Abstract
Background and purpose Hydroxyapatite (HA) coating is believed to improve bone-implant ingrowth and long-term survival of prostheses. Recent studies, however, have challenged this view. Furthermore, HA particles may produce third-body wear and initiate aseptic loosening of implants. We report the performance of HA- and porous-coated acetabular cups in a prospective randomized trial.
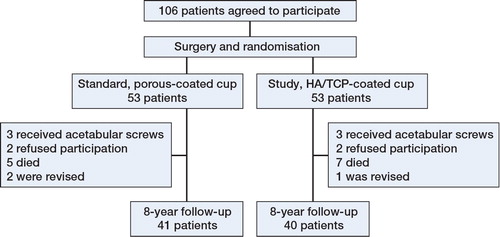
Methods This was an 8-year follow-up study of our previously published prospective randomized study to compare clinical outcomes, survival, periprosthetic bone mineral density, migration, and wear rates of HA- and porous-coated acetabular cups. Dual X-ray absorptiometry (DXA) and Ein Bild Roentgen Analyse (EBRA) measurements were used. 100 patients who underwent unilateral cementless total hip arthroplasty were randomized to either porous-coated cups or HA-coated cups. Patients were examined preoperatively and at 3, 6, and 9 months, and also 1, 3, and 8 years after surgery. 81 patients were available for 8-year follow-up, 40 with porous-coated cups and 41 with HA-coated cups.
Results Age, sex, bone mineral density, and clinical results (Harris hip score) were similar in the 2 groups. The survival, wear, and migration patterns of the cups were also similar in both groups. The results of periprosthetic bone mineral density scans in region of interest 2 was in favor of the porous-coated cups, but there were no differences between the 2 groups in all the remaining regions of interest.
Interpretation HA coating had no statistically significant effect on clinical results, survival, wear, or migration at the 8-year follow-up.
Loosening and polyethylene (PE) limit the longevity of joint replacements. One of the methods to overcome loosening is osteoconductive coatings of hydroxyapatite (HA), which is believed to enhance component fixation by providing initial stability and by improving bone ingrowth (Soballe et al. Citation1990, Furlong and Osborn. Citation1991, Soballe et al. Citation1992, Rahbek et al. Citation2005). The increasing use of HA-coated components has given rise to controversy regarding HA debris-induced osteolysis and PE wear (Morscher et al. Citation1998). Recently, a retrospective study on Mallory-Head cups confirmed that PE wear is greater in HA-coated cups, but without this having a negative influence on revision rate (Gottliebsen et al. Citation2012). The finding of increased wear in HA-coated cups partially confirms the mechanism of failure for these cups: third-body wear, osteolysis, and loosening. A previous Danish Hip Arthroplasty Register study compared HA-coated implants with non-HA-coated implants (Paulsen et al. Citation2007) and showed that HA coating did not reduce the overall risk of revision. The same conclusions were reached from a study in the Swedish Hip Arthroplasty Register regarding uncemented femoral stems (Lazarinis et al. Citation2011). On the other hand, another Swedish registry study found inferior results for acetabular cups coated with hydroxyapatite. The authors questioned the routine use of HA-coated cups in primary total hip arthroplasty (THA) and they were able to show an even higher risk of loosening of HA-coated cups that led to revisions (Lazarinis et al. Citation2010).
In our previously reported randomized study comparing HA- and porous-coated acetabular cups (Laursen et al. Citation2007), we found similar outcome, survival, and changes in bone mineral density (BMD) around cups at 3-year follow-up. Here we present data from the 8-year follow-up, adding results on migration and on analysis of PE wear.
Patients and methods
166 consecutive patients were invited to participate in the trial; 60 patients refused. 6 others were excluded because screws had been used for cup fixation. 100 patients who underwent unilateral cementless THA were enrolled in a controlled randomized study from October 1998 to May 2000. Inclusion criteria were primary hip osteoarthrosis, no previous hip surgery in the hip planned to be operated, and no competing medical conditions (neurological disorders or metabolic diseases) that could influence postoperative medication, hindering bone formation or an impending standard mobilization program. Patients were scheduled for unilateral cementless arthroplasty with press-fit fixated cups, and signed an informed consent document. For details of the surgery, the randomization procedure, and the cups used, see Laursen et al. (Citation2007).
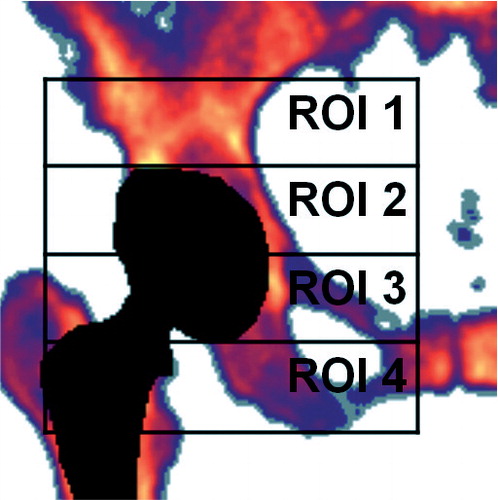
The patients were examined preoperatively, at 3, 6, and 9 months, and also 1, 3, and 8 years after surgery. Data regarding clinical results, periprosthetic bone mineral density (BMD) measurements, complications during the first 3-year follow-up, were given in our previous paper (Laursen et al. Citation2007). At the latest follow-up, the following data were collected: (1) Demographics, complications, prosthesis survival, and clinical results assessed according to the Harris hip score (HHS); (2) Dual X-ray absorptiometry (DXA) for measurement of BMD around the THA cups. Scans of the acetabular region were performed using the same Norland X-ray bone densitometer (Norland Corporation) as in the original study, but with upgraded software (Illuminatus DXA 4.2.0), allowing examination of 4 regions of interest (ROIs) according to Wilkinson et al. (Citation2001) (); (3) Cup migration and polyethylene liner wear using Ein Bild Roentgen Analyse (EBRA) software. The EBRA measurement system is based on measurement of 2D migrations from digitalized plain radiographs using a grid of transverse and longitudinal tangents on prominent landmarks of the pelvic contour. Based on the measurement error of the EBRA software, migration values less than 1 mm were not considered significant (Ilchmann et al. Citation1995, Wilkinson et al. Citation2002).
Figure 1. Four regions of interest for DEXA measurements, “the Wilkinson regions”. (Figure used from Laursen et al. Citation2007 with permission).
We used records from the Danish Hip Arthroplasty Registry and The Danish National Patient Registry to obtain information on patients who were lost to follow-up.
Statistics
The null hypotheses tested were: (1) There is no difference between the 2 treatment groups concerning migration of the cups, as measured by EBRA; and (2) There is no difference between the 2 treatment groups in any of the 4 ROIs concerning BMD change during the evaluation period. In both cases, a significance level of p < 0.05 was selected.
Sample size was chosen to find differences exceeding BMD of 0.25 g/cm2 (least relevant difference: δ = 0.25; SD 0.4), with a risk of type-1 error: α = 0.05 (5%); β = 0.2 (20%); statistical power (risk of type-2 error) = 1 – β = 80%. The statistical table sample size had 43 patients in each group. In our previous study, 50 patients were chosen in each group to allow for dropouts. Differences between groups were evaluated by calculating the 95% confidence limits of the changes in BMD.
Survival data were calculated as Kaplan-Meier survival estimates, and p-value was calculated from a log-rank test using R statistical software. BMD and EBRA results were calculated as repeated-measures ANOVA, and displayed as mean curves with 1.96 SD error bars. If the mean fell outside the error bar of the other curve, there was a statistically significant difference.
Ethics
The Ethics Committee of North Denmark Region approved the study (N-20080005). It was performed in compliance with the Helsinki Declaration. All patients signed an informed consent document. The protocol was registered at ClinicalTrials.Gov with the identifier NCT00159497.
Results
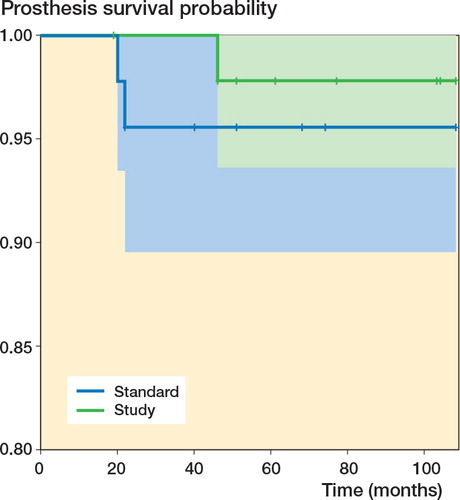
The patients who were invited and accepted to attend a follow-up had a mean follow-up period of 8.8 (7.9–9.6) years. 4 patients (2 from each group) refused participation in the 8-year follow-up; 13 had died of unrelated causes before the latest follow-up (5 in the standard (porous-coated) group and 7 in the study (HA/TCP-coated) group). Records from Danish national registries revealed that 1 of these patients had received an HA-coated cup and had undergone revision at another hospital due to aseptic loosing of the femoral stem. The patient was excluded from our study population because of the revision, but was included in the survival analysis. In 2 patients, both with non-HA-coated cups, revision surgery had been performed (due to aseptic loosing of the femoral stem in one case and recurrent luxation in other), leaving a study population of 81 patients (). Similar numbers of patients were included in the 2 groups, and age, sex, BMI, and clinical results by HHS were similar (Table). Also, Kaplan-Meyer survival analysis showed a similar distribution of patients in the 2 groups ().
Figure 3. Kaplan-Meier survival estimates with revision as end-point. “Standard” refers to porous-coated cups and “Study” refers to HA/TCP-coated cups. The colored areas represent 95% confidence intervals. Patients who died before the last follow-up appear as censored observations and are marked with crosses. The p-value, calculated from a log-rank test, was 0.5.
Demographic data and Harris hip score (HHS)
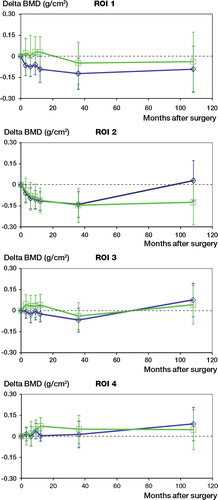
Changes in periprosthetic BMD were measured in 4 ROIs (). There was a decrease in periprosthetic bone density during the first postoperative years in all ROIs except ROI 4. In ROI 1, neither group reached early postoperative BMD levels (measured within 1 week after surgery), whereas in ROI 4, BMD levels rose during all 8 years of follow-up. We found a small but statistically significant difference in BMD in ROI 2 in favor of the standard (non-HA) cups. Results of periprosthetic BMD in ROIs 1, 3, and 4 revealed no differences between the 2 groups after 8 years.
Figure 4. BMD changes in 4 regions of interest (ROIs). Measurements in ROI 2 were significantly favorable for the porous-coated cup.
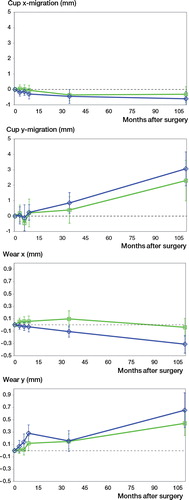
80 patients had radiographs that were adequate for the EBRA analysis. We measured migration of cups and wear rate (). Results were calculated as repeated-measures ANOVA, and we found greater migration and wear rates in the standard porous-coated group, but the differences between groups were not statistically significant.
Discussion
In the late 1980s, de Groot et al. (Citation1987) described a technique for direct covering of prosthetic surfaces with hydroxyapatite. This triggered a plethora of studies seeking an explanation of processes in the bone-coating/prostheses interface and looking for data which would support a superior performance for HA-coated components (Furlong and Osborn. Citation1991, Hardy et al. Citation1991, Soballe Citation1993, Overgaard et al. Citation1998, Rahbek et al. Citation2005). As the use of HA-coated implants increased, hydroxyapatite granules were suspected of becoming disintegrated from implants and migrating into the joint space, producing third-body wear, osteolysis, and loosening of components (Morscher et al. Citation1998, Stilling et al. Citation2009). Despite the fact that there have been many studies, definitive conclusions regarding the performance of HA-coated implants have not been reached because coating properties, implant roughness, and overall design all influence study results. Superior performance in long-term clinical or registry studies should be the most reliable predictor of the benefits of coating (Dumbleton and Manley. Citation2004). But register and long-term clinical studies also reveal discrepancies regarding the performance of HA-coated hip prostheses. Gottliebsen et al. (Citation2012) reported superior survival of HA-coated Mallory-Head cups in their 11-year follow-up clinical study, but a higher annual PE wear rate in the HA group than in the non-HA group. This was a retrospective non-randomized study and the mean age in the HA group was 6 years younger than that in the non-HA group, indicating that a higher activity level could contribute to the PE wear. Gottliebsen’s findings certainly highlight the risk of HA particles from the implant surface entering the bearing and causing excessive wear, as also shown by Stilling et al. (Citation2009) at 15-year follow-up in an RCT where the HA-coated cups had a higher revision rate than in the Ti-group (57% vs. 17%). An increased risk of need for revision of HA-coated acetabular cups was noted in a Swedish Hip Arthroplasty Register study (Lazarinis et al. Citation2010), but this could not be confirmed by data based on the Danish Hip Arthroplasty Register (Paulsen et al. Citation2007).
To come to conclusions based on reliable data, an impeccable study design and long-term follow-up are crucial. On this basis, the present study is of interest concerning the effectiveness of HA coating of acetabular components. The study has several limitations, however. It was statistically underpowered, the findings apply to one specific prosthesis, and there was a lack of patient-reported outcome measures. The strengths of the study were the similar-sized patient groups, blinded prospective randomization, identical prostheses, and 8-year follow-up. Our previous publication (Laursen et al. Citation2007) provided data obtained after a 3-year follow-up. These results showed similar BMD changes and clinical outcome between HA and non-HA (standard porous-coated) cups. We assumed that HA coating would show benefit after a longer follow-up. At the 8-year follow-up, both patient groups gave affirmative results with similar clinical outcome. Overall changes in periprosthetic BMD had no statistically significant differences and had no negative influence on clinical outcome. All our patients showed major loss of BMD in ROI 1, and the level of BMD afterwards did not reach the preoperative level in either group. This is surprising, because ROI 1 represents the most loaded pelvic area, and the body’s response to the load should induce most significant bone strengthening. Schmidt et al. (Citation2012) published a prospective 7-year follow-up, computed tomography-assisted study on cortical and cancellous BMD loss after press-fit HA-coated cup fixation. The authors observed a loss of BMD in the cranial periacetabular area, comparable to our ROI 1, during the follow-up period. Their patients showed no adverse effects on outcome due to BMD loss.
A Swedish registry study (Lazarinis et al. Citation2010) showed increased revision rates in patients with HA-coated cups. Our data do not support this finding. On the contrary, our results show a clear tendency of less migration and lower wear rates in the HA-coated group. Gottliebsen et al. (Citation2012) presented 11-year follow-up data comparing HA-coated and non-HA-coated Mallory-Head cups. They concluded that PE wear was increased in HA-coated cups. In our study and in that by Gottliebsen et al., the values for PE wear did not have a negative effect on the revision rate or on the clinical outcome. Having this controversial knowledge, we were uncertain as to whether the better sealing effect of HA coatings (Furlong and Osborn. Citation1991, Rahbek et al. Citation2005) has any effect on the wear, migration, and survival of prostheses. We used the same type of acetabular component (produced by one manufacturer) in all study patients. Thus, our findings apply to only this specific prosthesis. However, we suggest that both surgeons and the companies that manufacture HA-coated prostheses should consider whether use of these prostheses is justified considering the lack of scientific evidence to support their rational use.
In summary, we did not find any superior effect of Trilogy Calcicoat (HA-coated) cups over Trilogy porous-coated cups at 8-year follow-up. Both cups had excellent clinical outcome and equal overall revision rates, and there were no statistically significant differences regarding BMD loss in periacetabular bone, migration, or PE wear.
KV, KS, PTN, and MBL designed the study. KV and MBL gathered the data. KV reviewed all the patients, MBL did the EBRA analysis, and KV and MBL performed all the statistical analyses and interpretation of data. All the authors were involved in writing of the manuscript.
We thank nurse Ulla Hornum for her great help in managing patient contacts and outpatient clinic visits, and statistician Maria Rodrigo Domingo for statistical analysis and consultations. The previous study (Laursen et al. Citation2007) was supported by Zimmer Scandinavia, which produced Trilogy Calcicoat prostheses.
No competing interests declared.
- de Groot K, Geesink R, Klein CP, Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res 1987; 21 (12): 1375-81.
- Dumbleton J, Manley MT. Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J Bone Joint Surg (Am) 2004; 86 (11): 2526-40.
- Furlong RJ, Osborn JF. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J Bone Joint Surg (Br) 1991; 73 (5): 741-5.
- Gottliebsen M, Rahbek O, Ottosen PF, Soballe K, Stilling M. Superior 11-year survival but higher polyethylene wear of hydroxyapatite-coated mallory-head cups. Hip Int 2012; 22 (1): 35-40.
- Hardy DC, Frayssinet P, Guilhem A, Lafontaine MA, Delince PE. Bonding of hydroxyapatite-coated femoral prostheses. histopathology of specimens from four cases. J Bone Joint Surg (Br) 1991; 73 (5): 732-40.
- Ilchmann T, Mjoberg B, Wingstrand H. Measurement accuracy in acetabular cup wear. three retrospective methods compared with roentgen stereophotogrammetry. J Arthroplasty 1995; 10 (5): 636-42.
- Laursen MB, Nielsen PT, Soballe K. Bone remodelling around HA-coated acetabular cups: A DEXA study with a 3-year follow-up in a randomised trial. Int Orthop 2007; 31 (2): 199-204.
- Lazarinis S, Karrholm J, Hailer NP. Increased risk of revision of acetabular cups coated with hydroxyapatite. Acta Orthop 2010; 81 (1): 53-9.
- Lazarinis S, Karrholm J, Hailer NP. Effects of hydroxyapatite coating on survival of an uncemented femoral stem. A swedish hip arthroplasty register study on 4,772 hips. Acta Orthop 2011; 82 (4): 399-404.
- Morscher EW, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg (Br) 1998; 80 (>2): 267-72.
- Overgaard S, Lind M, Glerup H, Bunger C, Soballe K. Porous-coated versus grit-blasted surface texture of hydroxyapatite-coated implants during controlled micromotion: Mechanical and histomorphometric results. J Arthroplasty 1998; 13 (4): 449-58.
- Paulsen A, Pedersen AB, Johnsen SP, Riis A, Lucht U, Overgaard S. Effect of hydroxyapatite coating on risk of revision after primary total hip arthroplasty in younger patients: Findings from the danish hip arthroplasty registry. Acta Orthop 2007; 78 (5): 622-8.
- Rahbek O, Kold S, Bendix K, Overgaard S, Soballe K. Superior sealing effect of hydroxyapatite in porous-coated implants: Experimental studies on the migration of polyethylene particles around stable and unstable implants in dogs. Acta Orthop 2005; 76 (3): 375-85.
- Schmidt R, Kress AM, Nowak M, Forst R, Nowak TE, Mueller LA. Periacetabular cortical and cancellous bone mineral density loss after press-fit cup fixation: A prospective 7-year follow-up. J Arthroplasty 2012; 27 (7): 1358,1363.e1.
- Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. mechanical and histological studies in dogs. Acta Orthop Scand (Suppl 255) 1993: 1-58.
- Soballe K, Hansen ES, Brockstedt-Rasmussen H, Pedersen CM, Bunger C. Hydroxyapatite coating enhances fixation of porous coated implants. A comparison in dogs between press fit and noninterference fit. Acta Orthop Scand 1990; 61 (4): 299-306.
- Soballe K, Hansen ES, B-Rasmussen H, Jorgensen PH, Bunger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10 (2): 285-99.
- Stilling M, Rahbek O, Soballe K. Inferior survival of hydroxyapatite versus titanium-coated cups at 15 years. Clin Orthop 2009; (467) (11): 2872-9.
- Wilkinson JM, Peel NF, Elson RA, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 2001; 83 (2): 283-8.
- Wilkinson JM, Hamer AJ, Elson RA, Stockley I, Eastell R. Precision of EBRA-digital software for monitoring implant migration after total hip arthroplasty. J Arthroplasty 2002; 17 (7): 910-6.