Abstract
Background and purpose Dupuytren’s disease (DD) is a benign fibroproliferative process of the palmar aponeurosis showing similarities to wound healing. Communication of cells involved in wound healing is mediated by the composition of gap junction (GJ) proteins. We investigated the expression of 3 GJ proteins, connexins 26, 30, and 43 (Cx26, Cx30, and Cx43) in DD.
Patients and methods Fragments of Dupuytren’s tissue from 31 patients (mean age 56 (30–76) years, 24 male) were analyzed immunohistochemically and compared to control tissue for expression of the GJ proteins Cx26, Cx30, and Cx43 and also alfa-smooth muscle actin (α-SMA).
Results 14 of 31 samples could be attributed to the involutional phase (α-SMA positive) whereas 17 samples had to be considered cords in the residual phase (α-SMA negative). Expression of Cx26 and Cx43 was seen in 12 of the 14 samples from the involutional phase, and Cx30 was seen in 7 of these. Only 4 of the 17 samples from the residual phase showed any Cx, and there was none in the controls.
Interpretation The high expression of GJ proteins Cx26, Cx30, and Cx43 in α-SMA positive myofibroblast-rich nodules, which are characteristic of the active involutional phase of DD, suggests that connexins could be a novel treatment target for the treatment of DD.
Dupuytren’s disease (DD) is a benign progressive disease of the palmar aponeurosis that leads to a permanent and irreversible flexion contracture of the fingers. Various genetic aberrations (Dolmans et al. Citation2011) and environmental factors (Burge et al. Citation1997) have been linked to the development of DD. Local tissue damage can cause myofibroblast proliferation or tissue repair (Verjee et al. Citation2009, Shih and Bayat Citation2010).
Because of the similarities, parallels have been drawn between DD and wound healing (Tomasek et al. Citation2002, Howard et al. Citation2004, Shih and Bayat Citation2010, Holzer et al. Citation2013). Myofibroblasts are present in both DD and in wound healing, and play an important role throughout the wound healing process, eventually causing a large deposit of collagen III (Shih and Bayat Citation2010).
Wound healing is a complex, carefully regulated process requiring communication between different cell types. In normal tissues, fibroblasts are widely separated by extracellular matrix, but contact each other through elongated protoplasmic extensions. Communication is provided by gap junction (GJ) channels (Kumar and Gilula Citation1996, Mese et al. Citation2007, Churko and Laird Citation2013). These GJs allow the passage of small molecules, including ions and second messengers, between cells (Kumar and Gulila Citation1996). The protein subunits of GJ channels are called connexins. Their composition is important for their selectivity regarding passage of molecules and—as a result—communication between cells (Kumar and Gulila Citation1996). GJs have been shown to be important in cell proliferation, migration, and differentiation (Kumar and Gulila Citation1996, Mese et al Citation2007). In recent years, it has been shown that connexins play a critical role in wound healing, and Cx26, Cx30, and Cx43 are involved in this process (Brandner et al. Citation2004).
Connexin expression changes in the different stages of wound healing, and this is important in regulation of the process. Cx26, Cx30, and Cx43 expression is rapidly downregulated at the wound edge within 6 h of wounding. In the subsequent stages, cells at the edges continue to be Cx26- and Cx30-negative whereas cells behind the edges show upregulation. The loss of Cx43 staining in all cells of the regenerating epidermis appears to be important for induction of human wound healing (Coutinho et al. Citation2003, Brandner et al. Citation2004, Davis et al. Citation2013). Additionally, connexins have recently been shown to directly control gene expression and cell migration (Kardami et al. Citation2007).
3 connexins have been reported to have a role in human wound healing: Cx26, Cx30, and Cx43 (Brandner et al. Citation2004). We determined the expression of Cx26, Cx30, and Cx43 in Dupuytren’s tissue immunohistochemically, to investigate a possible involvement of GJ proteins in the pathogenesis of DD.
Patients and methods
31 Caucasian patients with DD (mean age 56 (30–76) years, 24 male) who were consecutively treated surgically between April and December 2008 were included in this study (). Clinically, DD was diagnosed according to Iselin’s 4-degree classification, as follows: first degree, palmar tubercles and small cords without signs of contracture; second degree, bending contracture within the metacarpophalangeal articulation; third degree, bending contracture affecting the proximal interphalangeal articulation; and fourth degree, severe contracture within the metacarpophalangeal articulation with hyperextension in the distal interphalangeal articulation, together with advanced secondary lesions in the osseous system (Iselin and Dieckmann Citation1951).
Table 1. Demographic data on patients with Dupytren’s disease and carpal tunnel syndrome
Patients had either partial or total fasciectomy, and tissue samples of pathological palmar aponeurosis (Dupuytren’s tissue) were taken during surgery, quick-frozen in Tissue-Tek Cryomold Standard (Miles Laboratories Inc., Kamkakee, IL) and stored frozen at –70°C. As a control, tissue was taken from the transverse carpal ligament of 9 patients (mean age 62 (44–79) years, 6 female) who were treated surgically for carpal tunnel syndrome.
The samples of Dupuytren’s tissue were classified histologically according to the 3 distinct phases proposed by Luck (Citation1959). These describe the 3 phases of disease progression: (1) proliferative, (2) involutional, and (3) residual. The proliferative phase is almost entirely composed of myofibroblasts in highly cellular nodules. In the involution phase, which is still highly cellular, cells begin to align themselves along the lines of stress within the tissue. In the residual phase, which is almost acellular, myofibroblasts disappear leaving mature fibroblasts combined with thick bundles of collagen.
Immunohistochemistry
To establish the myofibroblast phenotype and the relationship between the GJ proteins Cx26, Cx30, and Cx43, we prepared representative serial sections of tissue samples from patients with DD and from controls.
Frozen tissue specimens were cut into 5-µm thick sections and produced on lysine-coated slides. Before immunostaining, the slides were dryed for 30 min at room temperature and then fixed in acetone for 10 min. Endogenous peroxidase activity was eliminated by incubation in 0.3% hydrogen peroxide for 3 min. For alfa-smooth muscle actin (α-SMA) determination, we used monoclonal mouse anti α-SMA (cat. no. A 2547; Sigma-Aldrich, St. Louis, MO). For different connexins, tissue sections were incubated overnight with the following primary antibodies: mouse monoclonal anti-Cx26 diluted 1:100 (cat. no. 13-8100), rabbit polyclonal anti-Cx30 diluted 1:500 (cat. no. 71-2200), and rabbit polyclonal anti-Cx43 diluted 1:500 (cat. no. 70-0700; all primary antibodies were from Invitrogen, Life Technologies, Carlsbad, CA). As secondary antibodies, we used biotin-labeled rabbit anti-mouse antibodies to detect the monoclonal primary antibodies and biotinylated goat anti-rabbit antibodies to detect polyclonal primary antibodies. This was followed by incubation with streptavidin. The reaction products were developed with 0.5% Triton X-100 (SERVA) and 10 µL hydrogen peroxide per 100 mL staining fluid, and 0.06% diaminobenzidine (Fluka, Buchs, Switzerland). Slides were counterstained with hematoxylin. The same immunohistochemical protocol was followed for negative controls, with omission of the primary antibodies. Blood vessels at the periphery of the tissue samples were used as an internal positive control.
Staining intensity of immunohistochemically stained sections was evaluated semi-quantitatively. A zero score defined slides with no staining, while 1+ referred to slides with a faint staining. A weak positive result characterized by weak staining corresponded to a 2+ score, while a strong positive result was represented by 3+. Scores of 0 were classified as negative, while scores of 1+, 2+, and 3+ were regarded as positive.
The evaluation was performed by one individual (AC) who was blind regarding the clinical stages of the patients.
Ethics
The representative material and patient demographic data were collected after obtaining written informed consent. In all cases, sample acquisition complied with the 1975 Declaration of Helsinki. The study was approved by the Ethical Review Board of the Medical University of Vienna (EK 183/2008).
Statistics
All data are given as mean (range). Statistical analyses were performed using the chi-square test and Fisher’s exact test, and p-values of < 0.05 were considered significant.
Results
Although the clinical symptoms and the grade of contracture were used as indications for surgery, the distribution covered all clinical stages. According to Iselin’s classification (Iselin and Dieckmann Citation1951), 6 of 31 patients were classified as stage I, 8 as stage II, 12 as stage III, and 5 as stage IV.
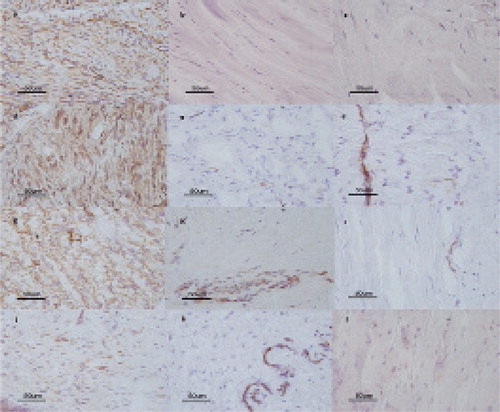
Histologically, 14 samples were attributed to the involutional phase of the disease and 17 samples to the residual phase, according to the Luck classification. Cellular-rich nodules mainly composed of myofibroblasts, which are characteristic of Dupuytren’s tissue, could be shown by positive staining for α-SMA in 14 of 31 samples (involutional phase) (Figure), whereas 17 samples were considered to be lesions in the residual phase. All control samples (CTS) were also negative for α-SMA.
Immunohistochemical expression of α-SMA (a, b, c); Cx26 (d, e, f); Cx43 (g, h, i), and Cx30 (j, k, l) in tissue sections from the involutional phase of DD (a, d, g, j); in tissue sections from the residual phase of DD (b, e, h, k), and in tissue sections from controls (patients with carpal tunnel syndrome) (c, f, i, l) (magnification 400×).
Clinical classification according to Iselin did not relate to histology according to Luck’s stages or to the expression pattern of connexins, either in the involutional phase or in the residual phase of the disease.
Immunohistochemical staining
At least 1 of the connexins was expressed in 12 of 14 α-SMA positive samples, whereas connexins were detected in only 4 of 17 α-SMA negative samples. Cx26 and Cx43 expression was seen in 12 of 14 samples in the involutional phase and Cx30 expression was seen in 7 of the 14 (Figure). In these 7 cases, all 3 connexins were detected. In the residual phase, GJ protein expression was low; Cx26 and Cx43 were found in 4 of 14 samples and 3 of 14 samples, respectively. No Cx30 expression was detected in the residual phase. None of the GJ proteins studied were expressed in the control samples ().
Table 2. Numbers of Cx26-, Cx30-, and Cx43-positive cases in the proliferative and residual phase and in controls
Expression of Cx26, Cx30, and Cx43 in the involutional phase was higher than in the residual phase (Cx26 and Cx43: p < 0.001; Cx30: p = 0.03) and in the controls (Cx26 and Cx43: p < 0.001; Cx30: p = 0.02). Intensity of staining was highest for Cx26 and Cx43.
Discussion
We found expression of Cx26, Cx30, and Cx43 mainly in the involutional phase of DD. This is different from spontaneous epithelial wound healing, where expression of all these connexins is downregulated and Cx26 and Cx30 expression is increased at later stages, at the edges of wound margins. Cx43 is expressed in DD, but loss of Cx43 is essential for the induction of wound healing. We have not found any previous studies that have examined the expression of GJ proteins in tissue specimens of DD.
DD shares many biological features with wound healing. Both processes are characterized by proliferation of fibroblasts and myofibroblasts and by the deposition of collagen III (Howard et al. Citation2004, Tomasek et al. Citation2002, Shih and Bayat Citation2010, Holzer et al. Citation2013). Several structural proteins, including collagen III and signaling molecules such as transforming growth factor-β, fibronection, and the heat shock protein HSP47 (Howard et al. Citation2004) are known to be expressed both during wound healing and in DD. Myofibroblasts are implicated in the contraction of both wound granulation tissue and cords characteristic of DD. These cells not only regulate the remodeling of the extracellular matrix, but also produce growth factors that can promote scar formation in the case of DD, or further tissue repair events during normal wound healing.
Wound healing requires communication between different cell types, which is provided by connexins, the protein subunits of GJ channels. The skin has an extensive GJ network, which ensures coordinated regional cellular activity (Mese et al Citation2007). 3 connexins have been described in wound healing: Cx26, Cx30, and Cx43 (Goliger and Paul Citation1995, Coutinho et al. Citation2003, Brandner et al. Citation2004).
Cx26 is expressed in proliferative epidermis during wound re-epithelization (Goliger and Paul Citation1995, Coutinho et al. Citation2003). Unlike the ear, where at least Cx26 appears to be essential for normal function, there may be redundancy in the skin. Thus, loss of any one connexin is not deleterious unless it also affects the other connexins with which it interacts. Mutations in both Cx26 and Cx30 have been associated with various skin phenotypes linked to non-syndromic deafness in different human populations (Xu and Nicholson Citation2013). Cx30 is strongly expressed in the scar epidermis and was found in the epidermis of patients presenting with different hyperproliferative skin disorders (Lemaître et al. Citation2006).
In spontaneous epithelial wound healing, the pattern of connexin expression changes (Kumar and Gilula Citation1996). Initially, there is a lack of staining of Cx26, Cx30, and Cx43 in cells at the wound margins and surroundings. This early lack of all 3 of these connexins appears to be important for spontaneous wound healing.
In the subsequent stages, cells at the edges of regenerating epidermis continue to be Cx26- and Cx30-negative, where cells behind the edges or at some distance show an upregulation of Cx26 and Cx30 expression. The loss of Cx43 staining in all cells of the regenerating epidermis at or near the wound margins during the early stages appears to be important for the induction of human wound healing (Coutinho et al. Citation2003, Brandner et al. Citation2004, Davis et al. Citation2013).
Cx43, the most abundant connexin, appears to have a key role in skin wound healing, and its expression is greatly reduced in keratinocytes migrating into the margins of healing wounds (Goliger and Paul Citation1995). In mouse skin, reducing the expression of Cx43 by conditional gene knockout leads to accelerated incisional wound closure, but is followed by an upregulation of various connexins (Mori et al. Citation2006).
In contrast, early upregulation of Cx26 and Cx30 and no loss of Cx43 at or near the wound margins is typical for chronic, non-healing wounds (Pollok et al. Citation2011, Becker at al. 2012). The missing downregulation of Cx43 may contribute to the failure to heal (Brandner et al. Citation2004).
Significant increases in skin wound healing rates occur by altering GJ-mediated intercellular communication, but the underlying cellular and molecular basis remains unclear (Wright et al. Citation2009). Both downregulation of connexin expression in migrating cells and interaction of connexins with the extracellular matrix may be vitally important for cell migration during wound closure. Connexin mimetic peptides have been shown to inhibit GJ in a variety of cell types such as smooth muscle cells, endothelial cells, and fibroblasts (Kumar and Gilula Citation1996, Mese et al. Citation2007). Inhibitors that disrupt GJs result in a significant decrease in myofibroblast activity, and hence contraction. Topical Cx43 knockdown results in significant enhancement of wound-healing rates in different model systems (Qiu et al. Citation2003, Pollok et al. Citation2011). Inhibitors that disrupt GJs results in higher skin wound healing rates (Verhoekx et al. Citation2013).
So, connexins and the change in their expression, especially of Cx43, may offer a therapeutic approach to downregulation of myofibroblast activity in cutaneous and musculoskeletal fibrotic disorders such as DD.
Local recurrence after treatment of DD is common (Rayan Citation2007). Targeting of specific connexins could provide a new approach to the modulation of cell behavior in DD, as has been seen in wound closure (Wright et al. Citation2009).
LAH designed the study, analyzed the data, and wrote the manuscript. AC analyzed the immunohistochemistry results and wrote part of the manuscript. GH designed the study, analyzed the data, and wrote the manuscript.
We thank G. Pfandlsteiner, MD, for providing the specimens and G. Brand (†) and R. Grübl for preparing the slices and doing the immunohistochemistry. LAH thanks CF Ebner, MD, for inspiring discussions on gap junction proteins. GH dedicates the paper to the memory of G. Brand.
No competing interests declared.
- Becker DL, Thrasivoulou C, Phillips AR. Connexins in wound healing; perspectives in diabetic patients. Biochim Biophys Acta 2012; 1818: 2068-75.
- Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol 2004; 122: 1310–20.
- Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren’s contracture. J Bone Joint Surg (Br) 1997; 79: 206–10.
- Churko JM, Laird DW. Gap junction remodeling in skin repair following wounding and disease. Physiology (Bethesda) 2013; 28: 190-8.
- Coutinho P, Qiu C, Frank S, Tamber K, Becker D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int 2003; 27: 525–41.
- Davis NG, Phillips A, Becker DL. Connexin dynamics in the privileged wound healing of the buccal mucosa. Wound Repair Regen 2013; 21: 571-8
- Dolmans GH, Werker PM, Hennies HC, Furniss D, Festen EA, Franke L, Becker K, van der Vlies P, Wolffenbuttel BH, Tinschert S, Toliat MR, Nothnagel M, Franke A, Klopp N, Wichmann HE, Nürnberg P, Giele H, Ophoff RA, Wijmenga C. Wnt signaling and Dupuytren’s disease. N Engl J Med 2011; 365: 307-17.
- Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol Biol Cell 1995; 6: 1491–501.
- Holzer LA, Cör A, Pfandlsteiner G, Holzer G. Expression of VEGF, its receptors and HIF-1α in Dupuytren’s disease. Acta Orthop 2013; 84: 420-5
- Howard JC, Varallo VM, Ross DC, Faber KJ, Roth JH, Seney S, Gan BS. Wound healing-associated proteins Hsp47 and fibronectin are elevated in Dupuytren’s contracture. J Surg Res 2004; 117: 232–8.
- Iselin M, Dieckmann GD. Therapy of Dupuytren’s contracture with total zigzag plastic surgery. Presse Med 1951; 59: 1394–5.
- Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol 2007; 94: 245–64.
- Kumar MN, Gilula NB. The gap junction communication channel. Cell 1996; 84: 381–8.
- Lemaître G, Sivan V, Lamartine J, Cosset JM, Cavelier-Balloy B, Salomon D, Waksman G, Martin MT. Connexin 30, a new marker of hyperproliferative epidermis. Br J Dermatol 2006; 155: 844-6.
- Luck JV. Dupuytren’s contracture; a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg (Am) 1959; 41: 635–64.
- Mese G, Richard G, White TW Gap junctions: basic structure and function. J Invest Dermatol 2007; 127: 2516–24.
- Mori R, Power KT, Wang CM, Martin P, Becker D. Acute downregulation of connexin43 at wound sites leads to reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 2006; 119: 5193–203.
- Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, Brandner JM. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J Cell Mol Med 2011; 15: 861–73.
- Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, Green CR, Becker DL. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 2003; 13: 1697–703.
- Rayan GM. Dupuytren Disease: Anatomy, pathology, presentation, and treatment. J Bone Joint Surg (Am) 2007; 89: 189-98.
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol 2010; 6: 715-26.
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002; 3: 349–63.
- Verhoekx J, Verjee LS, Izadi D, Chan J KK, Nicolaidou V, Davidson D, Midwood KS, Nanchahal J. Isometric Contraction of Dupuytren’s Myofibroblasts is Inhibited by Blocking Intercellular Junctions. J Investig Dermatol 2013; 133(12): 2664-71.
- Verjee LS, Midwood K, Davidson D, Essex D, Sandison A, Nanchahal J. Myofibroblast distribution in Dupuytren’s cords: correlation with digital contracture. J Hand Surg Am 2009; 34: 1785-94.
- Wright CS, van Steensel M AM, Hodgins MB, Martin P ME. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Rep Reg 2009; 17: 240–9.
- Xu J, Nicholson BJ. The role of connexins in ear and skin physiology— Functional insights from disease-associated mutations. Biochim Biophys Acta 2013; 1828: 167–78.

