Abstract
Background and purpose Coating of acetabular revision implants with hydroxyapatite (HA) has been proposed to improve ingrowth and stability. We investigated whether HA coating of revision cups can reduce the risk of any subsequent re-revision.
Methods We studied uncemented cups either with or without HA coating that were used at a primary acetabular revision and registered in the Swedish Hip Arthroplasty Register (SHAR). 2 such cup designs were identified: Harris-Galante and Trilogy, both available either with or without HA coating. These cups had been used as revision components in 1,780 revisions of total hip arthroplasties (THA) between 1986 and 2009. A Cox proportional hazards model including the type of coating, age at index revision, sex, cause of cup revision, cup design, the use of bone graft at the revision procedure, and the type of cup fixation at primary THA were used to calculate adjusted risk ratios (RRs with 95% CI) for re-revision for any reason or due to aseptic loosening.
Results 71% of the cups were coated with HA and 29% were uncoated. At a mean follow-up time of 6.9 (0–24) years, 159 (9%) of all 1,780 cups had been re-revised, mostly due to aseptic loosening (5%), dislocation (2%), or deep infection (1%). HA coating had no significant influence on the risk of re-revision of the cup for any reason (RR = 1.4, CI: 0.9–2.0) or due to aseptic loosening (RR = 1.1, 0.6–1.9). In contrast, HA coating was found to be a risk factor for isolated liner re-revision for any reason (RR = 1.8, CI: 1.01–3.3). Age below 60 years at the index cup revision, dislocation as the cause of the index cup revision, uncemented cup fixation at primary THA, and use of the Harris-Galante cup also increased the risk of re-revision of the cup. In separate analyses in which isolated liner revisions were excluded, bone grafting was found to be a risk factor for re-revision of the metal shell due to aseptic loosening (RR = 2.1, CI: 1.05–4.2).
Interpretation We found no evidence to support the notion that HA coating improves the performance of the 2 studied cup designs in revision arthroplasty. In contrast, patient-related factors such as younger age and dislocation as the reason for cup revision, and technical factors such as the choice of revision cup were found to influence the risk of subsequent re-revision of the cup. The reason for inferior results after revision of uncemented cups is not known, but it is possible that these hips more often had pronounced bone loss at the index cup revision.
The most common cause of re-revision of the hip after revision surgery is failure of the acetabular component (Swedish Hip Arthrolasty Register (SHAR) Citation2010). Acetabular revision with cemented implants has shown up to 20% failure at 10 years of follow-up, whereas contemporary designs of uncemented acetabular cups have substantially reduced this failure rate (Callaghan et al. Citation1985, Kavanagh et al. Citation1985, Katz et al. Citation1997, Lie et al. Citation2004). Loosening of the primary acetabular component often leaves substantial bone loss and a sclerotic acetabular bed. Fixation of revision cups is therefore demanding, and several techniques have been used to restore bone loss and achieve long-term fixation of the revision cup—such as screw fixation, bone grafting, and different cup designs (Palm et al. Citation2007, Pulido et al. Citation2011). The use of hydroxyapatite (HA) coating on cups used as revision implants has been proposed to be an improvement over uncoated cups (Dorairajan et al. Citation2005, Geerdink et al. Citation2007).
HA is the main inorganic component of human bone. It has therefore been hypothesized that coating of metallic implants with HA enhances ingrowth of bone and thus leads to improved stability (Soballe et al. Citation1999). Indeed, some HA-coated cups perform well and are still in use, while other HA-coated cups have shown high failure rates in the long term. For instance, inferior results were achieved with the Romanus cup where the combination of an inferior locking mechanism of the liner with an inferior type of polyethylene resulted in excessive osteolysis in the acetabular region (Puolakka et al. Citation1999, Lyback et al. Citation2004, Lazarinis et al. Citation2010, SHAR Citation2010). Due to the relatively small numbers of revision procedures, very few authors have reported results after hip revision arthroplasty using HA-coated implants. To our knowledge, there have been no registry studies specifically investigating the performance of HA-coated hip revision implants. Thus, the question of whether the use of HA coating on revision cups is beneficial remains to be answered.
In this study, we analyzed the outcome of acetabular revision surgery using uncemented cups with or without HA coating recorded in the Swedish Hip Arthroplasty Register (SHAR). Our hypothesis was that coating of revision acetabular cups with HA reduces the risk of re-revision of the acetabular component inserted. The primary endpoint was re-revision of the acetabular component for any reason and the secondary endpoint was re-revision of the cup due to aseptic loosening.
Patients and methods
Source of data
Our data were derived from the SHAR (SHAR Citation2010). Primary and revision hip arthroplasties performed in Sweden, both in public and private orthopedic units, have been reported to the Register since 1979. From 1992, data from primary procedures were linked to the personal ID number of each patient, providing information relevant to follow-up—such as changes of address, dates of emigration, or death. In this study we used the reoperation database, which includes personal ID numbers from the start of the Register in 1979. Information on the type of implant and fixation, and technical details, are recorded from the case records of each reoperation and entered into the database. In this database, reoperations are also recorded when the patient undergoes more than one operation of the same hip. For this study, only primary revisions using uncemented cups available with and without HA coating and any subsequent revision of these cups (irrespective of implant used at re-revision) were analyzed (index operation). The term “cup revision” was defined as an intervention where 1 or more components of the cup (shell, liner, or both) were removed. Thus, other types of reoperations where the implant was left untouched—or where only the stem was exchanged—were disregarded in this study. The SHAR has been repeatedly validated, and the completeness has been described to be about 99% for primary hip arthroplasties and 94% for revision hip arthroplasties (Söderman et al. Citation2000, Citation2001, SHAR Citation2010), although one study indicated a lower degree of completeness (Ornstein et al. Citation2009).
Study population and characteristics
We identified uncemented cups, with or without HA coating, that were registered in the SHAR reoperation database between 1979 and 2009 and used as components for the first acetabular revision performed after primary hip arthroplasty (1,780 hips in 1,772 patients). There were 2 such cup designs: Harris-Galante I and II (Zimmer Inc., Warsaw, IN; n = 340) and Trilogy (Zimmer Inc.; n = 1,440). They had been used in revision procedures between 1986 and 2009. The Harris-Galante I was introduced to the Swedish market in 1984. 4 years later, the Harris-Galante II with a thicker acetabular shell and wider screws became available. This design was gradually replaced by the Trilogy cup, with an improved locking mechanism in 1993.
All the cups investigated were hemispherical press-fit shells made of titanium alloy with a porous coating consisting of commercially pure titanium, and all were available either with or without HA coating. Thus, 4 different implants were available for analysis: HA-coated Harris-Galante, uncoated Harris-Galante, HA-coated Trilogy, and uncoated Trilogy. The ceramic coating on the Harris-Galante and Trilogy cups consists of a mixture of HA (70%) and tricalcium phosphate (30%) with a thickness of 70 µm and 50% crystallinity. The cups were combined with different types of liners that were all made of conventional polyethylene until 1999, when highly crosslinked polyethylene (XLPE) was introduced and gradually replaced the older type of polyethylene over a period of 8–9 years.
1,772 patients received uncemented acetabular revision cups of the uncemented designs mentioned above. 8 patients had revisions in both hips, resulting in a total of 1,780 hips for analysis.
Statistics
Follow-up started on the day of revision THA and ended on the day of re-revision, death, emigration, or December 31, 2009 (whichever came first). Unadjusted survival with revision for any reason or due to aseptic loosening was calculated according to Kaplan-Meier. A Cox proportional hazards model was used in order to analyze the relative risk (RR) of re-revision of the cup component inserted at the first revision—either for any reason or due to aseptic loosening. The absence or presence of HA coating, age at index cup revision (< 50, 50–59, 60–75, > 75 years), sex, cause of index cup revision (aseptic loosening, dislocation, infection, or other cause(s)), cup design (Harris-Galante or Trilogy), the use of bone graft at index cup revision, and the type of cup fixation in primary THA (cemented or uncemented) were considered relevant covariates. Other covariates were investigated in an exploratory manner: the type of hospital where the primary or the revision surgery was performed, age at primary THA, diagnosis underlying primary THA, and type of stem fixation in revision arthroplasty when a total revision (including stem revision) was performed. An initial analysis investigated the covariates mentioned above as singular variables, and a crude RR was calculated for each variable. Then the covariates mentioned above were entered in the regression model and mutually adjusted for other covariates. The assumption of proportional hazards was investigated by hazard function plots and log-minus-log plots of all covariates. There was no sign of insufficient proportionality in the hazard functions, and log-minus-log plots ran parallel for all covariates.
Re-revision of the cup was defined as exchange of the liner or exchange or removal of the liner together with the metal shell. In another set of analyses, we separately investigated procedures where either only the liner or both liner and metal shell had been exchanged or extracted, either for any reason or due to aseptic loosening.
8 patients had undergone bilateral revisions with use of either of the 4 cup designs selected for this study. In order to investigate dependency issues (Ranstam et al. Citation2011), we performed a separate analysis excluding the cup revision in the second hip in these patients but found that parameter estimates were not affected when 1,780 or only 1,772 hips were included.
Categorical data were investigated using the chi-square test. Statistical analyses were performed using SPSS software (version 19.0). The R software package (version 2.14.1) was used in order to plot survival functions according to Kaplan-Meier, and p-values less than 0.05 were considered significant.
Results
Characteristics of the study population
The group with HA-coated cups was 2.5 times larger than the group with uncoated cups. Primary osteoarthritis was the most common preoperative diagnosis for primary THA (). Sex distribution was similar between the groups with HA-coated cups and uncoated cups. The largest number of revisions was found in patients aged between 50 and 75 years. The main cause for the index cup revision was aseptic loosening (87%). 803 (45%) of the index revisions were isolated cup revisions, and in 977 (55%) the stem was revised together with the investigated cup. The diagnosis of previous pediatric hip disease at primary hip arthroplasty was more common in the group of patients with uncoated revision cups (p = 0.03). There was a larger proportion of older patients (aged > 75 years) in the group with HA-coated cups (p < 0.001) and there was a larger proportion of patients with dislocation as the cause of index cup revision in the group with HA-coated cups (p = 0.03) (). The use of bone grafts during the index cup revision was comparable for HA-coated cups and uncoated cups (p = 0.6) ().
Table 1. Type of diagnosis at primary THA within the 2 groups of patients with HA-coated and uncoated revision cups
Table 2. Characteristics of the study population
Table 3. Use of bone graft at index cup revision within the 2 groups (HA-coated and uncoated revision cups)
Re-revisions of cups
Mean follow-up for all cases was 6.9 (0–24) years. The mean observation time was 4.6 years for the HA-coated cups and 10.3 years for the uncoated cups. 103 patients died during the period studied, excluding patients who died after the cup had been re-revised. By 2009, 159 (9%) of all 1,780 cups had been re-revised, mostly due to aseptic loosening (5%), dislocation (2%), or deep infection (1%). In 80 hips, only the liner had been exchanged and in 79 both the liner and the shell had been exchanged or extracted. 87 (55%) of these re-revision procedures were isolated cup re-revisions, and 72 (45%) were total hip re-revisions where the stem was also revised.
Risk of cup re-revision for any reason
The crude RR of HA coating for the risk of cup re-revision for any reason was 1.0 (95% CI: 0.7–1.4) ( and ). After adjustment for all other covariates, HA coating still did not have any influence on the risk of cup re-revision for any reason, with an adjusted RR of 1.4 (CI: 0.9–2.0) (). Age below 60 years at the time of index cup revision, dislocation as the cause of index cup revision, and the use of the Harris-Galante cup statistically significantly increased the risk of cup re-revision for any reason (). In separate analyses where the endpoints were either isolated re-revisions of the liner or re-revisions of both liner and metal shell, we investigated whether HA coating had any influence on the risk of re-revision of these components. We found that HA coating was a risk factor for isolated liner re-revision, with an adjusted RR of 1.8 (CI: 1.01–3.3), but HA coating had no influence on the risk of cup re-revision when isolated liner revisions were excluded (adjusted RR = 1.0, CI: 0.6–1.7).
Table 4. Relative risk (RR) for cup re-revision for any reason
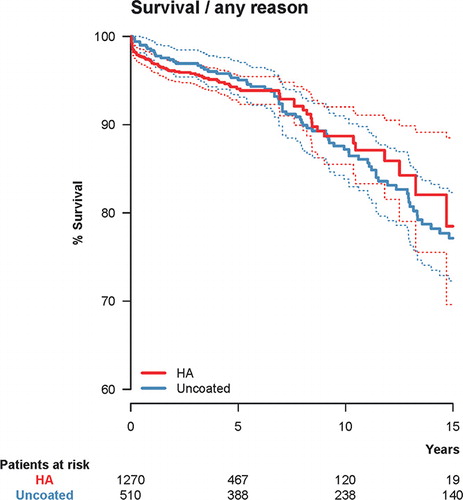
Figure 1. Unadjusted Kaplan-Meier survival with hydroxyapatite (HA) coating as the independent factor and cup re-revision due to any reason as the endpoint. 10-year survival was 88.7% (CI: 85.5–92.0) for the HA-coated cups (red) and 87.2% (CI: 83.8–90.7) for the uncoated cups (blue). The dashed lines represent 95% confidence intervals for the 2 groups of cups.
Risk of cup re-revision due to aseptic loosening
The crude RR of HA coating for the risk of cup re-revision due to aseptic loosening was 0.7 (CI: 0.4–1.1) ( and ) without adjustment for covariates. After adjustment for the covariates mentioned previously, we found that HA coating had no influence on the risk of cup re-revision due to aseptic loosening (adjusted RR = 1.1, CI: 0.6–1.9) (). The covariates age below 60 years at index cup revision, the use of a Harris-Galante cup, and the use of uncemented cup in primary arthroplasty were associated with an increased risk of cup re-revision due to aseptic loosening (). The analysis of the subgroup of re-revisions of metal shell and liner, excluding isolated liner re-revisions, indicated that HA coating had no influence on the risk of re-revision due to aseptic loosening: The presence of HA coating was associated with an adjusted RR of 1.0 (CI: 0.5–2.2) for re-revision of the metal shell. The use of bone graft at the index procedure did not influence the risk of cup re-revision (including liner exchanges) due to aseptic loosening (adjusted RR = 1.1, CI: 0.5–2.1) but when isolated liner revisions were excluded, bone grafting was found to be a risk factor for re-revision of the metal shell due to aseptic loosening (adjusted RR = 2.1, CI: 1.05–4.2).
Table 5. Relative risk (RR) for cup re-revision due to aseptic loosening
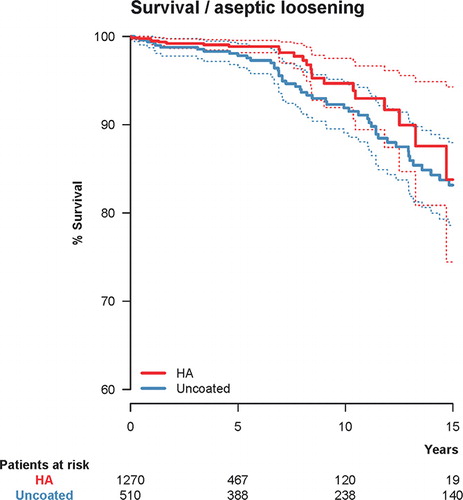
Figure 2. Unadjusted Kaplan-Meier survival with hydroxyapatite (HA) coating as the independent factor and cup re-revision due to aseptic loosening as the endpoint. 10-year survival was 94.7% (CI: 91.9–97.5) for the HA-coated cups (red) and 91.9% (CI: 89.1–94.8) for the uncoated cups (blue). The dashed lines represent 95% confidence intervals for the 2 groups of cups.
Exploratory analyses
Further exploratory analyses involving insertion of putatively relevant covariates into the Cox regression model were performed: (1) type of hospital where the primary surgery or the revision surgery was performed; (2) age at primary THA; (3) the diagnosis underlying primary arthroplasty; and (4) the type of stem fixation when the index cup revision was combined with a stem revision. None of the other factors mentioned above statistically significantly influenced the risk of cup re-revision—either for any reason or due to aseptic loosening—with the following exception. Insertion of the primary THA at a local hospital was a risk factor for cup re-revision for any reason ().
Table 6. Exploratory analyses using putatively relevant covariates. Adjusted relative risk (RR) for cup re-revision for any reason
The scenario of an unstable stem that was not revised together with the cup at the index revision procedure because it was wrongly thought to be stable could distort our results. We therefore determined whether the stem had been revised together with the cup during the index cup revision: The Cox regression model with the endpoint re-revision for any reason was applied to cases where only the cup had been revised during the index procedure (isolated cup revisions). The results of this analysis were similar to those found for the entire study population, i.e. inclusion of those cases where the stem had also been revised during the index revision procedure (total hip revisions; data not shown).
Patients who were less than 60 years of age at the time of the index cup revision had a greater risk of cup re-revision for any reason and due to aseptic loosening. A separate analysis only including patients younger than 60 years at the index cup revision revealed that HA coating did not affect the performance of the cups investigated, even in this subgroup of patients. However, the occurrence of “other diagnosis” (including fracture, technical reasons, pain only, or implant fracture) as the cause of the index cup revision and the use of an uncemented cup in primary arthroplasty were associated with an increased risk of cup re-revision for any reason and due to aseptic loosening in the group of younger patients (data not shown). In these patients, we also found that the use of bone grafting at the index revision was associated with a higher risk of cup re-revision due to aseptic loosening (data not shown).
Discussion
HA-coated implants are widely used in both primary and revision total hip arthroplasty. In primary hip arthroplasty, the use of HA on cups appears to be questionable. Some reports have indicated that the outcome is not affected by the presence of HA coating, whereas others have shown an increased risk of cup revision after the use of HA coating (Havelin et al. Citation2000, Reikerås and Gunderson Citation2002, Cheung et al. Citation2005, Stilling et al. Citation2009, Lazarinis et al. Citation2010). There is very little literature on revision hip arthroplasty, and it involves very few patients, but some studies investigating the outcome of acetabular revision using uncemented HA-coated components have shown promising results (Nivbrant and Kärrholm Citation1997, Dorairajan et al. Citation2005, Geerdink et al. Citation2007, Palm et al. Citation2007). However, to our knowledge there have been no studies comparing identical cups with or without HA coating used in revision arthroplasty. Taken together, our results show no evidence that HA coating of acetabular components used in revision THA improved the performance of the 2 cup designs under investigation.
Risk factors for cup re-revision
Age at revision arthroplasty of less than 60 years increased the risk of cup re-revision for any reason and due to aseptic loosening. That younger patients have an inferior outcome after primary THA is well known. In this group of patients, inferior survival of acetabular HA-coated cups has also been reported (Manley et al. Citation1998, Puolakka et al. Citation1999, Wangen et al. Citation2008, Lazarinis et al. Citation2010). Higher demands in young patients and increased wear may explain the higher risk of revision. However, in the SHAR there is no distinction between loosening and osteolysis as causes of revision.
HA coating was a risk factor for isolated liner re-revisions, something that may be explained by “third-body wear” induced by HA particles. This phenomenon has been described as a cause of early failure of HA-coated cups after primary THA (Morscher et al. Citation1998).
Use of the Harris-Galante cup enhanced the risk of cup re-revision due to aseptic loosening and for any reason when compared with the Trilogy cup. The Trilogy cup has shown good long-term results after primary THA (Lazarinis et al. Citation2010, SHAR Citation2010). In revision cases, Tanzer et al. (Citation1992) reported good medium-term results after using the Harris-Galante cup in acetabular revision, with only 1% component failure after an average follow-up time of 3.4 years. Long-term results (with 10–14 years of follow-up) for this cup used as a revision component have also been reported to be satisfactory (Templeton et al. Citation2001, Hallstrom et al. Citation2004). The Trilogy cup is a new version of the Harris-Galante design where problems associated with the locking mechanism that secures the polyethylene liner have been addressed (Röhrl et al. Citation2006).
Other factors affecting outcome after cup revision
The type of stem component and its fixation could influence cup survival and thereby possibly distort our findings. Different types of stems were combined with the cups investigated in our study; either they were left in situ at the time of the index revision surgery or they were exchanged during the same revision procedure (). The type of stem fixation varied, with the majority being cemented stems—giving hybrid revision THA. In order to investigate whether the type of stem fixation affected cup survival when both stem and cup were exchanged at the index revision, we performed a separate analysis including this variable (cemented or uncemented stem fixation) as a covariate. We found that stem fixation was not a statistically significant risk factor for cup re-revision for any reason () or due to aseptic loosening.
Table 7. Distribution of the 3 most commonly used stems combined with the cup types Trilogy (A) and Harris-Galante (B)
It is conceivable that some stems were thought to be stable at the time of the index operation and left in situ while in truth they were unstable. Furthermore, stems left in situ could influence the results, causing difficulties in correct balancing of the soft tissues, and increase the risk of subsequent taper corrosion or dislocation—with a possible influence on the risk of further revisions. To avoid this potential bias, we investigated 2 groups separately according to the type of index revision procedure, e.g. only cup revisions or combined cup and stem revisions. We found no differences between these groups.
Due to the large number of degrees of freedom when entering each individual stem type into a Cox regression model, such analyses were impossible to perform. We therefore studied the various cup and stem combinations in detail and found that HA-coated cups were not commonly used in combination with stems of inferior performance ().
The use of an uncemented cup in primary THA was a risk factor for cup re-revision due to aseptic loosening. To our knowledge, this phenomenon has not been described previously. The reason for this finding is unknown. It might be that uncemented cups were more often surrounded by focal osteolyses and osteoporosis due to stress shielding (Digas et al. Citation2006). Loosening of cemented cups is often associated with a generalized widening of the acetabulum bordered by a sclerotic rim, which can be partly used for fixation of an uncemented revision cup. Larger acetabular bone defects and inferior bone quality may thus be possible reasons for inferior fixation of revision cups inserted after removal of failed uncemented implants.
The reason underlying the index cup revision was included as a covariate in the Cox regression model. We found that cups that were revised due to aseptic loosening were not more likely to be re-revised due to aseptic loosening than cups that were originally revised for other reasons. In contrast, when analyzing the endpoint cup re-revision for any reason, cups that were originally revised due to dislocation were more likely to undergo re-revision. The fact that dislocation is a risk factor for re-revision for any reason is probably related to a higher risk of recurrent dislocation after revision surgery. Patients undergoing cup revision due to dislocation have been found to be at higher risk of re-dislocation, possibly because of soft tissue laxity or patient-related factors such as cognitive impairment or neuromuscular disorders, age greater than 80 years, and a propensity to fall (Patel et al. Citation2007). It appears that conventional revision cups such as those investigated in our study do not sufficiently address the problem of recurrent dislocation. Constrained or dual-mobility acetabular components should perhaps be considered, at least in some of these cases.
Factors such as medication with steroids, non-steroidal anti-inflammatory drugs, or bisphosphonates that are known to influence bone metabolism are not registered in the SHAR and cannot be analyzed in our study. The same applies to other possible confounding factors such as medical conditions that could have a direct or indirect influence on implant survival, e.g. osteoporosis; neurological, mental, and endocrine disorders; or overweight.
Limitations of the study
One limitation of our study was that only 2 cup types were investigated. However, these designs were the only ones available in the SHAR for studies of the effect of HA coating on cup survival after revision arthroplasty. On the other hand, the comparatively large number of revision operations in each group was a strength of our study. To our knowledge, no studies with comparable numbers of patients have been published.
A further limitation was the lack of detailed information on the extent of the acetabular defects and the amount and type of bone graft used during the index cup revision. Especially in Paprosky type III defects (Paprosky et al. Citation1994), the use of large-diameter implants—sometimes in conjunction with bone grafting—is often advocated. Supplementary screws and morselized or structural bone grafts are commonly applied to restore the defects that are present during cup revision surgery. In cases of severe acetabular defects, the outcome after revision arthroplasty has resulted in inferior outcomes compared with revisions of Paprosky type I and II acetabular defects (Issack et al. Citation2009, Pulido et al. Citation2011). That is also confirmed by our subgroup analysis where re-revision of the metal shell due to aseptic loosening was the endpoint. We found that the use of bone graft at the index cup revision was a risk factor for re-revision of the metal shell, possibly reflecting larger acetabular defects and inferior initial (and even long-term) stability. Even if details about the bone defects and grafting are lacking, we have no reason to believe that these circumstances were unevenly distributed between the 2 groups of implants. Importantly, we found no differences between the 2 groups of revision cups with or without HA coating with respect to the use of bone grafts (). Altogether, we have no reason to believe that the size of the acetabular defects at revision surgery skewed our results.
Other information such as the use of additional screw fixation of the cups or the type of polyethylene liner (XLPE or conventional models) is not registered in the revision database, and was therefore not considered in our analyses.
Conclusion
Our results, derived from registry data on 1,780 acetabular hip revisions, lend no support to the notion that HA coating improves the performance of revision cups. On the contrary, HA coating can increase the risk of liner revision, possibly due to third-body wear. Dislocation as the underlying diagnosis for the index revision and age below 60 years at the index cup revision are patient-related risk factors for subsequent cup re-revision. The type of stem fixation at index cup revision did not affect the risk of cup re-revision whereas other technical factors such as the use of a Harris-Galante cup at the index cup revision and uncemented cup fixation at primary THA increased the risk of subsequent re-revision of the cup. Bone grafting is a risk factor for re-revision of the metal shell due to aseptic loosening, probably reflecting a higher degree of acetabular bone deficiency at the time of index cup revision.
SL: data analysis, statistics, and writing and revision of the manuscript. JK: provision of all data from the Register, preparation of the database, data analysis, and revision of the manuscript. NPH: data analysis, statistics, and writing and revision of the manuscript.
We thank all the Swedish orthopedic surgeons and secretaries who contributed data, and not least the personnel of the Swedish Hip Arthroplasty Register for preparations that resulted in the primary database. We are also grateful to Niclas Eriksson of Uppsala Clinical Research Center for his help with R syntax.
No competing interests declared.
- Callaghan JJ, Salvati EA, Pellicci PM, Wilson PDJr., Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement 1979. A two to five-year follow-up. J Bone Joint Surg (Am) 1985;67 (7):1074-85.
- Cheung KW, Yung SH, Wong KC, Chiu KH. Early failure of smooth hydroxyapatite-coated press-fit acetabular cup—7 years of follow-up. J Arthroplasty 2005; 20 (5): 627-31.
- Digas G, Kärrholm J, Thanner J. Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop 2006; 77 (2): 218-26.
- Dorairajan A, Reddy RM, Krikler S. Outcome of acetabular revision using an uncemented hydroxyapatite-coated component: two- to five-year results and review. J Arthroplasty 2005; 20 (2): 209-18.
- Geerdink CH, Schaafsma J, Meyers WG, Grimm B, Tonino AJ. Cementless hemispheric hydroxyapatite-coated sockets for acetabular revision. J Arthroplasty 2007; 22 (3): 369-76.
- Hallstrom BR, Golladay GJ, Vittetoe DA, Harris WH. Cementless acetabular revision with the Harris-Galante porous prosthesis. Results after a minimum of ten years of follow-up. J Bone Joint Surg (Am) 2004; 86 (5):1007-11.
- Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand 2000; 71 (4): 337-53.
- Issack PS, Nousiainen M, Beksac B, Helfet DL, Scilco TP, Buly RL. Acetabular component revision in total hip arthroplasty. Part II: management of major bone loss and pelvic discontinuity. Am J Orthop (Belle Mead NJ). 2009; 38 (11):550-6.
- Katz RP, Callaghan JJ, Sullivan PM, Johnston RC. Long-term results of revision total hip arthroplasty with improved cementing technique. J Bone Joint Surg (Br) 1997; 79 (2):322-6.
- Kavanagh BF, Ilstrup DM, Fitzgerald RH, Jr. Revision total hip arthroplasty. J Bone Joint Surg (Am) 1985; 67 (4):517-26.
- Lazarinis S, Kärrholm J, Hailer NP. Increased risk of revision of acetabular cups coated with hydroxyapatite. Acta Orthop 2010; 81 (1): 53-9.
- Lie SA, Havelin LI, Furnes ON, Engesaeter LB, Vollset SE. Failure rates for 4762 revision total hip arthroplasties in the Norwegian Arthroplasty Register. J Bone Joint Surg (Br) 2004; 86 (4):504-9.
- Lyback CC, Lyback CO, Kyro A, Kautiainen HJ, Belt EA. Survival of Bi-Metric femoral stems in 77 total hip arthroplasties for juvenile chronic arthritis. Int Orthop 2004; 28 (6): 357-61.
- Manley MT, Capello WN, D’Antonio JA, Edidin AA, Geesink RG. Fixation of acetabular cups without cement in total hip arthroplasty. A comparison of three different implant surfaces at a minimum duration of follow-up of five years. J Bone Joint Surg (Am) 1998; 80 (8):1175-85.
- Morscher EW, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg (Br) 1998; 80 (2):267-72.
- Nivbrant B, Kärrholm J. Migration and wear of hydroxyapatite-coated press-fit cups in revision hip arthroplasty: a radiostereometric study. J Arthroplasty 1997; 12 (8): 904-12.
- Ornstein E, Linder L, Ranstam J, Lewold S, Eisler T, Torper M. Femoral impaction bone grafting with the Exeter stem—the Swedish experience: survivorship analysis of 1305 revisions performed between 1989. J Bone Joint Surg (Br) 2009;91 (4):441-6.
- Palm L, Jacobsson SA, Kvist J, Lindholm A, Ojersjo A, Ivarsson I. Acetabular revision with extensive allograft impaction and uncemented hydroxyapatite-coated implants. Results after 9 (7-11) years follow-up. J Arthroplasty 2007; 22 (8): 1083-91.
- Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994; 9 (1): 33-44.
- Patel PD, Potts A, Froimson MI. The dislocating hip arthroplasty: prevention and treatment. J Arthroplasty (4 Suppl 1)2007; 22:86-90.
- Pulido L, Rachala SR, Cabanela ME. Cementless acetabular revision: past, present, and future. Revision total hip arthroplasty: the acetabular side using cementless implants. Int Orthop 2011; 35 (2): 289-98.
- Puolakka TJ, Pajamaki KJ, Pulkkinen PO, Nevalainen JK. Poor survival of cementless Biomet total hip: a report on 1,047 hips from the Finnish Arthroplasty Register. Acta Orthop Scand 1999; 70 (5): 425-9.
- Ranstam J, Kärrholm J, Pulkkinen P, Makela K, Espehaug B, Pedersen AB, Statistical analysis of arthroplasty data. II. Guidelines. Acta Orthop 2011; 82 (3): 258-67.
- Reikerås O, Gunderson RB. Failure of HA coating on a gritblasted acetabular cup: 155 patients followed for 7-10 years. Acta Orthop Scand 2002; 73 (1): 104-8.
- Röhrl SM, Nivbrant B, Snorrason F, Kärrholm J, Nilsson KG. Porous-coated cups fixed with screws: a 12-year clinical and radiostereometric follow-up study of 50 hips. Acta Orthop 2006; 77 (3): 393-401.
- SHAR. Swedish Hip Arthroplasty Register. Annual Report 2010;http://www.shpr.se/Default.aspx
- Soballe K, Overgaard S, Hansen ES, Brokstedt-Rasmussen H, Lind M, Bunger C. A review of ceramic coatings for implant fixation. J Long Term Eff Med Implants 1999; 9 (1-2): 131-51.
- Söderman P, Malchau H, Herberts P, Johnell O. Are the findings in the Swedish National Total Hip Arthroplasty Register valid? A comparison between the Swedish National Total Hip Arthroplasty Register, the National Discharge Register, and the National Death Register. J Arthroplasty 2000; 15 (7): 884-9.
- Söderman P, Malchau H, Herberts P, Zugner R, Regner H, Garellick G. Outcome after total hip arthroplasty: Part II. Disease-specific follow-up and the Swedish National Total Hip Arthroplasty Register. Acta Orthop Scand 2001; 72 (2): 113-9.
- Stilling M, Rahbek O, Soballe K. Inferior survival of hydroxyapatite versus titanium-coated cups at 15 years. Clin Orthop 2009; 467 (11): 2872-9.
- Tanzer M, Drucker D, Jasty M, McDonald M, Harris WH. Revision of the acetabular component with an uncemented Harris-Galante porous-coated prosthesis. J Bone Joint Surg (Am) 1992; 74 (7):987-94.
- Templeton JE, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Revision of a cemented acetabular component to a cementless acetabular component. A ten to fourteen-year follow-up study. J Bone Joint Surg (Am) 2001; 83 (11):1706-11.
- Wangen H, Lereim P, Holm I, Gunderson R, Reikerås O. Hip arthroplasty in patients younger than 30 years: excellent ten to 16-year follow-up results with a HA-coated stem. Int Orthop 2008; 32 (2): 203-8.
