Abstract
Background Biofilms are considered the key factor in the development of implant-related infections. However, only a few reports have dealt with the ability of organisms isolated from such infections to develop biofilms in vitro.
Methods We evaluated different phenotypic techniques (2 microtiter plate assays and confocal laser scanning microscopy (CLSM) and genotypic techniques (detection of the ica operon) related to biofilm development by clinical isolates of Staphylococcus spp.
Results All 26 strains tested (from 23 specimens) were biofilm producers. Stepanovic test detected biofilm formation in 85% of the strains, microtiter plate assay in 65%, and CLSM in 39%. The ica operon was detected in 73% of all strains (all 13 S. aureus strains and 6 of the 13 coagulase-negative Staphylococcus strains). 7 ica-negative strains were biofilm-positive by phenotypic methods.
Interpretation The detection of ica genes could not be related to the phenotypic ability of the strains to develop a biofilm in vitro, so both studies (genetic and phenotypic) are required for a better evaluation of the biofilm-producing ability of clinical strains of Staphylococcus isolated from orthopedic infections.
Prosthetic joint infection is an emerging problem due to the increase in surgical techniques that involve the use of biomaterials. It has been estimated that infection rates of orthopedic prostheses of the hip and knee range from 1% to 2%, but they can be as high as 9% for elbow prostheses (Darouiche Citation2004). The main pathogenic factor for the development of the infection is the ability of bacteria to form a biofilm. Biofilm has been defined as a multicellular community composed of prokaryotic and/or eukaryotic cells embedded in a matrix composed, at least partially, of material synthesized by the sessile cells in the community (Costerton Citation2007). Biofilm development has important consequences for the management of the patient: sessile bacteria become antibiotic-resistant, and treatment is therefore more complicated than treatment of acute bacterial infections where biofilms are not involved (Patel Citation2005). Moreover, bacteria in biofilms are more difficult to isolate, so the diagnosis of these infections requires techniques different from those used in conventional microbiology laboratories (Trampuz et al. Citation2007, Esteban et al. Citation2008). The main extracellular substance involved when staphylococci are embedded in biofilms, which has received much attention, is the polysaccharide intercellular adhesin (PIA) (Cafiso et al. Citation2004, Gara Citation2007). PIA is encoded by the genes of the ica operon (icaA, icaD, icaB, and icaC), which are negatively regulated by icaR (Arciola et al. Citation2002, Cafiso et al. Citation2004, Gara Citation2007).
Many studies on biofilm formation by clinically relevant bacteria have been performed. However, there is limited information available from studies performed on clinical isolates from prosthetic infections where several techniques have been used to evaluate biofilm formation. Here we report a study comparing phenotypic and genotypic techniques that have been developed to study biofilm formation. These were applied to clinically relevant strains of Staphylococcus spp.
Material and methods
Bacterial strains
26 clinical isolates of Staphylococcus spp. were included in the study. The strains were isolated from retrieved orthopedic prostheses from 22 consecutive patients with staphylococcal deep prosthetic joint infections at two major university hospitals (Fundación Jiménez Díaz and La Princesa, Madrid, Spain). All the patients were diagnosed as having prosthetic joint infection according to clinical criteria (Cordero-Ampuero et al. Citation2007).
The strains were isolated using a sonication protocol previously described (Esteban et al. Citation2008). The isolates were identified by conventional biochemical tests (coagulase) and commercial identification galleries (API-STAPH; bioMérieux, Marcy L'Etoile, France) and were maintained frozen at –20ºC in skimmed milk until experiments were performed. S. epidermidis ATCC strain 35984 (a biofilm-producing strain) and S. aureus strain 15981, kindly provided by Dr. Lasa (Valle et al. Citation2003), were used as biofilm-positive control strains. Sterile PBS was used as negative control.
The informed consent in our hospital includes authorization to handle samples from the patients, including implants obtained during surgery. No other approval by our Institutional Review Board was necessary because only bacterial isolates were analyzed, and neither samples nor patients were studied in this work.
Biofilm formation studied by microtiter plate assay
We performed the microtiter plate assay (MP) described by Christensen et al. (Citation1985), with modifications. A suspension equivalent to the McFarland 0.5 turbidity standard was prepared in Müller-Hinton broth (Becton Dickinson, Franklin Lakes, NJ) for each strain. Accuracy of bacterial counts in the suspension was confirmed by serial dilution in log steps. 100 μL from each bacterial suspension was inoculated onto 96-well Costar tissue culture microtiter plates (Corning). These were incubated at 37ºC for 24 h in a normal atmosphere. After incubation, the medium was removed and replaced with 100 μL sterile Müller-Hinton broth. Samples were incubated for another 24 h under the same conditions to obtain enough growth from strain P-95, which showed slow growth characteristics and almost no growth after only 24 h of incubation. The medium was then removed, and the wells were washed 3 times with sterile distilled water. 150 μL of crystal violet (Panreac, Barcelona, Spain) was added to each well and left for 45 min at room temperature. The dye was then removed, and this was followed by 5 washings with sterile distilled water. The preparations were then destained with 200 μL of 95% ethanol for 3 min. Finally, 100 μL of colored ethanol from each sample was transferred to another microtiter plate. The optical density (OD) of the ethanol-dye suspension was measured at 540 nm with an Opsys MR spectrophotometer (Dynex Technologies, Richfield, MN). The ODs obtained were compared with those of negative controls (wells without bacterial inoculum). We considered the ODs 0.150 to be negative (mean value of all negative controls included in the tests), and those with OD > 0.150 to be positive. All the strains were tested in triplicate in 3 experiments, and the average value for each sample was calculated.
We also used the Stepanovic method as a control for our protocol, using the same medium. This was also repeated twice and all the strains were tested in triplicate. Evaluation of the results was performed using the scale described by Stepanovic et al. (Citation2007).
Confocal laser scanning microscopy (CLSM)
A McFarland 0.5 standard suspension was prepared for each strain in Müller-Hinton broth. 6-for-4 microtiter plates (Nunc, Roskilde, Denmark) with a sterile Thermanox disk for each well were inoculated with 1 mL of each suspension. The plates were incubated for 24 h at 37ºC. The medium was then changed using 1 mL of Müller-Hinton broth and the preparations were re-incubated for another 24 h. After incubation, the medium was removed and the wells were washed 3 times with sterile distilled water. The disks were then removed and stained with Live/Dead BacLight stain (Invitrogen), using the instructions provided by the manufacturer. Stained disks were examined with a Leica DM IRB confocal laser scanning microscope at 40× magnification. The experiment was performed in triplicate and repeated 2–3 times for all the strains. Several photographs were taken for all strains. The percentage of covered surface was calculated using ImageJ software for live and dead bacteria separately, and then the result was used to calculate the proportion of both types in the biofilm.
Detection of the ica gene
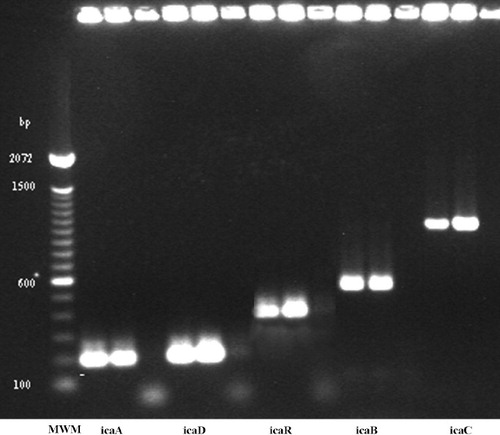
After isolation of chromosomal DNA from the strains studied, amplification of icaR and the 4 genes of the ica operon was performed by PCR with specific primers (), according to previously described protocols (Cafiso et al. Citation2004, Gara Citation2007). S. epidermidis strain ATCC 35984 (RP62A), a biofilm-forming strain, and a biofilm-negative one (ATCC 12228) were used as positive and negative controls, respectively (Christensen et al. Citation1982a, Citationb). The PCR products were analyzed by agarose gel electrophoresis.
Table 1. Sequences of the primers used, sizes of the expected PCR products, and references
Statistics
Statistical analysis was performed for S. aureus and S. epidermidis isolates, and in some cases between S. aureus and all other coagulase-negative staphylococci. Comparison between sensitivity of tests and species was performed using Chi-square and Fisher's exact tests. The calculations were performed using EPI-INFO software version 3.5.1 (Centers for Disease Control and Prevention, Atlanta, GA).
Results
Of the 26 strains included in the study, isolated from 23 clinical specimens (22 patients), 13 were Staphylococcus aureus and 13 were coagulase-negative staphyloccoci (CoNS) (10 S. epidermidis, 1 S. hominis, 1 S. warneri, and 1 S. lugdunensis). 13 of the strains had been isolated from clinical specimens where more than one bacterium was recovered (polymicrobial infections), some of them including other bacterial genera (data not shown).
All 26 strains were positive for biofilm development (). The overall sensitivity of the Stepanovic method (STEP) was 89%, that of MP was 65%, and that of CLSM was 39%. For S. aureus isolates, the sensitivity of the techniques was as follows: STEP 85%, MP 62%, and CLSM 39%. For S. epidermidis isolates, the results were: STEP 90%, MP 70%, and CLSM 50%.
Table 2. Results obtained with each technique
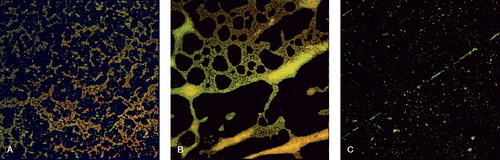
Although CLSM had the lowest percentage of positive results, it allowed us to study the percentage of live and dead bacteria (). Among the CLSM-positive strains, the mean percentage of covered surface was 52 (SD 11, range 77–44). CLSM images showed either mature biofilm (with the strains considered biofilm-positive) or isolated bacteria without any biofilm development (the biofilm-negative strains) (). The mean percentage of covered surface was 43% (SD 18, range 21–67).
Figure 1. CLSM images. A. Biofilm-positive S. aureus (strain P-1). B. Biofilm-positive S. epidermidis (strain P-6.5). C. Biofilm-negative S. aureus (strain P-41).
19 of the 26 strains were ica-positive, including all 13 of the S. aureus strains, half of the S. epidermidis strains, and altogether 6 of the 13 CoNS. A statistically significant difference for ica detection was detected between S. aureus and S. epidermidis (p = 0.007, Fisher's exact test) (). This difference was also found to be significant when all CoNS strains were included in the comparison (p = 0.002, Fisher's exact test).
Figure 2. Agarose gel electrophoresis of PCR products. Each PCR run included the reference ATCC 35984 ica-positive strain, and a positive and a negative strain from our collection.
6 ica-positive strains also carried icaR (2 S. aureus and 4 CoNS), and among them, 3 were biofilm-positive by both phenotypic methods (). No statistically significant difference was found in detection of ica R between these groups. 7 ica-negative CoNS strains (5 S. epidermidis, 1 S. lugdunensis, and 1 S. warneri) were biofilm-positive.
There were no statistically significant differences between S. aureus and S. epidermidis in the results of phenotypic tests.
Discussion
Biofilms have been increasingly implicated as a key pathogenic factor in biomaterial infections (Costerton Citation2005, Citation2007). Recently, new diagnostic methods have been designed based on the characteristics of biofilms, with better results than the classical methods of assessment (Trampuz et al. Citation2007, Esteban et al. Citation2008). To evaluate phenotypic and genotypic methods that can be used in the study of clinically relevant staphylococci that can produce biofilms, we performed this study which shows that different methods are required to fully detect biofilm-forming potential in isolates.
Of the different techniques used to evaluate the ability of different bacterial strains to produce biofilms, the crystal violet microplate assay technique—initially described by Christensen et al. (Citation1985)—is one of the most popular. Based on the property of biofilms of retaining the dye despite washing with water, destaining with ethanol allows quantification of the amount of biofilm if one measures the amount of dye present in the ethanol by spectrophotometry. The usefulness of this technique has been evaluated using different organisms, and became popular because of its simplicity. A recent study using S. epidermidis isolated from orthopedic samples showed a higher number of positive results than molecular biology techniques (Arciola et al. Citation2006). This discrepancy may be due to the different sequences selected as targets for the molecular detection, because several genes are involved in biofilm development, and primer selection cannot detect all possibe genes involved.
The Stepanovic method is another technique with similar principles, although more standardized than the Christensen method. Here we found some discrepancies between these two techniques. The most notorious was an S. aureus strain that had the highest OD readings by the modified Christensen test but which appeared to be negative with the Stepanovic test (strain P-95). This particular strain had a lower growth rate than other strains, with minute colonies after 24 h at 37ºC, and it was also negative by the CLSM technique. Our modification of the Christensen method included a longer incubation period; this could be an explanation for this discrepancy, because biofilm development takes more time for this strain. This is a very important issue, since it is reported that S. aureus strains involved in osteoarticular infections (especially small-colony variants, or SCVs) are slowly growing strains (von Eiff Citation2008), and biofilm detection probably requires longer periods of time than those used for common strains.
CLSM has become one of the reference techniques for the study of biofilms (Donlan Citation2001, Lawrence et al. Citation1991). This technique allows analysis of the internal structure of biofilms through the use of microscopy combined with fluorescent stains, such as Backlight Live-Dead stain (Boulos et al. Citation1999). Despite this, no studies have been published concerning its usefulness as a screening technique for a broad number of clinical strains of Staphylococcus spp. In our study, CLSM detected a lower number of biofilm-producing strains than the crystal violet microtiter plate assay. This difference was more evident for CoNS than for S. aureus, where the number of biofilm-positive strains was the same with both techniques. This could be due to technical problems, which can be attributed to the thermanox slides; these may have different adherence properties than other polymers. However, CLSM allowed us to determine the percentage of live/dead bacteria in our isolates, so we believe that this technique is still useful for the study of more detailed aspects of biofilms, although selection of the substrate appears to be important for the study of particular strains, because of the observed differences between them. However, because of its low sensitivity, the method cannot be used to evaluate the ability of different strains to produce biofilms in a screening study.
Molecular detection of ica genes revealed the presence of the operon in most clinical strains (19 of 26). In addition, of the 6 ica-positive strains carrying icaR, 3 were negative for biofilm formation only by one of the phenotypic methods used. This indicates that carriage of icaR in not always related to inactivation of the ica operon, as reported elsewhere (Cafiso et al. Citation2004). However, 7 CoNS strains were biofilm-positive, even though they did not carry the ica operon. This has been described previously, and can be explained by the presence of other genetic markers (Fitzpatrick et al. Citation2005). This suggests the existence of ica-independent mechanisms of biofilm formation (Gara Citation2007).
In conclusion, all strains tested in our study could produce biofilm that could be detected with at least one of the techniques used. According to our results, molecular detection of the ica operon is associated with biofilm production. However, the absence of ica genes did not exclude the phenotypic ability of the strains to develop biofilm in vitro, so both approaches (genetic and phenotypic) are required for optimum evaluation of the biofilm producing ability of clinical strains of Staphylococcus isolated from orthopedic infections.
JE designed and coordinated the study, and participated in both analysis of the results and editing of the manuscript. DMM performed the phenotypic characterization and participated in editing of the manuscript. IS designed the genetic study, evaluated the results, and participated in editing of the manuscript. JCA and EGB diagnosed the patients, performed the surgery, and collaborated in the data analysis and in the editing of the manuscript. RFR participated in analysis of the results and in editing of the manuscript. AF performed the genetic characterization study.
This work was funded by grants from the CICyT (MAT2006-12603-CO2-O2), Comunidad de Madrid (S-0505/MAT/0324), and from the CONSOLIDER-INGENIO program (FUNCOAT-CSD2008-00023). DMM was funded by a grant from the Fundación Conchita Rábago de Jiménez Díaz.
No competing interests declared.
- Arciola CR, Campoccia D, Cervellati M, Donati E, Montanaro L. Detection of slime production by means of an optimised Congo red agar plate test based on a colourimetric scale in Staphylococcus epidermidis clinical isolates genotyped for ica locus. Biomaterials 2002; 23:4233-9.
- Arciola CR, Campoccia D, Baldassarri L, Donati ME, Pirini V, Gamberini S, . Detection of biofilm formation in Staphylococcus epidermidis from implant infections. Comparison of a PCR-method that recognizes the presence of ica genes with two classic phenotypic methods. J Biomed Mater Res 2006; 76A:425-30.
- Boulos L, Prevost M, Barbeau B, Coallier J, Desjardins D. LIVE/DEAD BacLight: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J Microbiol Meth 1999; 37:77-86.
- Cafiso V, Bertuccio T, Santagati M, Campanile F, Amicosante G, Perilli MG, . Presence of the ica operon in clinical isolates of Staphylococcus epidermidis and its role in biofilm production. Clin Microbiol Infect 2004; 10(12):1081-8.
- Cordero-Ampuero J, Esteban J, García-Cimbrelo E, Munuera L, Escobar R. Low relapse with oral antibiotics and two-stage exchange for late arthroplasty infections in 40 patients after 2-9 years. Acta Orthop 2007; 78(4):511-9.
- Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop 2005; (437):7-11.
- Costerton JW. The Biofilm Primer. Springer-Verlag: Berlin 2007.
- Christensen GD, Binso AL, Parisi JT, McLaughlin B, Hester MG, Luther RW. Nosocomial septicemia due to multiply antibiotic-resistance Staphylococcus epidermidis. Ann Intern Med 1982a; 96:1-10.
- Christensen GD, Simpson WA, Binso AL, EHB. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 1982b; 37:318-26.
- Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, . Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. JClin Microbiol 1985; 22:996-1006.
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350:1422-9.
- Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 2001; 33:1387-92.
- Esteban J, Gómez-Barrena E, Cordero J, Martín-de-Hijas NZ, Kinnari TJ, Fernández-Roblas R. Evaluation of quantitative cultures from sonicated retrieved orthopaedic implants in the diagnosis of orthopaedic infection. J Clin Microbiol 2008; 46(2):488-92.
- Fitzpatrick F, Humphreys H, O'Gara JP. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant staphylococcus aureus clinical isolates. J Clin Microbiol 2005; 43(4):1973-6.
- Gara JP. Ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and staphylococcus aureus. FEMS Microbiol Lett 2007; 270:179-88.
- Lawrence JR, Korber DR, Hoyle BD, Costerton JW, Caldwell DE. Optical sectioning of microbial biofilms. J Bacteriol 1991; 173(20):6558-67.
- Patel R. Biofilms and antimicrobial resistance. Clin Orthop 2005; (437):41-7.
- Stepanovic S, Vukovic D, Hola V, Di Bonaventura G, Djukic S, Cirkovic I, . Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007; 115 (8): 891-9.
- Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Douglas RO, . Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007; 357:654-63.
- Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penadés JR, . SarA and not sigmaB is essential for biofilm development by staphylococcus aureus. Mol Microbiol 2003; 48:1075-87.
- von Eiff C. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int J Antimicrob Agents 2008; 31(6):507-10.
