Abstract
Background and purpose YKL-40 is a glycoprotein that is expressed in many types of cancer cells. In some cancers, there is a correlation between high serum YKL-40 levels on the one hand and more aggressive disease and early death on the other. YKL-40 has never been studied in patients with soft-tissue sarcomas (STSs). We investigated whether YKL-40 is expressed in STS tissue and ascertained that the degree of expression is related to survival and/or the histological grade of the malignancy (FNCLCC).
Patients and methods We included archived tissue from 49 patients (40 with STS and 9 with atypical lipomatous tumor, 20 female and 29 male, mean age 58 (4–89) years) who were treated with tumor resection in 2004 or 2005 at the Department of Orthopedics, Rigshospitalet. The minimum length of follow-up with respect to survival was 5–7 years. Immunohistochemical analysis with anti-YKL-40 antibody using tissue microarray was performed on resected tumors, and a semiquantitative measure of the intensity of YKL-40 staining was performed.
Results 41 of the 49 tumors were positive for YKL-40, and of these, 36 had moderate to intense staining. 24 of the patients died within the follow-up period, and the intensity of YKL-40 staining was significantly higher in tumors from patients who had died in the follow-up period than in tumors from those who survived (p = 0.01). The staining intensity was different for the 3 grades of malignancy (p = 0.004): it was higher in highly malignant tumors (FNCLCC grade 2 and grade 3) than in low-malignancy tumors (grade 1).
Interpretation YKL-40 is expressed in soft-tissue sarcomas. There is a correlation between expression of YKL-40 in STS and both histological grade of the malignancy and survival. Whether or not YKL-40 expression is an independent prognostic variable could not be determined in the present study.
YKL-40 (also called chitinase 3-like protein 1 (CHI3L1) and human cartilage glycoprotein 39 (HC gp39)) is a 40-kDa heparin-binding glycoprotein (Rehli et al. Citation1997, Johansen et al. Citation2009) that is produced by a variety of normal cells and cancer cells (Rehli et al. Citation1997, Johansen Citation2006, Johansen et al. Citation2009). YKL-40 is a growth and differentiation factor—particularly for cartilage cells, bone cells, and fibroblasts (Johansen et al. Citation2007)—that protects against cell death (Lee et al Citation2009), and it plays a role in angiogenesis (Shao et al Citation2009, Faibish et al Citation2011) and fibrosis (Lee et al Citation2011). In cancer cells, the production of YKL-40 is stimulated by stress influences such as hypoxia and radiotherapy, and it probably has a role in cancer cell growth, survival, and proliferation (Johansen et al. Citation2009).
Expression of YKL-40 in sarcomas has previously been studied by immunohistochemistry in chondrosarcomas (Daugaard et al. Citation2009), and improved standardization of the staining method using tissue microarray (TMA) has been used in breast and ovarian cancer tissue (Roslind et al. Citation2008, Høgdall et al. Citation2009), but little is known about the expression and prognostic role of YKL-40 in soft-tissue sarcomas (STSs). We therefore investigated the expression of YKL-40 in STS and atypical lipomatous tumors (ALTs) by immunohistochemistry and TMA, and the prognostic significance of the YKL-40 expression was determined.
Patients and methods
Patients
We included 49 patients aged 58 (4–89) years at diagnosis, 20 females and 29 males, with newly diagnosed STS (n = 40) or ALT (n = 9) of the trunk wall and limbs who had been diagnosed and treated surgically in the period of 2004–2005 at the Department of Orthopedics, Rigshospitalet, Copenhagen (). With the exception of the ALTs, we used the histological diagnosis from the original pathology reports. We also used the grading from the original pathologist’s report, performed according to Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) (Guillou et al. Citation1997). During the years 2004–2005 and until 2013, ALT was classified as an STS (ALT/well-differentiated liposarcoma), but in the most recently published WHO tumor classification ALT of the trunk wall and limbs is no longer classified as an STS (Dei Tos and Pedeutour 2002, 2013). ALT of the trunk wall and limbs has no metastatic potential unless dedifferentiation occurs, but it is still considered a locally aggressive mesenchymal neoplasm (Dei Tos and Pedeutour 2013), so we did not exclude ALT from the present study.
Patient and tumor characteristics including immunohistochemical YKL-40 staining results in 49 soft-tissue tumors
The patients included constituted a cohort that could be followed retrospectively for a minimum of 5 years, or until death. In our pathology database 118 patients were identified, and of these 69 were excluded, 45 because they had previously been diagnosed with STS (or ALT) and had presented with a local recurrence (n = 36) or metastatic lesion (n = 9) in 2004–2005. The tumor tissue samples from the patients included had to be large enough to allow performance of TMA analysis, and 18 patients were excluded because (1) not enough tumor tissue was stored due to the surgery performed at our department being a re-excision after primary surgery elsewhere (n = 9), (2) only biopsy material was stored (n = 6), or (3) the primary tumor removed was very small (n = 3). Finally, 5 patients were excluded due to lack of follow-up, and 1 patient was excluded because the tumor was finally diagnosed as a bone sarcoma. Thus, 49 tumors remained for analysis.
Tissue and preparation of the TMA blocks
TMA paraffin blocks were prepared and stained in a standardized manner similar to that described previously for ovarian carcinomas (Høgdall et al. Citation2009). After evaluation of all available sections, 1 representative area from a chosen section was marked by a pathologist, and 2 tissue cylinders of 2.0 mm were punched out from this area in each tumor block. These were transferred along with the other cylinders to a recipient paraffin block, the TMA block, and the biopsies were aligned and marked for identification. 98 biopsies were obtained, 2 from each tumor. Synovial tissue, which is known to be positive for YKL-40, was used as a positive control. The stability of the staining results has previously been checked using TMAs and regular tumor sections (Høgdall et al. Citation2009). At the time of diagnosis, the sarcomas had been classified according to the WHO histological classification of soft-tissue tumors and histological grading was performed using the FNCLCC grading system (Trojani et al. Citation1984). The 49 tumors consisted of ALTs and 14 different histological subtypes of STS (Table).
YKL-40 staining
The staining procedure has been described previously in detail (Ringsholt et al. Citation2007, Høgdall et al. Citation2009).
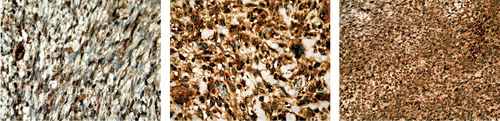
Figure 1. Immunohistochemical analysis of YKL-40 protein expression in paraffin sections of different types of soft-tissue sarcomas. The cytoplasmatic glycoproteins in both the tumor cells and the tumor stroma cells take up the stain, and it is therefore the intensity of all parts of the tumor that is evaluated. Positive immunostaining appears as a cytoplasmic, granular brown-colored staining. Left panel: a peripheral nerve sheet tumor, Trojani grade 2, with an average YKL-40 intensity score of 1. Middle panel: an unclassifiable sarcoma, Trojani grade 3, with an average YKL-40 intensity score of 2. Right panel: a synovial sarcoma, Trojani grade 3, with an average YKL-40 intensity score of 3.
Estimation of staining intensity
The slides were evaluated independently by 2 investigators (MLH and LHC), and they were scored in a semiquantitative manner as: negative for YKL-40 (0 = no staining) or positive for YKL-40 (staining intensity graded as: 1 = weak, 2 = moderate, and 3 = high). The final score of the YKL-40 staining in each patient was calculated as the average value of the scores given by the 2 investigators. Thus, an average staining intensity (ASI) score of 0 was considered to be negative for YKL-40, while an ASI score of 0.5-1 was considered weakly positive, an ASI score of 2.5-1 moderately positive and an ASI score of 2.5-3 highly positive for YKL-40. Two tumors were each missing in one of the TMA sections. Remaining tissue sections from each of these were then stained individually, and evaluation of the reactivity and staining intensity, which was used for the statistical analysis, was performed on these. The evaluation of the reactivity and staining intensity was then performed on the remaining section from each tumor and that score was used for the statistical analysis.
Statistics
Data are presented as mean (range). For comparison of YKL-40 staining level between the 3 histological malignancy grades, nonparametric analysis of variance for unpaired data (the Kruskal-Wallis test) and nonparametric testing for unpaired data (the Mann-Whitney U test) was used.
Survival data were extracted from the Danish Centralized Civil Register, and these were not disease-specific data but rather overall survival data (until death from any cause). Patient survival was analyzed using the Kaplan-Meier method and the influence of YKL-40 staining intensity and the histological malignancy grade on survival was evaluated, with calculation of the 95% confidence interval (CI) for the mean survival rate.
The study was approved by the Ethics Committee of the Capital Region of Denmark (HD-2007-075).
Results
8 tumors were negative for YKL-40 staining and 41 were positive with weak (n = 4), moderate (n = 16), or high (n = 21) staining intensity (Table). 25 patients (with a mean follow-up of 6 (5–7) years) were still alive and 24 (with a mean follow-up of 2 (0.5–4) years) had died by the end of the follow-up period, and of the patients who died, 19 had developed metastatic disease. The mean ASI YKL-40 score was higher in tumor biopsies from patients who died (2.4) than in those from patients who were alive (1.5) at the end of the follow-up period (p = 0.01).
The YKL-40 staining intensity was different in the different histological grades of malignancy (p = 0.004) with higher values in the highly malignant tumors (grades 2 and 3) than in the low-malignancy tumors (grade 1). Similar ASI YKL-40 scores was found between groups, when the material was divided into groups with tumor size below 5 cm (n = 15) or above 5 cm (n = 34). Deep-seated tumors (n = 39) had an ASI YKL-40 score of 2.1, as compared to 1.2 in tumors with a subcutaneous location (p = 0.04).
The probability of 5-year survival of the whole group of patients (n = 49) was 51% (). When the material was divided into 3 groups with (1) negative-staining or low ASI YKL-40 staining tumors (n = 12), (2) moderate staining-intensity tumors (n = 16), and (3) high staining-intensity tumors (n = 21), the mean survival rate was higher in the group with negative-staining or low staining-intensity tumors (6.4 years, CI: 5.5–7.3) than in the groups with moderate staining-intensity tumors (3.8 years, CI: 2.3–5.1) or high staining-intensity tumors (3.8 years, CI: 2.8–4.9) ().
Figure 2. Kaplan-Meier survival analysis. A. Survival analysis of all 49 patients with atypical lipomatous tumor (n = 9) or soft-tissue sarcoma (n = 40). B. Survival analysis of the 49 patients with atypical lipomatous tumor or soft-tissue sarcoma divided into 3 subgroups with negative or low ASI YKL-40 staining-intensity tumors (n = 12) (blue line), moderate staining-intensity tumors (n = 16) (brown line), and high staining-intensity tumors (n = 21) (green line). C. Survival analysis of the 49 patients with atypical lipomatous tumor or soft-tissue sarcoma divided into 3 subgroups based on the histological malignancy grade: grade-1 tumors (n = 14, blue line), grade-2 tumors (n = 14, green line), and grade-3 tumors (n = 21, brown line).
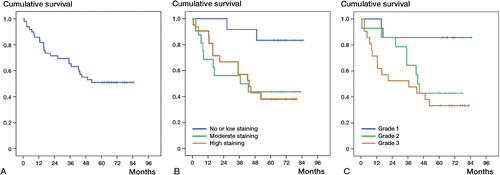
The histological grade of malignancy also influenced the probability of mean survival with grade-1 tumors (n = 14, mean survival rate 6.2 years, CI: 5.2–7.3) and grade-3 tumors (n = 21, mean survival rate 3.4 years, CI: 2.2–4.6) having significantly different mean survival rates. The mean survival rate for grade-2 tumors (n = 14, mean survival rate 4.2 years, CI: 3.1–5.4) was not significantly different to those for grade-1 and grade-3 tumors ().
Discussion
To our knowledge, this is the first report on YKL-40 expression in STS and ALT. We found that 41 of the tumors were positive for YKL-40. Only 8 tumors were negative for YKL-40. All 8 of these negative tumors were an ALT. The only ALT that was positive for YKL-40 had an osteochondroid dedifferentiation. A study on the expression of YKL-40 in chondroid tumors has shown a universal immunoreactivity for YKL-40 in chondrosarcomas (Daugaard et al. Citation2009). The osteochondroid dedifferentiation of the ALT might explain the immunoreactivity to YKL-40. YKL-40 is associated with cancer aggressiveness, and it might be a predictor of a more malignant transformation of cancer cells (Bi et al. Citation2009), which might explain why the ALT did not express YKL-40 at all.
The probability of 5-year survival for the whole group was 51%. The ASI YKL-40 score was higher in tumors from patients who had died during the follow-up period than in tumors from the patients who survived. When divided into subgroups according to the ASI score, the mean survival rate was higher in the group with staining-negative or low-intensity stained tumors. This suggests that YKL-40 expression in STS might be used as a prognostic factor for survival. This study is, however, too small to provide solid evidence, but our finding is similar to the results of other studies of YKL-40 expression in other tumors: glioblastomas (Pelloski et al. Citation2005), epithelial ovarian cancer (Yang et al. Citation2009), and gastric cancer (Bi et al. Citation2009) with an association between YKL-40 expression and shorter survival. Other studies have established that tumor expression of YKL-40 is related to stage and histology, but has no correlation to overall survival (Roslind et al. Citation2008, Høgdall et al. Citation2009, Shao et al. Citation2009). The histological malignancy grade showed the same pattern as the ASI YKL-40 score on survival, with longer mean survival of patients with grade-1 tumors (including ALT) than of patients with grade-3 tumors. This correlates with the ASI YKL-40 score, which was higher in grade-3 tumors than in grade-1 tumors.
The ASI YKL-40 score was similar for tumors with size below 5 cm or larger than 5 cm, and between subcutaneous and deep-seated tumors. Tumor size along with histological type and grade is normally considered to be a prognostic factor (Singer et al. Citation1994, Levine Citation1999, Ramanathan et al. Citation2001). A study of 490 STSs showed that depth had no major effect on survival when tumor grade and size were taken into account (Rydholm and Gustafson Citation2003). We had to exclude 18 patients due to having too little tissue; this may have biased our results regarding size and survival. A study by Gustafson (Citation1994) showed that size is of no prognostic value in liposarcomas (including ALT). The present study had 19 lipomatous tumors of various types (ALT, liposarcomas and myxiod liposarcomas), all of which were greater than 5 cm. 19 tumors of 49 is a large proportion, which may have influenced the association between ASI YKL-40 score and size.
YKL-40 concentration can be determined in serum by ELISA (Johansen et al. Citation2008) and it has been measured in more than 7,000 patients with different types of cancer. High serum YKL-40 is common in patients with bone, lung, and liver metastases (CitationJohansen 2006, Johansen et al. Citation2009). Moreover, studies have shown that high serum YKL-40—compared to levels in healthy individuals—is an independent prognostic biomarker signaling short time to worsening of the disease and short time to death in patients with localized or disseminated cancer of the colon, breast, ovary, lung, prostate, kidney, skin, melanoma, blood cells, and bone marrow (Schmidt et al. Citation2006, Johansen et al. Citation2009). Several studies have shown that serum YKL-40 is useful for monitoring of recurrence and progression in cancer patients after surgery (Johansen Citation2006, Johansen et al. Citation2009).
The present study has shown that YKL-40 is expressed in STS, and in the future the degree of YKL-40 expression may be shown to be a clinically important prognostic marker of the severity of the disease. Degree of YKL-40 expression showed consistency with the FNCLCC grading, and survival in patients suffering from ALT/STS. However, in this study we could not determine whether YKL-40 expression is an independent prognostic factor that can add something new to the prognostic value of FNCLCC grading.
The YKL-40 expression that we found may contribute to the serum levels of YKL-40 in patients with STS and, if so, serum YKL-40 may have potential as a prognostic serum biomarker in STS patients. Further studies with a much larger sample size are warranted to determine whether YKL-40 expression (in a Cox proportional hazards regression analysis) still remains a significant prognostic factor, when analyzed together with the parameters that make up the FNCLCC grading system and perhaps even other parameters. Furthermore, the role of serum YKL-40 as a biomarker should be evaluated in larger prospective studies.
MLH collected clinical and pathological data, estimated staining intensity, analyzed data, and wrote the article. LHC estimated staining intensity and reviewed the article. MR performed YKL-40 staining and wrote the part of the text on YKL-40 staining. GSL wrote the protocol and reviewed the article. MMP supervised the project, analyzed data, and helped write the article.
Sincere thanks to consultant Søren Daugaard, MD, and to head of department Vera Timmermans Wielenga, MD, DMSc of the Department of Pathology, Rigshospitalet, for help and guidance with the materials and for stimulating discussions.
No competing interests declared.
- Bi J, Lau SH, Lv ZL, Xie D, Li W, Lai YR, Zhong JM, Wu HQ, Su Q, He YL, Zhan WH, Wen JM, Guan XY. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Human Pathology 2009; 40: 1790-7.
- Daugaard S, Christensen LH, Høgdall E. Markers aiding the diagnosis of chondroid tumors: an immunohistochemical study including osteonectin, bcl-2, cox-2, actin, calponin, D2-40 (podoplanin), mdm-2, CD117 (c-kit), and YKL-40. APMIS 2009; 117: 518-28.
- Dei Tos AP, Pedeutour F. Atypical lipomatous tumour / well differentiated liposarcoma. In: World Health Organisation classification of tumours. Pathology and genetics of tumours of soft tissue and bone (Fletcher CDM, Unni KK, Mertens F, eds). IARCPress, Lyon 2002: 35–7.
- Dei Tos AP, Pedeutour F. Atypical lipomatous tumour. In: WHO classification of tumours of soft tissue and bone. International agency for research on cancer (Fletcher CDM, Brigde JA, Hogendoom PCW, Mertens F, eds). Lyon 2013: pp. 33–6.
- Faibish M, Francescone R, Bentley B, Yan W, Shao R. A YKL-40 neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011; 10 (5): 742–51.
- Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A, Jacquemier J, Duplay H, Sastre-Garau X, Costa J. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997; 15: 350–62.
- Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand (suppl 259) 1994: 1-31.
- Høgdall EV, Ringsholt M, Høgdall CK, Christensen IJ, Johansen JS, Kjaer SK, Blaakaer J, Ostenfeld-Møller L, Price PA, Christensen LH. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer 2009; 9: 8.
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodeling, fibrosis and cancer. Dan Med Bull 2006; 53: 172-209.
- Johansen JS, Høyer PE, Larsen LA, Price PA, Møllgård K. YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem 2007; 55: 1213-28.
- Johansen JS, Lottenburger T, Nielsen HJ, Jensen JE, Svendsen MN, Kollerup G, Christensen IJ. Diurnal, weekly and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol Biomarkers Prev 2008; 17: 2603-8.
- Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future Oncol 2009; 5: 1065-82.
- Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, Coyle A, Chupp G, Reed J, Flavell RA, Elias JA. Role of breast regression protein 39 (BRP-39)/Chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med 2009; 206: 1149-66.
- Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 2011; 73: 479-501
- Levine EA. Prognostic factors in soft tissue sarcoma. Semin Surg Oncol 1999; 17: 23–32.
- Pelloski CE, Mahajan A, Maor M, Chang EL, Woo S, Gilbert M, Colman H, Yang H, Ledoux A, Blair H, Passe S, Jenkins RB, Aldape KD. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res 2005; 1: 3326–34.
- Ramanathan RC, A’Hem R, Fisher C, Thomas JM. Prognostic Index for Extremity Soft Tissue Sarcomas With Isolated Local Recurrence. Ann Surg Oncol 2001; 8: 278–89.
- Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997; 43: 221-5
- Ringsholt M, Høgdall EV, Johansen JS, Price PA, Christensen LH. YKL-40 protein expression in normal adult human tissues – an immunohistochemical study. J Mol Histol 2007; 38: 33-43.
- Roslind A, Knoop AS, Jensen M, Johansen JS, Nielsen DL, Price PA, Balslev E. YKL-40 protein expression is not a prognostic marker in patients with primary breast cancer. Breast Cancer Res Treat 2008; 112: 275–85.
- Rydholm A, Gustafson P. Should tumor depth be included in prognostication of soft tissue sarcoma? BMC Cancer 2003; 3: 17.
- Schmidt H, Johansen JS, Gehl J, Geertsen PF, Fode K, von der Maase H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with Mmetastatic melanoma. Cancer 2006; 106: 1130-9.
- Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009; 28: 4456-68.
- Singer S, Corson JM, Gonin R, Labow B, Eberlein TJ. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg 1994; 219: 165-73.
- Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft tissue sarcoma of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984; 33: 37-42.
- Yang GF, Cai PY, Li XM, Deng HX, He WP, Xie D. Expression and clinical significance of YKL-40 protein in epithelial ovarian cancer tissues. Chinese J Cancer 2009; 28: 142-5.

