Abstract
Background and purpose — Glenoid reconstruction and inverted glenoid re-implantation is strongly advocated in revisions of failed reverse shoulder arthroplasty (RSA). Nevertheless, severe glenoid deficiency may preclude glenoid reconstruction and may dictate less favorable solutions, such as conversion to hemiarthropasty or resection arthropasty. The CAD/CAM shoulder (Stanmore Implants, Elstree, UK), a hip arthroplasty-inspired implant, may facilitate glenoid component fixation in these challenging revisions where glenoid reconstruction is not feasible. We questioned (1) whether revision arthroplasty with the CAD/CAM shoulder would alleviate pain and improve shoulder function in patients with failed RSA, not amenable to glenoid reconstruction, (2) whether the CAD/CAM hip-inspired glenoid shell would enable secure and durable glenoid component fixation in these challenging revisions.
Patients and methods — 11 patients with failed RSAs and unreconstructable glenoids underwent revision with the CAD/CAM shoulder and were followed-up for mean 35 (28–42) months. Clinical outcomes included the Oxford shoulder score, subjective shoulder value, pain rating, physical examination, and shoulder radiographs.
Results — The average Oxford shoulder score and subjective shoulder value improved statistically significantly after the revision from 50 to 33 points and from 17% to 48% respectively. Pain rating at rest and during activity improved significantly from 5.3 to 2.3 and from 8.1 to 3.8 respectively. Active forward flexion increased from 25 to 54 degrees and external rotation increased from 9 to 21 degrees. 4 patients required reoperation for postoperative complications. No cases of glenoid loosening occurred.
Interpretation — The CAD/CAM shoulder offers an alternative solution for the treatment of failed RSA that is not amenable to glenoid reconstruction.
Reverse shoulder arthroplasty (RSA) has become an established treatment for painful and debilitating shoulder pathologies associated with rotator-cuff insufficiency (Boileau et al. Citation2005, Citation2006, Frankle et al. Citation2005). The preoperative condition of shoulders requiring RSA and the technically demanding nature of the procedure make RSA challenging, with an overall complication rate of 15–50% in recently reported series (Guery et al. Citation2006, Gerber et al. Citation2009, Kempton et al. Citation2011). Complications related to the glenoid component (e.g. loosening, mechanical baseplate failure, dissociation) have been reported in 4–16% of cases (Gurey et al. Citation2006, Fevang et al. Citation2009, Farshad and Gerber Citation2010). Aseptic loosening is the most common glenoid-sided complication requiring revision following RSA (Fevang et al. Citation2009), and is often associated with considerable scapular bone loss (e.g. inferior scapular notching, glenoid deficiency after implant removal), which further complicates surgical revision (Antuna et al. Citation2001, Boileau et al. Citation2005, Elhassan et al. Citation2008, Gerber et al. Citation2009).
Re-implantation of a glenoid component has been found to provide better clinical results than conversion to hemiarthroplasty or resection arthroplasty in revisions of both anatomical (Antuna et al Citation2001, Elhassan et al. Citation2008) and reverse shoulder arthroplasties (Farshad et al. Citation2012, Favard Citation2013), and it is strongly advocated. However, achievement of secure fixation of a glenoid implant may not be feasible in the presence of severe glenoid bone loss. Glenoid reconstruction with bone graft has been used to facilitate glenoid implant fixation in poor glenoid bone stock in primary shoulder arthroplasty (Hill and Norris Citation2001) and revision shoulder arthroplasty (Holcomb et al. Citation2009, Patel et al. Citation2012). The inconsistent clinical results and durability of fixation achieved with this technique have led to increasing interest in more reliable surgical alternatives for this challenging problem.
The CAD/CAM (computer-assisted design/computer-assisted manufacture) shoulder (Stanmore Implants, Elstree, UK) is a constrained hip arthroplasty-inspired shoulder implant that was designed to facilitate glenoid implant fixation by securing a large glenoid shell to the scapula around the deficient glenoid, rather than to the deficient glenoid itself. Unlike Grammont-type implants, the CAD/CAM shoulder has an increased glenohumeral offset (less medialized implant), which has been shown to improve rotational movements of the shoulder (by recruiting anterior and posterior deltoid fibers and re-tensioning of the remaining rotator cuff) and to minimize scapular notching (Holcomb et al. Citation2009, Valenti et al. Citation2011)
The purpose of this study was (1) to determine whether revision arthroplasty with the CAD/CAM shoulder would alleviate pain and improve shoulder function in patients with failed RSA and severe glenoid deficiency that is not amenable to reconstruction and inverted glenoid re-implantation; and (2) to determine whether the CAD/CAM hip-inspired glenoid shell would enable secure and durable glenoid component fixation in these challenging revisions. To our knowledge, no previous study has evaluated the use of such implants in revision surgery for failed glenoid-deficient RSA.
Patients and methods
Study design and setting
This retrospective study was conducted at the Shoulder and Elbow Service of our university-affiliated hospital. The study was planned according to the STROBE Guideline and was approved by our Institutional Ethics Committee (Reg. no. SE.13.014).
Patient population
Between 2007 and 2011, 17 patients with inverted glenoid implant failure (i.e. baseplate migration with or without screw breakage) following Grammont-type RSA (11 Delta III (DePuy); 3 Aequalis reverse prosthesis (Tornier); 2 SMR reverse prosthesis (Lima Corporate); and 1 TESS reversed shoulder (Biomet)) were referred to us. All the patients had severe pain and limited function related to their shoulder condition; they had not improved with nonoperative treatment over a period of 6–12 months. 11 of these patients (8 females)—whose glenoid bone stock was assessed to be insufficient for glenoid reconstruction and inverted glenoid re-implantation (all had combined severe deficiency (Antuna et al. Citation2001))—underwent revision with the CAD/CAM shoulder (Stanmore Implants, Elstree, UK) and were followed-up for mean 35 (28–42) months. The average age of the study group was 72 (58–84) years. Revision was performed at a mean of 58 (30–84) months after the index RSA (). 3 patients whose glenoid bone stock was assessed to be sufficient for glenoid reconstruction and re-implantation were revised with another reverse-polarity implant, and were not included in the study. 2 patients declined further surgery and another patient was declared unfit for elective shoulder surgery.
Table 1. Patient characteristics and clinical details
Preoperative planning
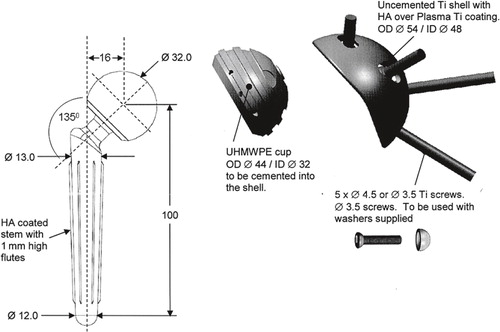
Glenoid bone deficiency was assessed preoperatively by conventional radiography and CT scan, and was confirmed intraoperatively after implant removal, according to Antuna et al. (Citation2001). Patients whose glenoid was assessed to be inadequate for reconstruction were treated with the CAD/CAM shoulder. The implants were custom-made by Stanmore Implants according to the patient’s lateral scapular morphology, based on the CT scan. The CAD/CAM shoulder implant consists of the following: (1) a hemispheric, 2-mm wall-thickness titanium glenoid shell with multiple longitudinal slots for screw fixation to the scapula around the deficient glenoid and hydroxyapatite coating to facilitate uncemented fixation; (2) a high-molecular-weight polyethylene liner which is cemented into the titanium shell (designed to allow for a minimum of 2 mm of cement mantle between its outer diameter and the shell). The liner depth is 2 mm deeper than the radius of the prosthetic head (available in 28 mm or 32 mm), which thereby forms a constrained, inherently stable prosthetic articulation; (3) a cobalt-chrome tapered humeral stem designed to fit the patient’s proximal humerus morphology with press-fit or cemented fixation; (4) a cobalt-chrome humeral head (28 mm or 32 mm) inserted on the Morse-tapered neck of the humeral stem and mated into the polyethylene liner. The maximum range of motion of the implant is 60 degrees in each direction before impingement of the neck on the liner occurs; and (5) 3.5 mm titanium fixation screws (DePuy Synthes, Solothurn, Switzerland) with hemispheric washers to permit variable-angle fixation of the shell to the scapula ().
Figure 1. The CAD/CAM shoulder (Stanmore Implants, Elstree, UK). A custom-made, constrained hip arthroplasty-inspired implant. The implant comprises an uncemented titanium glenoid shell mated to a cobalt-chrome tapered humeral stem (cemented or uncemented with a 28-mm or 32-mm head) with a high-molecular-weight polyethylene liner (cemented into the glenoid shell). A. A preoperative drawing. B. The actual implant.
Operative technique
All procedures were performed under general anesthesia in the reclining position through a deltopectoral approach, by 1 of 2 senior shoulder consultants. The original incision was used whenever possible and it was extended as necessary. When present, the remnant of the subscapularis tendon was divided medially to its humeral insertion and, if possible, it was repaired at the end of the procedure. Releases around the subscapularis tendon and inferior capsule were carried out under nerve stimulator control. The RSA was exposed and removed using standard instruments. No humerus osteotomy was required in this series. A “neo-fossa” was created for CAD/CAM glenoid shell insertion by exposing the glenoid base, the lateral border of the coracoid, and the anterior margin of the scapular spine with standard spherical (acetabular) reamers to a size 2 mm less than the eventual shell diameter. The glenoid shell was impacted into the “neo-fossa” to gain provisional stability within the lateral fornix of the scapula and secured to the scapula into the “neo-fossa” in a divergent quadruped fashion using 3.5-mm titanium cortical screws into the lateral column of the scapula, scapular spine, base of coracoid, and glenoid body if this was possible. The polyethylene liner was cemented into the glenoid shell using gentamicin-loaded cement. The humeral stem was re-inserted with cement in 8 patients. In 3 patients who had proximal humerus medial and lateral cortical thickness of ≥ 4 mm and no medical conditions affecting bone healing (e.g., diabetes mellitus, heavy smoking, corticosteroids medication) the humeral stem was re-inserted without cement. The prosthesis was then mated, followed by range of motion and stability assessment. The subcutaneous tissue and skin were closed with absorbable sutures (). Antibiotic prophylaxis was given before anesthetic induction and was continued for 24 hours after the operation.
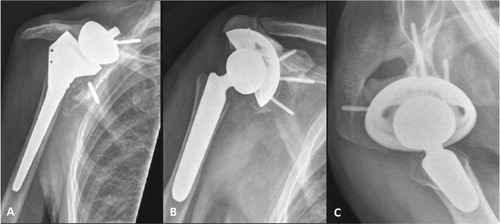
Figure 2. A 72-year-old man (patient 6) presented with severe pain and limited function 6 years after reverse shoulder arthroplasty for primary cuff-tear arthropathy. Shoulder radiographs at presentation showed loosening and migration of the baseplate (panel A). The prosthesis was revised using the CAD/CAM shoulder with significant pain relief and improvement in functional scores at the 36-month follow-up (panels B and C).
Postoperatively, the patients were immobilized in a shoulder sling in adduction and internal rotation for 6 weeks to encourage osseointegration (or at least fibrous integration) of the uncemented glenoid shell with the cortico-cancellous “neo-fossa”. Passive shoulder forward flexion to the shoulder level and external rotation to neutral position were initiated on the first postoperative day. Active assisted range of motion and isometric strengthening exercises were initiated at 8 weeks, followed by stretching exercises and an anterior deltoid eccentric strengthening program at 3 months postoperatively.
Outcome measures
Pre-revision and post-revision outcome measures at the most recent follow-up were retrospectively collected from the patients’ records. Patients’ subjective outcome measures included the Oxford shoulder score as originally described (Dawson et al. Citation1996), i.e. ranging from 12 (least difficulties) to 60 (most difficulties); subjective shoulder value (on a 0–100% scale) (Gilbart and Gerber Citation2007); and pain score at rest and during activity on a numeric scale from 0 to 10 (where 0 means no pain and 10 means excruciating pain). We have found these patients’ self-assessed clinical scores to save valuable time in the clinic and we use them routinely. Active range of motion of the shoulder was measured with a goniometer in forward flexion (FF), external rotation (ER), in adduction, and internal rotation (IR) measured with the thumb behind the back on a scale from 0 to 6 (i.e. 1-thigh, 2-buttock, 3-sacroiliac joint, 4-waist, 5-thoracic-lumbar junction, 6-scapula). Patients’ ability to reach their face, opposite armpit, and ipsilateral buttock (“functional triangle”) with the affected arm was also assessed (and considered positive only if all 3 could be reached).
Anterior-posterior and axillary shoulder radiographs at the most recent follow-up were compared with immediate postoperative radiographs and previous shoulder imaging. Radiolucency of more than 2 mm around the entire bone-prosthesis interface (of either or both the uncemented glenoid shell and humeral stem) was considered to be evidence of definitive loosening. Similar radiolucency around the bone-cement/cement-prosthesis interface of cemented stems or component migration (shift or subsidence) in consecutive radiographs was also considered to be evidence of definitive loosening.
Statistics
Descriptive statistics are reported as mean (SD) for continuous parameters and as proportions for categorical parameters. Comparisons between pre-revision outcome and post-revision outcome are presented with 95% confidence intervals (CIs) around the difference. Pre-revision and post-revision outcome measures were compared using paired 2-tailed t-test for continuous parameters and 2-tailed McNemar test for categorical parameters. Statistical analysis was performed using SPSS for Windows version 16.0. Any p-value of < 0.05 was considered to be statistically significant.
Results
The average Oxford shoulder score improved from 50 (SD 4) to 33 (SD 6) following the revision (p < 0.001). Subjective shoulder value improved from 17 (SD 11) to 48 (SD 17) (p < 0.001). Pain level at rest and during activity decreased from 5.3 (SD 1.6) and 8.1 (SD 1.6) before revision to 2.3 (SD 1.3) and 3.8 (SD 1.7) after revision (p < 0.001). Active FF increased from 25 (SD 12) degrees to 54 (SD 17) degrees (p < 0.001) and active ER in adduction increased from 9 (SD 10) degrees to 21 (SD 9) degrees (p = 0.002). Active IR remained similar before and after revision. 3 patients were able to use their affected arm in the “functional triangle” before the revision, as compared to 6 patients after the revision (p = 0.3) ().
Table 2. Summary of outcome measures before and after revision
At the latest follow-up, all the CAD/CAM glenoid shells remained well fixed clinically and radiographically, and no cases of glenoid loosening occurred. Radiolucent lines of less than 1 mm around the fixation screw to the scapular spine were noticed in 2 cases (but no radiolucent lines were noticed around any of the glenoid shells).
Postoperative complications occurred in 4 patients. 1 patient developed aseptic loosening of her cemented CAD/CAM humeral stem 12 months after the revision. The loose stem was re-cemented and remained stable at the 40-month follow-up. Another patient had early prosthetic dislocation 2 weeks after the revision, which required an open reduction in theater. The shoulder remained stable at the latest follow-up (34 months). A third patient sustained periprosthetic humeral fracture at the tip of the humeral stem, caused by indirect trauma due to a fall 6 months after the revision. The prosthetic stability was not compromised, and the fracture was internally fixed with a locking plate and healed uneventfully within 5 months. The last patient had persistent mechanical pain postoperatively, which was believed to be related to a large lump of cement mantle protruding at the posterior aspect of the glenoid shell into the posterior deltoid muscle, with no improvement at the 12-month postoperative follow-up. The protruding cement was removed surgically 18 months after the revision, with some relief of symptoms.
Discussion
Glenoid reconstruction followed by re-implantation of a glenoid component has shown superior clinical outcomes in revision shoulder arthroplasty than conversion to hemiarthroplasty or resection arthroplasty (Antuna et al. Citation2001, Elhassan et al. Citation2008, Holcomb et al. Citation2009, Farshad et al. Citation2012, Favard Citation2013). Despite the evidence supporting glenoid reconstruction and re-implantation, retention of a stable glenoid component may not be possible in revisions of glenoid-deficient shoulders—and less favorable surgical solutions (e.g. hemiarthroplasty, resection arthroplasty) may be required (Antuna et al. Citation2001, Elhassan et al. Citation2008, Farshad and Gerber Citation2010, Zumstein et al. Citation2011). Zumstein et al. (Citation2011) found that glenoid component re-implantation was not possible in 14 of 79 cases of inverted glenoid revision. Conversion to hemiarthroplasty or resection arthroplasty was carried out in these cases.
The purpose of our study was to determine whether the CAD/CAM shoulder would enable retention of a stable glenoid component and improve the clinical outcomes in this challenging subgroup of patients with failed RSA and deficient glenoid, not amenable to glenoid reconstruction and re-implantation with other currently available implants.
After revision with the CAD/CAM shoulder, our patients’ pain ratings and functional scores improved substantially. Active FF increased from 25 to 54 degrees and ER increased from 9 to 21 degrees. The average gain in FF of 29 degrees in our patients seems modest compared to Holcomb et al. (Citation2009) who reported gain in FF of 67 degrees. Poorer initial shoulder condition (i.e. severe glenoid deficiency, poorer pre-revision FF) in our patients and possibly inferior mechanical properties of the CAD/CDM shoulder (i.e. constrained design, maximal range of motion of 60 degrees around the center of the prosthesis) compared to the prosthesis used by Holcomb et al. (Reverse Shoulder Prosthesis; Encore Medical) may explain this difference. On the other hand, the average post-revision gain in ER in our patients (12 degrees) was roughly similar to the findings of Holcomb et al. (14 degrees) and supports the hypothesis that less medialized reverse shoulder design has a beneficial effect in restoring active ER (Valenti et al. Citation2011).
The use of an acetabulum-like, over-sized glenoid shell (i.e. Epoca Reco Glenoid; Synthes) has been reported previously by Jeske et al. (Citation2012) as an alternative to RSA in 23 patients with cuff-tear arthropathy (the status of the glenoid bone stock was not discussed). At an average postoperative follow-up of 3.5 years, patients’ clinical scores and range of motion of the shoulder improved. However, they had an unacceptable glenoid loosening rate (10 of 23 patients) and half of these cases required revision of the glenoid implant. In contrast to these results, the CAD/CAM glenoid shell in our study remained well fixed, with no evidence of loosening at a similar postoperative follow-up time. We believe that the properties of the CAD/CAM glenoid shell (e.g. hemispheric design, hydroxyapatite coating) and our surgical technique (e.g. creating a “neo-fossa” using spherical acetabular reamers) may explain our favorable results in terms of the glenoid shell fixation.
Complications requiring reoperation occurred in 4 of our patients, in concordance with the complication rates following revision shoulder arthroplasty reported in other studies with similar duration of follow-up (Antuna et al. Citation2001, Holcomb et al. Citation2009, Zumstein et al. Citation2011, Farshad et al. Citation2012). This high rate of complications at short- to medium-term follow-up highlights the complexity of revision shoulder arthroplasty and the need to weigh up the surgical risk against the expected benefit on an individual basis.
Our study had several limitations. First, it was a retrospective study with a limited cohort and relatively short-term follow-up. Second, it was a single-arm study with no control group. It is therefore difficult to conclude whether our approach would be superior to other alternatives, particularly conversion to hemiarthroplasty. Third, the outcome measures we used are different from scores used by others. Lack of standardization in outcome assessment tools precluded meaningful comparisons between our results and those of other studies.
OU designed the study and data collection tools, collected the data, analysed and interpreted of the data and drafted the manuscript. IB contributed substantially to the interpretation of the data and revised the manuscript critically for important intellectual content. SL designed the study and data collection tools, contributed substantially to the interpretation of the data and to the manuscript drafting and revised the manuscript critically for important intellectual content.
No competing interests declared.
- Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg 2001; 10 (3): 217-24.
- Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg (1 Suppl S) 2005; 14: 147S-161S.
- Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award. 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006; 15: 527-40.
- Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. J Bone Joint Surg (Br) 1996; 78 (4): 593-600.
- Elhassan B, Ozbaydar M, Higgins LD, Warner JJ. Glenoid reconstruction in revision shoulder arthroplasty. Clin Orthop 2008; (466) (3): 599-607.
- Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop 2010; 34 (8): 1075-82.
- Farshad M, Grogli M, Catanzaro S, Gerber C. Revision of reversed total shoulder arthroplasty. Indications and outcome. BMC Musculoskelet Disord 2012; 13: 160.
- Favard L. Revision of total shoulder arthroplasty. Orthop Traumatol Surg Res (1 Suppl) 2013; 99: S12-21.
- Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop 2009; 80 (1): 83-91.
- Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg (Am) 2005; 87: 1697-705.
- Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2009; 17: 284–95.
- Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg 2007; 16 (6): 717-21.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg (Am) 2006; 88: 1742–7.
- Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg (Am) 2001; 83 (6): 877-83.
- Holcomb JO, Cuff D, Petersen SA, Pupello DR, Frankle MA. Revision reverse shoulder arthroplasty for glenoid baseplate failure after primary reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18 (5): 717-23.
- Jeske HC, Wambacher M, Dallapozza C, Hengg C, Schoepf R, Oberladstaetter J, et al. Functional and clinical outcome of total shoulder arthroplasty with oversized glenoid. Arch Orthop Trauma Surg 2012; 132 (7): 927-36.
- Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop 2011; (469) (9): 2496-504.
- Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21 (11): 1478-83.
- Valenti P, Sauzières P, Katz D, Kalouche I, Kilinc AS. Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop 2011; (469) (9): 2550-7.
- Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011; 20 (1): 146-57.

