Abstract
Background and purpose Metal ion toxicity both locally and systemically following MoM hip replacements remains a concern. Cobalt ions have been shown to induce secretion of proinflammatory chemokines locally; however, little is known about their effect systemically. We investigated the in vitro effect of cobalt ions on a variety of cell lines by measuring production of the proinflammatory chemokines IL-8 and MCP-1.
Method Renal, gastrointestinal, and respiratory epithelium and also neutrophils and monocytes were exposed to cobalt ions at 4, 12, 24, and 48 hours.
Results We found that cobalt ions enhanced the secretion of IL-8 and MCP-1 in renal epithelial cells, gastric and colon epithelium, monocytes and neutrophils, and small airway epithelial cells but not in alveolar cells. Secretion of IL-8 and MCP-1 was markedly elevated in renal epithelium, where a 16-fold and 7-fold increase occurred compared to controls. There was a 6-fold and 4-fold increase in IL-8 and MCP-1 secretion in colon epithelium and a 4-fold and 3-fold increase in gastric epithelium. Small airway epithelial cells showed a maximum increase in secretion of 8-fold (IL-8) and of 4-fold (MCP-1). The increase in chemokine secretion observed in alveolar cells was moderate and did not reach statistical significance. Monocytes and neutrophils showed a 2.5-fold and 2-fold increase in IL-8 secretion and a 6-fold and 4-fold increase in MCP-1 secretion at 48 and 24 hours, respectively.
Interpretation These data demonstrate the potent bioactivity of cobalt ions in a variety of cell types and the potential to induce a proinflammatory response.
In an attempt to reduce polyethylene-induced osteolysis and improve implant longevity, there has been renewed interest in the use of metal-on-metal (MoM) arthroplasty. The improved wear performance profiles result in substantially less wear debris and subsequent periprosthetic osteolysis (Glant et al. Citation1993, Shanbhag et al. Citation1995, Yao et al. Citation1995). As a result, many MoM implants have been shown to last over 2 decades, or are still functioning in patients who received the implant at a young age (Schmalzried et al. Citation1996). MoM bearings are now commonly used in younger patients where implant longevity is particularly important (Haynes et al. Citation1993). However, metal ion toxicity—both locally in the periprosthetic space and systemically—remains a concern (Horowitz et al. Citation1996, Haynes et al. Citation1997, Fritz et al. Citation2005, Citation2006). It has been well established that substantial levels of metallic products can be transferred into the host environment from implanted metallic devices (Coleman et al. Citation1973, Merritt and Brown Citation1993). Cobalt-chrome-molybdenum is the alloy used for MoM bearings, due to its wear performance profile. Cobalt and chromium ions are produced as a result of the MoM articulation (Cobb et al. Citation2006). In this study, we have focused on the effect of cobalt ions, as we have previously demonstrated a greater level of toxicity of these ions compared to chromium ions when in contact with primary human osteoblasts (Queally and Devitt et al. Citation2009).
Local effects of cobalt ions in the periprosthetic tissue include cytotoxicity, and these ions have been demonstrated to induce apoptosis, necrosis, and chemokine secretion (interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1)) and tumor necrosis factor-α secretion in both macrophages and osteoblasts (Merrit et al. Citation1993, Catelas et al. Citation2001, Citation2003, Citation2005, Huk et al. Citation2004, MacDonald Citation2004, Petit et al. Citation2004, Fleury et al. Citation2006, Keegan et al. Citation2007, Queally and Devitt et al. Citation2009). In comparison, the systemic effect of metal ions is relatively unknown. Cobalt ions can be generated in the periprosthetic space due to electrochemical corrosion of metal particles or possibly as a result of phagocytosis of CoCrMo particles by cells and exposure of these particles to a series of oxidative mechanisms designed to destroy the foreign body (Galle et al. Citation1992, Lundborg et al. Citation1992, Case Citation2001). The ions then bind to serum proteins (mainly albumin) and are transported systemically before being excreted in the urine (Catelas et al. Citation2001, Willert et al. Citation2005, Rasquinha et al. Citation2006, Grubl et al. 2007). Elevated serum levels of cobalt ions have been demonstrated, but the clinical implications of this have not been fully elucidated yet (Catelas et al. Citation2003, Petit et al. Citation2004).
The cells chosen for our experiments are representative of 3 main organ systems that can be susceptible to chronic inflammation: renal, gastrointestinal, and respiratory. Cobalt ions are excreted through the renal and gastrointestinal systems (Cobb and Schmalzreid Citation2006). In the distal tubule of the kidney, proximal tubule epithelial cells are in direct contact with cobalt ions during excretion. The gastrointestinal epithelial cells are avascular but are in intimate contact with the gastric submucosa and colon submucosa, which possess arterial and venus plexuses that supply and drain the vessels of the mucosa, respectively. The diffuse blood supply within this layer enables the passage of ions to the mucosa. The respiratory system was also investigated, as previous studies have shown an increased incidence of asthma and inflammatory conditions following occupational exposure to cobalt (Nemery Citation1990). The respiratory system can be divided into two major parts, a conducting portion and a respiratory portion. For the purpose of our experiments, two varieties of cells representative of each portion were chosen.
Neutrophils and monocytes represent the first line of defense against acute inflammatory processes. As the cobalt ions are in intimate contact with these constituents of blood during their transit through the vascular system, we also wanted to examine the effect that these ions have on monocytes and neutrophils.
The aim of our study was to investigate the in vitro effect of cobalt ions on a variety of human cell lines representing potential systemic targets of cobalt ion toxicity. The primary aim was to examine the inflammatory response elicited by cobalt ions when exposed to these cells. To achieve this, we examined IL-8 and MCP-1, two immediate-early stress response chemokines that function to attract neutrophils and monocytes respectively. These chemokines initiate the progression of an inflammatory response, which is governed to a large extent by the continued availability of infiltrating leukocytes that are recruited to the site of inflammatory injury. Previous studies have shown that cobalt ions lead to increased chemokine secretion in primary human osteoblasts with the potential to induce osteolysis by recruiting inflammatory leukocytes (Queally and Devitt et al. Citation2009). We hypothesized that cobalt ions have a similar pro-inflammatory effect on other systemic cell lines, thus potentially inducing an inflammatory condition or exacerbate an existing one. Specifically, we investigated the production of proinflammatory chemokines (IL-8 and MCP-1) by these cell types in response to cobalt ion exposure.
Materials and methods
Cell culture and metal ion exposure ()
A variety of cell lines were chosen to represent different organ systems. The cells were cultured as monolayers in their respective recommended media, in 6-well plates. All experiments were carried out once the cells had reached 80–90% confluence and at their optimal passage number. The cells were stimulated with 10 parts per million (ppm) cobalt ions (Co²+) (CoCl2; Sigma-Aldrich) in growth medium (). This concentration has been used in vitro by other authors in assessing the effect of cobalt ions on macrophages (Catelas et al. Citation2001, Citation2005, Petit et al. Citation2004, Huk et al. Citation2004, Fleury et al. Citation2006). Each experiment was carried out in triplicate. Supernatants were collected at 4, 12, 24, and 48 h. The medium alone without Co2+ was used as a negative control for each cell line.
Table 1. Cell cultures and culture medium used
Epithelial cells of the renal proximal tubule
Human Kidney-2 (HK-2) (American Type Culture Collection (ATCC) CRL-2190) cells are an immortalized proximal tubule epithelial cell line derived from normal adult kidney, thus providing an ideal means to assess the mechanism of proximal tubule cell physiology and pathophysiology (Ryan et al. Citation1994).
Epithelial cells of the gastrointestinal tract
The in vitro cells used to represent gastric epithelium were the AGS cell line (ATCC CRL-1739), which is derived from gastric adenocarcinoma (Barranco et al. Citation1983). In addition, T84 cells derived from colonic carcinoma were used. These cells have been found to show very similar physiological characteristics to those of normal colon epithelium, both morphologically and functionally (Dharmsathaphorn et al. Citation1984).
Epithelial cells of the respiratory tract
Two kinds of cells were used, each one representative of each portion of the respiratory tract: human small airway epithelial cells (representing the conducting portion) and alveolar epithelial cells (representing the respiratory portion).
Human small airway epithelial cells (ScienCell 3230) are located at the interface between the alveoli and the conducting airways. Airway epithelial cells, which form a continuous lining of the airways, have a unique role as a protective physical and functional barrier to deleterious external agents. These cells are ideal cultures for experimental applications in asthma, inhalation toxicology, and pulmonary inflammatory responses (Crapo et al. Citation1983).
A549 cells (ATCC CCL-185) are human alveolar basal epithelial cells. A549 cells fall under the squamous subdivision of epithelial cells, which are associated with the diffusion of water, electrolytes, and other substances. The cell line was derived from a lung carcinoma established from an explanted lung tumor (Giard et al. Citation1973).
Human neutrophils and monocytes
Circulating neutrophils and monocytes were extracted from the whole blood of healthy donors by Histopaque density centrifugation as previously described (Boyum Citation1968). Whereas monocytes were stimulated with cobalt ions (10 ppm) at time points up to 48 h, neutrophils were only exposed for up to 24 h due to their shorter half-life.
Preparation and analysis of secreted protein
Enzyme-linked immunosorbent assays (ELISAs) for the detection of “free” IL-8 and MCP-1 were carried out on the supernatant samples according to the manufacturer's protocol (Promocell).
Statistics
For studies on chemokine response, the data shown are from 1 of 3 experiments, all of which yielded similar results. For any given experiment, each data point represents the mean + SD of 6 individual cultures. Data were analyzed by one-way ANOVA using Analyze-it software. Only p-values ≤ 0.05 were considered significant.
Results
The results of chemokine secretion by the different cell lines in response to treatment with cobalt ions are summarized in .
Table 2. Apprixomate fold increase in chemokine secretion relative to contrtol levels
Cobalt ions stimulated IL-8 and MCP-1 secretion in renal epithelial cells
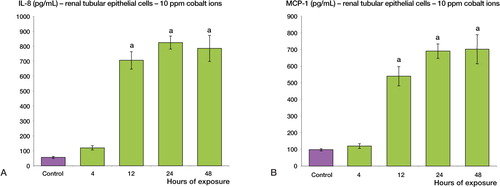
Following 12, 24, and 48 h of treatment with cobalt ions (at 10 ppm), renal tubular epithelial cells showed enhanced secretion of both IL-8 and MCP-1 relative to the control sample (p < 0.001) (). A 16-fold maximal increase was observed for IL-8 secretion, whereas the maximal increase for MCP-1 secretion was 7-fold.
Figure 1. Cobalt ions (10ppm) induce enhanced secretion of IL-8 and MCP-1 chemokines in human renal tubular epithelial cells (HK-2) at 12, 24 and 48 h. Renal tubule epithelial cells were treated with 10 ppm cobalt ions for 4, 12, 24 and 48h along with a negative control. A: There is a significant increase in the secretion of IL-8 protein post exposure at 12, 24 and 48 h relative to the control sample (a = p < 0.001). B: There is also a significant increase in the secretion of MCP-1 protein at 12, 24, and 48h compared to negative control (a = p < 0.001).
Cobalt ions stimulated secretion of IL-8 and MCP-1 in gastrointestinal epithelial cells
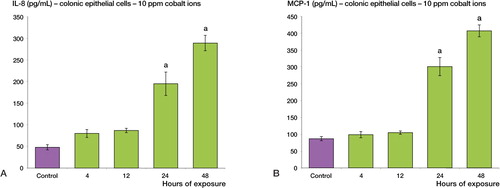
Following treatment with cobalt ions (10 ppm), gastric epithelial cells showed enhanced secretion of both IL-8 and MCP-1 relative to the control sample (p < 0.001) (). A 4-fold maximal increase was observed for IL-8 secretion and a 3-fold maximal increase was observed for MCP-1 secretion. A similar increase in chemokine secretion was observed in colon epithelial cells. Following 24 and 48 h of treatment with cobalt ions (10 ppm), colon epithelial cells showed enhanced secretion of both IL-8 and MCP-1 relative to the control sample (p < 0.001) (). A 6-fold maximal increase was observed for IL-8, whereas the maximal increase in MCP-1 secretion was 4-fold.
Figure 2. Cobalt ions (10ppm) induce enhanced secretion of IL-8 and MCP-1 chemokines in human gastric epithelial cells (AGS). Gastric epithelial cells were treated with 10 ppm cobalt ions for 4, 12, 24 and 48h along with a negative control. A: There is a significant increase in the secretion of IL-8 protein post exposure at 24 and 48h relative to the control sample (a = p < 0.001). B: There is also a significant increase in the secretion of MCP-1 protein at 12, 24, and 48h compared to negative control (a = p < 0.001).
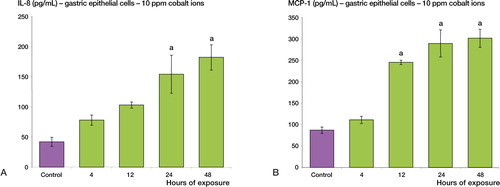
Figure 3. Cobalt ions (10ppm) induce enhanced secretion of IL-8 and MCP-1 chemokines in human colonic epithelial cells (T-84). Colonic epithelial cells were treated with 10 ppm cobalt ions for 4, 12, 24 and 48h along with a negative control. A: There is a significant increase in the secretion of IL-8 protein post exposure at 24 and 48h relative to the control sample (a = p < 0.001). B: There is also a significant increase in the secretion of MCP-1 protein at 24 and 48h compared to negative control (a = p < 0.001).
Cobalt ions stimulated secretion of IL-8 and MCP-1 in small airway epithelial cells but not in alveolar epithelial cells
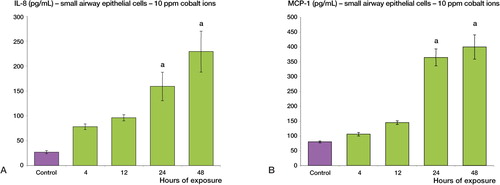
Following 24 and 48 h of treatment with cobalt ions (10 ppm), maximal increases of 8- and 4-fold were observed in the secretion of IL-8 and MCP-1, respectively, by small airway epithelial cells (p < 0.001) (). An increase in chemokine secretion was observed in alveolar epithelial cells but this increase was not statistically significant (p = 0.06) (Figure 5, supplementary data).
Figure 4. Cobalt ions (10ppm) induce enhanced secretion of IL-8 and MCP-1 chemokines in human small airway epithelial cells. Human small airway epithelial cells were treated with 10 ppm cobalt ions for 4, 12, 24 and 48h along with a negative control. A: There is a significant increase in the secretion of IL-8 protein post exposure at 24 and 48h relative to the control sample (a = p < 0.001). B: There is also a significant increase in the secretion of MCP-1 protein at 12, 24 and 48h compared to negative control (a = p < 0.001).
Cobalt ions stimulated secretion of IL-8 and MCP-1 in neutrophils and monocytes
Following treatment with cobalt ions (10 ppm), neutrophils showed enhanced secretion of both IL-8 and MCP-1 relative to the control sample (p < 0.001) (Figure 6, supplementary data). A 2-fold maximal increase was observed for IL-8 whereas the maximal increase in MCP-1 secretion was 4-fold. A similar increase in chemokine secretion was observed in monocytes. Following treatment with cobalt ions (10 ppm), monocytes showed enhanced secretion of both IL-8 and MCP-1 relative to the control sample (p < 0.001) (Figure 7, supplementary data). A 2.5-fold maximal increase was observed for IL-8 whereas the maximal increase in MCP-1 secretion was 6-fold.
Discussion
Metal-on-metal articulations consist of a cobalt-chromium-molybdenum alloy and are known to release wear particles and corrosion products into the periprosthetic tissue; they are also transported from the joint and distributed systemically (Coleman et al. Citation1973, Urban et al. Citation2000, Jacobs et al. Citation2004). There is a measurable increase in cobalt ion levels in the serum, erythrocytes, and urine of patients with MoM bearings (Coleman et al. Citation1973, Jacobs et al. Citation1998, Savarion et al. 2002, Brodner et al. Citation2003, MacDonald et al. Citation2003, Rasquinha et al. Citation2006).
The potential effects of elevated levels of metal ions are poorly defined. A number of adverse biological reactions have been linked to the dissemination of metal ions, including soft tissue toxicity, hypersensitivity reactions, bone loss, and risk of carcinogenesis (Gillespie et al. Citation1996, Shimmin et al. Citation2005, Willert et al. Citation2005, Keegan et al. Citation2007, Lidgren Citation2008). In vitro studies have clearly demonstrated that cobalt particles can be highly cytotoxic when produced in high enough concentrations (Merritt and Brown Citation1993). There is also much concern about the effect of long-term exposure and the potential for chromosomal aberrations in the patient, as well as the risk of passing chromosomal abnormalities to the next generation (Case et al. Citation1996, Doherty et al. Citation2001, Ladon et al. Citation2004, Bordner et al. 2004a, Papageorgiou et al. Citation2007, Ziaee et al. Citation2007).
The inflammatory response to a foreign material is of particular relevance. This is the area on which we have focused our research. The response to a foreign material involves a cascade of events. The typical inflammatory response is marked by the accumulation of polymorphonuclear leukocytes at the implant site. We have previously demonstrated that osteoblasts play an integral role in the initiation of this process by secreting proinflammatory chemotactic cytokines, which are sufficient to induce the migration of monocytes and neutrophils (Queally and Devitt et al. Citation2009). However, metal ions are not restricted to the periprosthetic space and they have direct access to other tissues as result of their movement through the blood. In this study, we have investigated the specific chemokine responses in a variety of human cell lines that represent potential systemic targets of cobalt ion toxicity, following exposure to cobalt ions (Firriolo et al. Citation1999, Urban et al. Citation2000, IARC Monographs) Chemokines are the major mediators of chemotactic signaling in the recruitment and activation of inflammatory cells (Miller et al. Citation1992, Taub et al. Citation1994). IL-8 and MCP-1 are immediate-early stress response chemokines that function to attract neutrophils and monocytes, respectively.
Unlike most organic chemicals, metals cannot be eliminated from tissues by metabolic degradation. Thus, they can only be eliminated from tissues by renal or gastrointestinal excretion (Cobb and Schmalzreid Citation2006). It is widely regarded, although without evidence-based research, that MoM bearing surfaces are contraindicated in patients with chronic renal failure. In such cases, there is a potential for elevated cobalt levels within the circulation as a result of reduced excretion (Brodner et al. Citation2000). However, of equal concern is the effect that cobalt ions have on renal tissue and whether this effect contributes to renal failure in itself. In light of this, we explored the effect of exposure of proximal tubule epithelial cells to cobalt ions. In this study, we have demonstrated for the first time that cobalt ions enhance protein secretion of IL-8 and MCP-1 in renal proximal tubule epithelial cells. The enhanced secretion was substantial, representing a 16-fold and 7-fold increase, respectively, compared to control levels.
Proinflammatory mediators recruit neutrophils and monocytes to the site of insult, which drive the inflammatory response. In the setting of chronic renal failure, the added insult of a further inflammatory stimulus may be sufficient to tip the balance into a vicious circle of reduced excretion and increased circulating levels of cobalt ions. The effect may also be compounded by the fact that neutrophils and monocytes, having been recruited, also give rise to enhanced IL-8 and MCP-1 secretion following exposure to cobalt ions (10 ppm). Whether this is sufficient to induce full renal failure remains to be seen, but it is certainly an area of concern and warrants further research.
Urban et al. have previously demonstrated the dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with a hip or knee replacement following retrieval studies (Urban et al. Citation2000). The gastrointestinal tract is intimately related to these three routes of dissemination, via the portal venous system and lymphatic drainage (Strandring 2008). In the present study, elevated secretion of these proinflammatory chemokines was seen in gastric and colon epithelium. Gastric epithelium showed a 4-fold and 3-fold maximal enhanced secretion of IL-8 and MCP-1, respectively, while colon epithelium showed a maximal 6-fold increase in IL-8 secretion and a 4-fold increase in MCP-1 secretion. It is difficult to assess the importance of this enhanced secretion from gastrointestinal epithelium. A link may perhaps be drawn between this and another chronic relapsing inflammatory condition that can occur in the gastrointestinal tract, inflammatory bowel disease. This disease is characterized by continuous infiltration of affected tissue by inflammatory cells from the circulation. This influx results in further tissue-destructive inflammatory processes. The recruitment and activation of immunocytes is mediated by chemokines, including IL-8 and MCP-1 (Uguccioni et al. Citation1999, McCormack et al. Citation2001). Chemokine secretion is low or non-existent in resting cells but rapidly becomes upregulated during inflammation (Luster Citation1998). The potential therefore exists for quiescent inflammatory bowel disease to become reactivated as a result of an inflammatory insult, thus perpetuating the disease process (McCormack et al. Citation2001). Is it then possible that exposure to high levels of cobalt ions in an individual with inflammatory bowel disease may precipitate a relapse of the inflammatory condition. This is simply a theory, and to date there have been no case reports to corroborate this. It is certainly something to consider in a young patient with inflammatory bowel disease requiring a total hip replacement, perhaps as a result of steroid-induced avascular necrosis of the hip (Klingenstein et al. Citation2005).
Frequent contact with inhaled cobalt from occupational exposure has been shown to cause an increased incidence of asthma and chronic inflammatory pulmonary conditions, either from the particles themselves or from solubilized cobalt ions (Nemery Citation1990, IARC Monographs). The toxic responses of the respiratory system in metal workers are largely related to inhalation exposure, and are therefore difficult to extrapolate to the vascular route. The respiratory system can be differentiated into two major parts: a conducting portion, consisting of structures that deliver air to the lungs, and a respiratory portion, consisting of structures within the lungs where oxygen is exchanged for carbon dioxide in the blood. The respiratory system has lining epithelia, supporting structures, glands, and other features that are characteristic of each part. The blood flow to the intraparenchymal airways is predominately from the bronchial circulation (Bernard et al. Citation1996). As a result, cobalt ions bound to proteins have the potential to come in contact with respiratory epithelium through systemic transmission. Our experiments demonstrate that exposure of small airway epithelial cells to cobalt ions can give rise to an 8-fold increase in IL-8 secretion and a 4-fold increase in MCP-1 secretion. Regarding human alveolar epithelial cells, roughly a 2-fold (maximal) increase in both IL-8 and MCP-1 secretion was observed, but this did not reach statistical significance. These results suggest that the conduction portion of the respiratory system induces an inflammatory response following exposure to cobalt ions while the respiratory portion remains relatively quiescent. Once again, the significance of these data in the clinical setting is unknown and to date there have been no case reports documenting any deterioration in chronic pulmonary conditions following MoM hip replacement. Further work is required to determine the long-term implications of elevated cobalt ion levels in patients with pulmonary disease.
Finally, we explored the effect of exposure of monocytes and neutrophils found within the bloodstream to cobalt ions. We found that monocytes have a rapid response to cobalt ions, inducing the secretion of IL-8 and MCP-1 at 4, 12, 24, and 48 h. Likewise, neutrophils showed a significant increase in secretion of these cytokines. These cells are found both in the circulation and deposited in tissues. They appear to have dual roles as responders to inflammation and recruiters of further leukocytes once stimulated by cobalt ions, thereby perpetuating the inflammatory cascade.
Metallic debris may exist as particles, in ionic form, or as inorganic salts. In this study, we used cobalt ions at a concentration of 10 ppm. The exact concentration of these ions in the periprosthetic space and systemically remains unclear, as there has been variation in study design, in the sources of samples (serum, whole blood, erythrocytes, and urine), and in the method of laboratory analysis (Catelas et al. Citation2005). This concentration has been used in vitro by other authors in assessing the effect of cobalt ions on macrophages (Catelas et al. Citation2001, Citation2005, Huk et al. Citation2004, Petit et al. Citation2004, Fleury et al. Citation2006). Serum blood levels are less than 10 ppm; however, these higher levels may be seen in patients with “runaway” wear (see below) or in the setting of renal failure (Hur et al. Citation2008, Lee et al. Citation2008). There is recent evidence to suggest that cobalt levels are influenced by factors such as the type, design, and positioning of the implant (Savarino et al. Citation2002, Brodner et al. Citation2003, Back et al. Citation2005a). The position of the implant, which is influenced by the skill of the surgeon, plays a major role in the degree of metal ion release. Brodner reported a 10- to 53-fold increase in cobalt levels in patients with an acetabular cup inclination of between 58° and 63° (Brodner et al. Citation2004b). The classical “run-in” period is well recognized with MoM prostheses, as peak serum levels of cobalt show a 10-fold increase at six months relative to the preoperative levels (Back et al. Citation2005b, Daniel et al. Citation2007). These peaks are followed by a gradual decline over the next 15 months as the bearing surface enters “steady-state” wear. Bowsher et al. (Citation2009) have also described the concept of “runaway” wear, which is a puzzling phenomenon that can result in a 2- to 19-fold increase in wear in prostheses compared to identical bearings (Bowsher et al. Citation2009).
In summary, the data presented here demonstrate the potent bioactivity of cobalt ions in a variety of systemic cell lines. This process is most likely representative of an overall toxic effect of high-dose exposure to cobalt ions. The significance of these findings has particular relevance in the context of patients undergoing hip replacement surgery with pre-existing renal failure, where there is a greater degree of exposure to high levels of cobalt ions due to reduced excretion. It may also be important in cases where the hip implant cup inclination is placed in excessive abduction or where “runaway” wear is experienced. In addition, due consideration should be given to the choice of bearing surface in patients with inflammatory bowel disease due to the possibility of triggering a relapse.
www.actaorthop.org
Download PDF (522.5 KB)BMD: author and primary investigator. JMQ: collation of data. MV: statistical analysis. JSB: cell culture. DM: study design. PPD: chief scientific supervisor. JMO'B: senior author.
Funding for this research was provided by the Cappagh Trust, Cappagh National Orthopaedic Hospital, Finglas, Dublin, Ireland.
There are no financial or professional affiliations between any of the authors and any third party that may have biased the content of this article.
- Back DL, Dalziel R, Young D, Shimmin AJ. Early results of primary Birmingham hip resurfacings. An independent prospective study of the first 230 hips. J Bone Joint Surg (Br) 2005a; 87:324-9.
- Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop 2005b; (438): 177-81.
- Barranco SC, Townsend CM Jr, Casartelli C, Macik BG, Burger NL, Boerwinkle WR, Gourley WK. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res 1983; 43(4):1703-9.
- Bernard SL, Glenny RW, Polissar NL, Luchtel DL, Lakshminarayan S. Distribution of pulmonary and bronchial blood supply to airways measured by fluorescent microspheres. J Appl Phys 1996; 80(2):430-6.
- Bowsher JG, Clarke IC, William PA, Donaldson TK. What is “normal” wear pattern for metal-on-metal hip bearings? J Biomed Mater Res B Appl Biomater 2009; 91 (1): 297-308.
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1g. Scand J Clin Lab Invest (Suppl) 1968; 97; 77-89.
- Brodner W, Grohs JG, Bitzan P, Meisinger V, Kovarik J, Kotz R. Serum cobalt and serum chromium level in 2 patients with chronic renal failure after total hip prosthesis implantation with metal-metal gliding contact. Z Orthop Ihre Grenzgeb 2000;138 (5): 425-9.
- Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplsty. J Bone Joint Surg (Am) 2003: 85: 2168-73.
- Brodner W, Grohs JG, Bancher-Todesca D, Dorotka R, Meisinger V, Gottsauner-Wolf F, Kotz R. Does the placenta inhibit the passages of chromium and cobalt after metal-on-metal total hip arthroplasty? J Arthroplasty 2004a; 19:102-6.
- Brodner W, Grubl A, Jankovsky R, Meisinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty (Suppl 3) 2004b; 19(8):66-70.
- Case CP. Chromosomal changes after surgery for joint replacement. J Bone Joint Surg (Br) 2001; 83(8):1093-5.
- Case CP, Langkamer VG, Howell RT, Webb J, Standen G, Palmer M, Kemp A, Learmonth ID. Preliminary observations on possible premalignant changes in bone marrow adjacent to worn total hip arthroplasty implants. Clin Orthop 1996; (329):269-79.
- Catelas I, Petit A, Zukor DJ, Huk OL. Cytotoxic and apoptotic effects of cobalt and chromium ions on J774 macrophages - Implication of caspase-3 in the apoptotic pathway. J Mater Sci Mater Med 2001; 12(10-12):949-53.
- Catelas I, Petit A, Zukor DJ, Antoniou J, Huk OL. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials 2003; 24(3):383-91.
- Catelas I, Petit A, Vali H, Fragiskatos C, Meilleur R, Zukor DJ, Antoniou J, Huk OL. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials 2005; 26(15):2441-53.
- Cobb AG, Schmalzreid TP. The clinical significance of metal ion release from cobalt-chromium metal-on-metal hip joint arthroplasty. Proc Inst Mech Eng [H] 2006; 220:385-98.
- Coleman RF, Herrington J, Scales JT. Concentration of wear products in hair, blood, and urine after total hip replacement. Br Med J 1973; 1:527-9.
- Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO. Morphometric characteristics of cells in the alveolar region of mammalian lungs. Am Rev Respir Dis 1983; 128:42-6.
- Daniel J, Ziaee H, Pynsent PB, McMinn DJW. The validity of serum levels of a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg (Br) 2007; 89:736-41.
- Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol 1984; 246:G204-8.
- Doherty AT, Howell RT, Ellis LA, Bisbinas I, Learmonth ID, Newson R, Case CP. Increased chromosome translocations and aneuploidy in peripheral blood lymphocytes of patients having revision arthroplasty of the hip. J Bone Joint Surg (Br) 2001; 83:1075-81.
- Firriolo JM, Ayala-Fierro F, Sipes IG, Carter DE. Absorption and disposition of cobalt naphthenate in rats after a single oral dose. J Tox Environ Health 1999: 58 (6): 383-95.
- Fleury C, Petit A, Mwale F, Antoniou J, Zukor DJ, Tabrizian M, Huk OL. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: morphology, cytotoxicity, and oxidative stress. Biomaterials 2006; 27(18):3351-60.
- Fritz EA, Jacobs JJ, Glant TT, Roebuck KA. Chemokine IL-8 induction by particulate wear debris in osteoblasts is mediated by NF-kappaB. J Orthop Res 2005; 23(6):1249-57.
- Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Chemokine gene activation in human bone marrow-derived osteoblasts following exposure to particulate wear debris. J Biomed Mater Res A 2006; 77A:192-201.
- Galle P, Berry JP, Galle C. Role of alveolar macrophages in precipitation of mineral elements inhaled as soluble aerosols. Environ Health Perspect 1992; 97; 145-7.
- Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumours. J Natl Cancer Inst 1973; 51:1417-23.
- Gillespie WJ, Henry DA, O'Connell DL, Kendrick S, Juszczak E, McInneny K, Derby L. Development of hematopoietic cancers after implantation of total joint replacement. Clin Orthop 1996: (329): 290-6.
- Glant TT, Jacobs JJ, Molnar G, Shanbhag AS, Valyon M, Galante JO. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res 1993; 8:1071-9.
- Grübl A, Marker M, Brodner W, Giurea A, Heinze G, Meisinger V, Zehetgruber H, Kotz R. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res 2007; 25(7):841-8.
- Haynes DR, Rogers SD, Hay SJ, Pearcy MJ, Howie DW. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium alloy wear particles. J Bone Joint Surg (Am) 1993; 75:825-34.
- Haynes DR, Hay SJ, Rogers SD, Ohta S, Howie DW, Graves SE. Regulation of bone cells by particle-activated mononuclear phagocytes. J Bone Joint Surg (Br) 1997; 79:988-94.
- Horowitz SM, Gonzales JB. Inflammatory response to implant particulates in a macrophage/osteoblast coculture model. Calcif Tissue Int 1996; 332:223-31.
- Huk OL, Catelas I, Mwale F, Antoniou J, Zukor DJ, Petit A. Induction of apoptosis and necrosis by metal ions in vitro. J Arthroplasty (Suppl 3) 2004; 19(8):84-7.
- Hur CI, Yoon TR, Cho SG, Song EK, Seon JK. Serum ion level after metal-on-metal THA in patients with failure. Clin Orthop 2008; (466):696-9.
- IARC Monographs on the evaluation of carcinogenic risks to humans. Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. 2006; 86: 131-2. ISBN 928321286X.
- Jacobs JJ, Skipor AK, Patterson LM, Hallab NJ, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled longitudinal study. J Bone Joint Surg (Am) 1998; 80:1447-58.
- Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty 2004; 19 (8): 59-65.
- Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J Bone Joint Surg (Br) 2007; 89:567-73.
- Klingenstein G, Levy RN, Kornbluth A, Shah AK, Present DH. Inflammatory disease related osteonecrosis; a report of a large series with a review of the literature. Aliment Pharmacol Ther 2005; 21(3):243-9.
- Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty 2004; 19:78-83.
- Lee R, Essner A, Wang A. Tribological considerations in primary and revision Metal-on-Metal arthroplasty. J Bone Joint Surg (Am) 2008: 90: 118-24.
- Lidgren L. Chronic inflammation, joint replacement and malignant lymphoma. J Bone Joint Surg (Br) 2008; 90:7-10.
- Lundborg M, Falk R, Johansson A, Kreyling W, Camner P. Phagolysosomal pH and dissolution of cobalt oxide particles by alveolar macrophages. Environ Health Perspect 1992; 97:153-7.
- Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. NEJM 1998; 338:436-45.
- MacDonald SJ. Metal-on-metal total hip arthroplasty: the concerns. Clin Orthop 2004; (429):86-93.
- MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop 2003: (406): 282-96.
- McCormack G, Moriarty D, O'Donoghue DP, McCormick PA, Sheahan K, Baird AW. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res 2001; 50:491-5.
- Merritt K, Brown SA. Effects of metal particles and ions on the biological system. Techniques Orthop 1993; 8:228-36.
- Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol 1992; 12:17-46.
- Nemery B. Metal toxicity and the respiratory tract. Eur Respir J 1990; 3:202-19.
- Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 2007; 28:2946-58.
- Petit A, Mwale F, Zukor DJ, Catelas I, Antoniou J, Huk OL. Effect of cobalt and chromium ions on bcl-2, bax, caspase-3, and caspase-8 expression in human U937 macrophages. Biomaterials 2004; 25(11):2013-8.
- Queally JM, Devitt BM, Butler JS, Malizia AP, Murray D, Doran PP, O'Byrne JM. Cobalt ions induce chemokine secretion in primary human osteoblasts. J Orthop Res 2009; 27(7):855-64.
- Rasquinha VJ, Ranawat CS, Weiskopf J, Rodriguez JA, Skipor AK, Jacobs JJ. Serum metal levels and bearing surfaces in total hip arthroplasty. J Arthroplasty 2006; 21:47-52.
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult kidney. Kidney Int 1994; 45(1):48-57.
- Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: a comparison with metal-on-polyethylene bearings. J Biomed Mater Res 2002; 63:467-74.
- Schmalzried TP, Peters PC, Maurer BT, Bragdon CR, Harris WH. Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces. J Arthroplasty 1996; 11(3):322-31.
- Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Human monocyte response to particulate biomaterials generated in vivo and in vitro. J Orthop Res 1995; 13:792-801.
- Shimmin AJ, Back D. Femoral neck fractures following Birmingham hip resurfacing: a national review of 50 cases. J Bone Joint Surg (Br) 2005; 87:463-4.
- Standring S. Gray's anatomy: The anatomical basis of cinical practice. Fortieth edition. Elsevier Limited. 2008: 1141. ISBN 978-0-8089-2371-8.
- Taub DD, Oppenheim JJ. Chemokines, inflammation and the immune system. Ther Immunol 1994; 1(4):229-46.
- Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol 1999; 155(2):331-6.
- Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M, Dissemination of wear particles to the liver, spleen, an abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg (Am) 2000; 82:457-76.
- Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and hisotomorphological study. J Bone Joint Surg (Am) 2005; 87:28-36.
- Yao J, Glant TT, Lark MW, Mikecz K, Jacobs JJ, Hutchinson NI, Hoerrner LA, Kuettner KE, Galante JO. The potential role of fibroblasts in periprosthetic osteolysis: fibroblast response to titanium particles. J Bone Miner Res 1995; 10:1417-27.
- Ziaee H, Daniel J, Datta AK, Blunt S, McMinn DJ. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty: a controlled study. J Bone Joint Surg (Br) 2007; 89:301-5.
