1 and 6 months of aging. The changes in toxicity with aging were ascribed to silver ions released during aging as measured during dissolution experiments. Contrary to our study, at no time there was a decrease in toxicity with aging. This could be due to the use of different aquatic media, as Kittler et al. (Citation2010) aged AgNPs in pure water while we used the algal medium. According to MINTEQ estimations, silver ions in the present study will react with medium components such as chloride, sulfide and phosphate (data not shown) and may cause complexation and/or precipitation of sparingly soluble salt, as also suggested by Kittler et al. (Citation2010) and Liu et al. (Citation2010). Manier et al. (Citation2013) studied the agglomeration or aggregation state along with the algal toxicity of CeO2 in ISO algal growth medium being non-aged or aged for 3 and 30 days under stirring and lighting. Agglomeration/aggregation already occurred after 24 h aging, which is much faster than the 3–4 days of aging required for AgNPs in our study. This is not surprising though, since different NPs are expected to possess different physicochemical properties and aggregation behavior. Opposite to our findings, Manier et al. (Citation2013) reported that aging of CeO2 NPs did not influence the toxicity toward P. subcapitata, indicating that aging processes differs among NP types and the influence of aging in ecotoxicity studies needs to be considered in a case by case manner.
We propose the 2-h algal 14C-assimilation test for screening NP toxicity for several reasons: (1) the 14C-uptake is measured by scintillation counting, which contrary to cell counting and the fluorometric techniques recommended in the ISO standard and OECD guidelines for algal growth inhibition tests, is not biased by the presence of NPs, (2) the 2-h exposure allows very limited time for algal proliferation, thus minimizing the amount of exudates and oxygen produced, which may play a role in NP aggregation (Unrine et al., Citation2012) and (3) compared to algal growth tests, less nutrients are needed, enabling use of diluted media for toxicity testing as suggested by Roemer et al. (Citation2011).
Currently, aquatic toxicity testing of NPs appears to be going in the opposite direction toward more complex exposure scenarios, chronic exposures and investigations of long-term effects, for the purpose of providing information crucial for the hazard and risk assessment of NPs. Although shortening the exposure duration in itself does not increase the environmental relevance of the guideline testing, the possible increased control gained serves an important purpose in the attempt to identify and rank the toxicity of NPs.
Conclusions
Our results highlight the importance of understanding the exposure concentration dynamics in ecotoxicity testing of AgNPs. The proposed 2-h algal 14C-assimilation test was found applicable for testing the toxicity of dissolved silver and AgNPs and it constitutes a fast and easy screening tool for NP toxicity testing. Although the shortened exposure did not provide improved control of the exposure to freshly prepared AgNP suspensions, the introduction of an aging step in algal medium prior to testing, yielded clear concentration–response relationships and a higher degree of reproducibility. The length of the aging period influenced the algal response of the 2-h test, as toxicity increased with aging up till 48 h, whereas toxicity declined with further aging until 7 days. Overall, these findings stress the importance of considering the influence of time-dependent transformation processes that NPs undergo upon suspension into aquatic media, when nanomaterial dispersion protocols for ecotoxicity testing are developed and applied.
Declaration of interest
This work is part of the project ENVNANO (Environmental Effects and Risk Evaluation of Engineered Nanoparticles) supported by the European Research Council (grant no. 281579). The authors are responsible for writing of the article and report no conflicts of financial, consulting and personal interests.
Supplementary material available online
Supplementary Tables S1–S3 and Figures S1–S3
SUPPLEMENTAL
Download MS Word (43.6 KB)Acknowledgements
Silver nanoparticles with citric acid capping (AgNP1) were generously provided by Dr. Jonathan Veinot, University of Alberta, Canada and NM-300 K as a part of the European Union MARINA Project. The authors thank Sacha J.V. Laruelle (DTU Environment) and Signe Qualmann (DTU Environment) for their contribution to the algal testing and NP characterization, Christian Engelbrekt (DTU Chemistry) for assistance with TEM and Torben Dolin (DTU Environment) for completing the illustrations. Dr. Steffen Foss Hansen and Dr. Nanna Bloch Hartmann (both DTU Environment) are thanked for their constructive commenting on the manuscript.
References
- Arensberg P, Hemmingsen V, Nyholm N. 1995. A miniscale algal toxicity test. Chemosphere 30:2103–15
- Christensen ER, Kusk KO, Nyholm N. 2009. Dose–response regressions for algal growth and similar continuous endpoints: calculation of effective concentrations. Environ Toxicol Chem 28:826–35
- Coutris C, Joner EJ, Oughton DH. 2012. Aging and soil organic matter content affect the fate of silver nanoparticles in soil. Sci Total Environ 420:327–33
- Cunningham S, Brennan-Fournet ME, Ledwith D, Byrnes L, Joshi L. 2013. Effect of nanoparticle stabilization and physicochemical properties on exposure outcome: acute toxicity of silver nanoparticle preparations in zebrafish (Danio rerio). Environ Sci Technol 47:3883–92
- D'Agata A, Fasulo S, Dallas LJ, Fisher AS, Maisano M, Readman JW, Jha AN. 2014. Enhanced toxicity of ‘bulk' titanium dioxide compared to ‘fresh' and ‘aged' nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 8:549–58
- DTU Environment. 2013. The nanodatabase. Available at: http://nanodb.dk/products. Accessed on 17 December 2013
- Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. 2011. Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–31
- Gustafsson JP. 2010. Visual MINTEQ ver. 3.0. Department of Land and Water Ressources Engineering. The Royal Institute of Technology, Sweden. Available at: http://www.lwr.kth.se/english/OurSoftware/vminteq/. Accessed on 15 October 2013
- Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, Sabo-Attwood T, et al. 2012a. Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem 31:15–31
- Handy RD, van den Brink N, Chappell M, Muehling M, Behra R, Dusinska M, et al. 2012b. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicology 21:933–72
- Hansen SF, Baun A. 2012. When enough is enough. Nat Nanotechnol 7:409–11
- Hartmann NB. 2011. Ecotoxicity of engineered nanoparticles to freshwater organisms [PhD thesis]. Kgs. Lyngby, Denmark: DTU Environment, Technical University of Denmark
- Hartmann NB, Engelbrekt C, Zhang J, Ulstrup J, Kusk KO, Baun A. 2013. The challenges of testing metal and metal oxide nanoparticles in algal bioassays: titanium dioxide and gold nanoparticles as case studies. Nanotoxicology 7:1082–94
- Hartmann NB, Von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A. 2010. Algal testing of titanium dioxide nanoparticles – testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 269:190–7
- Hiriart-Baer VP, Fortin C, Lee D, Campbell PGC. 2006. Toxicity of silver to two freshwater algae, Chlamydomonas reinhardtii and Pseudokirchneriella subcapitata, grown under continuous culture conditions: influence of thiosulphate. Aquat Toxicol 78:136–48
- ISO. 2004. Water Quality – Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. International Organization for Standardization. ISO Standard 8692. Geneva, Switzerland
- Kim J, Kim S, Lee S. 2011. Differentiation of the toxicities of silver nanoparticles and silver ions to the Japanese medaka (Oryzias latipes) and the cladoceran Daphnia magna. Nanotoxicology 5:208–14
- Kittler S, Greulich C, Diendorf J, Koeller M, Epple M. 2010. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–54
- Klein CL, Comero S, Stahlmecke B, Romazanov J, Kuhlbusch TAJ, Van Doren E, et al. 2011. NM-300 Silver Characterisation, Stability, Homogeneity. NM-Series of Representative Manufactured Nanomaterials. Luxembourg: European Commission Joint Research Centre, Institute for Health and Consumer Protection
- Kusk K, Nyholm N. 1992. Toxic effects of chlorinated organic-compounds and potassium dichromate on growth-rate and photosynthesis of marine-phytoplankton. Chemosphere 25:875–86
- Lee Y, Kim J, Oh J, Bae S, Lee S, Hong IS, Kim S. 2012. Ion-release kinetics and ecotoxicity effects of silver nanoparticles. Environ Toxicol Chem 31:155–9
- Liu J, Hurt RH. 2010. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol 44:2169–75
- Liu J, Sonshine DA, Shervani S, Hurt RH. 2010. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 4:6903–13
- Manier N, Bado-Nilles A, Delalain P, Aguerre-Chariol O, Pandard P. 2013. Ecotoxicity of non-aged and aged CeO2 nanomaterials towards freshwater microalgae. Environ Pollut 180:63–70
- Mayer P, Cuhel R, Nyholm N. 1997. A simple in vitro fluorescence method for biomass measurements in algal growth inhibition tests. Water Res 31:2525–31
- Miao A, Luo Z, Chen C, Chin W, Santschi PH, Quigg A. 2010. Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS One 5:e15196
- Miao A, Schwehr KA, Xu C, Zhang S, Luo Z, Quigg A, Santschi PH. 2009. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ Pollut 157:3034–41
- Misra SK, Dybowska A, Berhanu D, Luoma SN, Valsami-Jones E. 2012. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ 438:225–32
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. 2008. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42:8959–64
- Newton KM, Puppala HL, Kitchens CL, Colvin VL, Klaine SJ. 2013. Silver nanoparticle toxicity to Daphnia magna is a function of dissolved silver concentration. Environ Toxicol Chem 32:2356–64
- OECD. 2000. Guidance Document on the Aquatic Toxicity Testing of Difficult Substances and Mixtures. Organisation for Economic Co-operation and Development. OECD Environmental Health and Safty Publications, Series on testing and assessment No. 23. Paris: Organisation for Economic Co-operation and Development
- Oukarroum A, Bras S, Perreault F, Popovic R. 2012. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol Environ Saf 78:80–5
- Petersen S, Kusk K. 2000. Photosynthesis tests as an alternative to growth tests for hazard assessment of toxicant. Arch Environ Contam Toxicol 38:152–7
- Pettitt ME, Lead JR. 2013. Minimum physicochemical characterisation requirements for nanomaterial regulation. Environ Int 52:41–50
- Piccapietra F, Allue CG, Sigg L, Behra R. 2012. Intracellular silver accumulation in Chlamydomonas reinhardtii upon exposure to carbonate coated silver nanoparticles and silver nitrate. Environ Sci Technol 46:7390–7
- Ratte H. 1999. Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem 18:89–108
- Reidy B, Haase A, Luch A, Dawson KA, Lynch I. 2013. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials 6:2295–350
- Rejeski D. 2009. Nanotechnology and consumer products. Available at: http://www.nanotechproject.org/process/assets/files/8278/pen_submission_cpsc.pdf. Accessed on 18 September 2013
- Roemer I, White TA, Baalousha M, Chipman K, Viant MR, Lead JR. 2011. Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests. J Chromatogr A 1218:4226–33
- Rosenkrantz RT. 2013. Influence of test conditions and exposure duration on the result of ecotoxicological tests [PhD thesis]. Kgs. Lyngby, Denmark: DTU Environment, Technical University of Denmark
- SCENIHR. 2014. Nanosilver: Safety, Health and Environmental Effects and Role in Antimicrobial Resistance. Scientific Committee on Emerging and Newly Identified Health Risks. Luxembourg: European Commission, SANCO, Health & Consumers – Directorate C: Public Health
- Stensberg MC, Madangopal R, Yale G, Wei Q, Ochoa-Acuna H, Wei A, et al. 2014. Silver nanoparticle-specific mitotoxicity in Daphnia magna. Nanotoxicology 8:833–42
- Tejamaya M, Roemer I, Merrifield RC, Lead JR. 2012. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol 46:7011–17
- Turner A, Brice D, Brown MT. 2012. Interactions of silver nanoparticles with the marine macroalga, Ulva lactuca. Ecotoxicology 21:148–54
- Unrine JM, Colman BP, Bone AJ, Gondikas AP, Matson CW. 2012. Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles. Part 1. Aggregation and dissolution. Environ Sci Technol 46:6915–24
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, et al. 2009. Nano-silver – a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3:109–38
- Zhang W, Yao Y, Sullivan N, Chen Y. 2011. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ Sci Technol 45:4422–8
- Zhao C, Wang W. 2012. Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 6:361–70

