| Abbreviations | ||
| ADL | = | Activity of Daily Living |
| ANCOVA | = | Analysis of Covariance |
| ASA | = | American Society of Anaesthesiologists (Physical status classification system) |
| ASES | = | American Shoulder and Elbow Surgeons (shoulder score) |
| CI | = | Confidence Intervals |
| CMS | = | Constant Murley Score |
| DSR | = | Danish Shoulder Arthroplasty Registry |
| ES | = | Effect Size |
| ICC | = | Intraclass Correlation |
| MRI | = | Magnetic Resonance Images |
| NSAID | = | Nonsteroidal Anti-Inflammatory Drugs |
| OSS | = | Oxford Shoulder Score |
| RCT | = | Randomized Clinical Trial |
| SF-36 | = | Short Form-36 |
| SRM | = | Standardized Response Mean |
| SD | = | Standard Deviation |
| UCLA | = | University of California Los Angeles (score) |
| WOOS | = | Western Ontario Osteoarthritis of the Shoulder (index) |
Introduction
Glenohumeral osteoarthritis
Glenohumeral osteoarthritis is a gradual, progressive wear and breakdown of articular cartilages and underlying bone with flatting of the humeral head and formation of marginal osteophytes. It may involve degeneration of soft tissue structures including the synovium, joint capsule, ligaments and adjacent rotator cuff tendonsCitation1.
The incidence of glenohumeral osteoarthritis is unknown but it is, after the hip and knee, the third most common joint to require joint replacementCitation2. The incidence increases with age and glenohumeral osteoarthritis has an older average age of onset than hip and knee osteoarthritisCitation3. For unknown reasons glenohumeral osteoarthritis is more often seen in women than in menCitation3.
Glenohumeral osteoarthritis is divided into primary and secondary forms. The exact etiology of primary glenohumeral osteoarthritis is poorly understood but there are several risk factors including age, overweight, genetics, and occupations with heavy and/or overhead workCitation4. Secondary glenohumeral osteoarthritis has a known etiology such as malunion of a proximal humeral fracture, instability (acute or recurrent dislocation) or a history of inflammation (rheumatoid arthritis or previous septic arthritis)Citation4. Primary glenohumeral osteoarthritis is most prevalent, especially in patients over the age of 60. Younger patients are more often diagnosed with secondary glenohumeral osteoarthritisCitation4,5.
The pathological changes are initiated by a destruction of the articular cartilage changing the load distribution across the joint. This can lead to changes in the subchondral bone with flattening of the humeral head and/or glenoid wear. Loss of sphericity and concentricity can then result in a chronic posterior subluxation with an impaired range of motion. The range of motion can also be mechanically impaired by the formation of osteophytes around the glenoid and the humeral head (). Soft tissue structures are often involved in glenohumeral osteoarthritis. Initially, the synovium is affected but in the later stages the joint capsule and the ligaments thickenings, and joint stiffness may lead to capsular contracture resulting in a further impaired range of motion. Adjacent rotator cuff tendons, especially the subscapularis tendon, can be affected with contracture secondary to a long-term disuse of the shoulderCitation1, 3.
Figure 1. Pathological changes associated with glenohumeral osteoarthritis. Flattening of the humeral head; glenoid wear; posterior subluxation; and osteophytes around the glenoid and the humeral head.
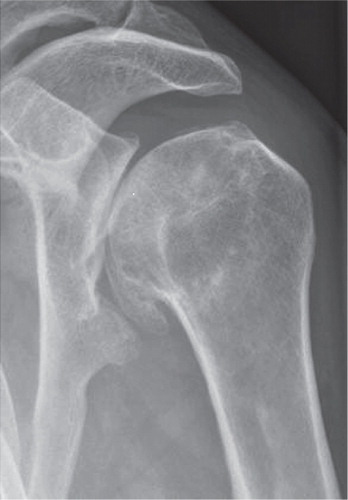
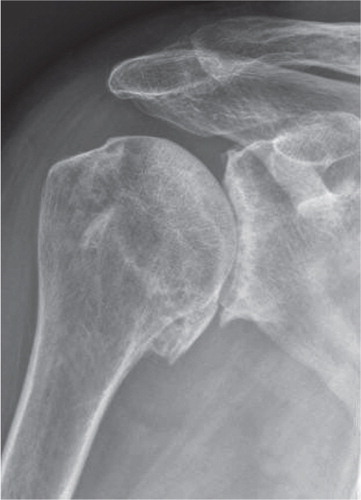
Glenohumeral osteoarthritis is associated with pain, decreased range of motion, stiffness and loss of shoulder function and in more severe cases disturbed sleep and impaired quality of life. It is diagnosed by symptoms and a plain radiograph showing subchondral sclerosis; joint space narrowing; marginal osteophytes or bone cystsCitation4 (). The integrity of the rotator cuff can be evaluated using a clinical examination (visualization of atrophy, range of motion and strength), radiographs, ultrasound and/or MRICitation6, 7.
Figure 2. A radiograph showing the classical findings of subchondral sclerosis, joint space narrowing, marginal osteophytes and subchondral bone cysts in a patient with glenohumeral osteoarthritis.
The first treatment of choice is traditionally non-surgical with physiotherapy (manual mobilization, well-structured exercise and acupuncture), modification in lifestyle, occupational changes, oral analgesics in particular NSAID or intraarticular injectionsCitation4,5. However, documentation of the efficacy of physiotherapy and NSAID in the treatment of glenohumeral osteoarthritis is sparseCitation8-10 and long-time use of NSAID in the treatment of osteoarthritis is associated with sever adverse effects including gastrointestinal bleeding, renal insufficiency and cardiovascular disordersCitation11. Injections with corticosteroids may induce temporary relief of unspecific shoulder pain but the effect in the treatment of diagnosed glenohumeral osteoarthritis is less favorable and poorly documentedCitation12. Intraarticular injections of hyaluronate have been examined in a large double-blinded RCT and may induce temporary pain-relief but it is not routinely usedCitation13. To my knowledge, there is no study comparing the effect of non-surgical and surgical treatment in patients with glenohumeral osteoarthritis.
Surgical treatment is considered when symptoms are interfering with quality of life and remain unresponsive to non-surgical treatment. In the earlier stages of glenohumeral osteoarthritis it is possible to perform joint preserving surgery including arthroscopic debridement to relieve pain and improve shoulder functionCitation14,15. It can be performed as an isolated procedure or combined with bursectomy and subacromial decompression14. In more severe cases of glenohumeral osteoarthritis shoulder replacement may be the only treatment option.
Historical use of shoulder replacement
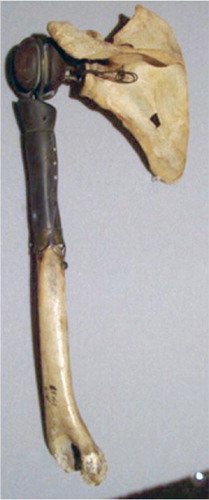
The first joint prostheses were designed by Themistocles Gluck (1853–1942) in the late 1880s and used in the treatment of tuberculous arthritis in the knee, wrist and elbow. Inspired by the ingenuity of Gluck’s implants the French surgeon Jules Émile Péan (1830–1898) developed and implanted the first metallic shoulder prosthesis in 1893Citation16 (). However, shoulder arthroplasties were not used routinely until the early 1950s where the first modern shoulder arthroplasties were developed and implanted by Charles Neer (1917–2011) in the treatment of displaced proximal humeral fracturesCitation17.
Figure 3. The first known shoulder arthroplasty developed and implanted by Jules Émile Péan in 1893. It is made of a platinum tube and fixated with use of screw holes at the distal end. The proximal ball consists of rubber, hardened by boiling in paraffin. The arthroplasty is on display in the Smithsonian Institute in Washington D.C., USA (Reprinted with permission from the American Journal of Roentgenology).
The first experiences with shoulder replacement for the treatment of osteoarthritis were described by Neer in 1974Citation18. He reported an excellent or satisfactory outcome in 41 out of 47 patients diagnosed with primary or secondary osteoarthritis treated with stemmed hemiarthroplasty with a mean follow-up of 6 years. Alongside this study a glenoid component was develop and total shoulder arthroplasty was increasingly used in the following years. An excellent or satisfactory result in 129 out of 150 patients with a minimum of two years follow-up was reported by Neer in 1982Citation19. Since that time, advantages in surgical technique and arthroplasty design had led to a widely use of shoulder arthroplasties in order to obtain pain-relief and increased mobility in patients with end-stage osteoarthritisCitation20-26.
Stephen Copeland began his work with resurfacing replacement in the late 1970s with pioneering anatomical and biomechanical studies. The first Copeland Mark I resurfacing arthroplasty was used in 1986. A screw fixated humeral head component was used together with a high-density polyethylene glenoid componentCitation27. In the following years osseous ingrowth technology rapidly improved and the modern hydroxyapatite coated Copeland Mark III resurfacing arthroplasty with a central grooved impact-fit taper peg for cementless fixation was introduced in 1993Citation28. The first results of 30 resurfacing hemiarthroplasties and 39 total resurfacing arthroplasties were promising with mean non-adjusted Constant Scores of 58 and 62 after minimum 2 years of follow-upCitation28.
The Danish Shoulder Arthroplasty Registry (DSR)
The DSR was established in January 2004 as an initiative of the Danish Society for Surgery of the Shoulder and the Elbow. During the first years the reporting of information was voluntarily but from 2006 reporting became mandatory. All Danish hospitals and private clinics performing shoulder arthroplasties are reporting. It is financed by the Danish counties and has no dependency on commercial parties. Data related to the patient and the operation is reported by the surgeon at the time of surgery by an internet based systemCitation29.
Compared with the data from the National Patient Registry, the Statistical Department of the Danish National Board of Health, 90% of all shoulder arthroplasty operations were reported to the registry between January 2006 and December 2010.
Patient reported outcome is assessed by mail survey 12 months postoperatively (range 10–14 months) using WOOSCitation30which is sent to the patients by a secretary located at the Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark. The same secretary handles the returned questionnaires without involving orthopaedic surgeons. For economical reasons WOOS is only sent to the patients once without any planned mid-term or long-term follow-up.
Revision rates are calculated by checking reported revisions to the DSR and by checking deaths with the Danish National Register of Persons. A revision is defined as the removal or exchange of any component or the addition of a glenoid component. The reason for revision is reported by the surgeon performing the revision procedure.
Incidence of shoulder replacement
Data on the incidence of shoulder replacement in Denmark since 2005 is available through the annual reports from the DSR. Based on data from the National Patient Registry, the Statistical Department of the Danish National Board of Health, the use of primary shoulder replacement in Denmark increased from 12 replacements per 100,000 inhabitants in 2005 to 18 replacements per 100,000 inhabitants in 2012. Patients with osteoarthritis form an increasing proportion of the increased total number of shoulder replacements. 416 shoulder replacements (38%) were performed in patients with osteoarthritis in 2012 compared to 240 (29%) in 2008 and osteoarthritis is now the most frequent reason for shoulder replacement in Denmark.
Clinical background
There are several arthroplasty designs available when shoulder replacement is considered in the treatment of osteoarthritis. The choice of arthroplasty design depends on age, glenoid wear, bone stock quality and the integrity of the rotator cuff. Resurfacing hemiarthroplasty and stemmed hemiarthroplasty are often preferred in DenmarkCitation29 whereas total shoulder arthroplasty is more frequently used in the United States, New Zealand and AustraliaCitation31-33. The different and inconsistent use of shoulder arthroplasty designs is most likely related to national traditions, surgeon preference, and lack of evidence.
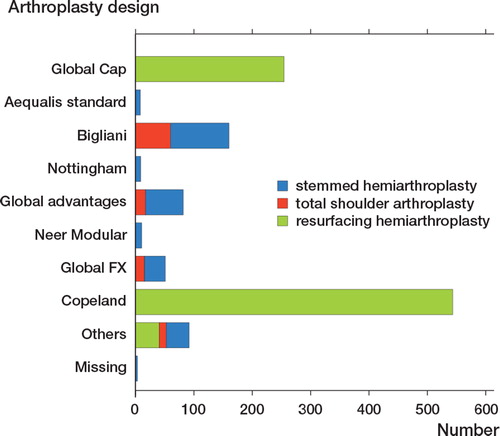
Stemmed hemiarthroplasty, evolved from the early mono-block design of NeerCitation18, is still widely used. During the last decades it has been modified to a modular prosthesis with the head connected to the stem by a taper locking system with the possibility of different head-stem combinations depending on the size of humerus (). There are several similar brands and they can be implanted with or without cemented technique. The most frequently used brand in Denmark since 2006 is the Bigliani-Flatow (Zimmer, Warsaw, In, USA)Citation29. The results of stemmed hemiarthroplasty have been reported in numerous studies showing significant pain-relief and improvement in shoulder functionCitation22,25,34-37. Nevertheless, some patients fail to benefit from stemmed hemiarthroplasty and continuously pain and an impaired shoulder function may be related to a frequently occurring glenoid wearCitation38.
Figure 4. A modular stemmed hemiarthroplasty with the head connected to the stem by a taper locking system with the possibility of different head-stem combinations depending on the size of humerus.

This has led to a controversy regarding the use of a glenoid component. Total shoulder arthroplasty may be the preferred treatment due to a superior functional outcome compared to stemmed hemiarthroplasty but the risk of glenoid loosening has been worryingCitation20,26,37,39. Thus, a failed total shoulder arthroplasty has been considered to have a poor prognosis in the absence of a good revision arthroplasty. Nevertheless, during recent years it has been possible to revise total shoulder arthroplasty using reverse total replacement with promising resultsCitation40-42.
Since the introduction in 1993, the modern resurfacing hemiarthroplasty has been increasingly used. There are several similar brands but the most frequently used brand in Denmark continues to be the Copeland resurfacing arthroplasty (Biomed Merck, Swindon, UK)Citation29. The current designs have a central taper peg that varies in size and shape and they are made of either cobalt-chromium alloy or titanium alloy (). They are implanted with use of a press-fit technique enhanced by osseous ingrowth due to hydroxyapatite coating.
Figure 5. A hydroxyapatite coated resurfacing arthroplasty with a central grooved impact-fit taper peg for cementless fixation.

Several advantages of resurfacing arthroplasty has been suggested including lower risk of periprosthetic fracture; shorter operation time; and minimal bone resection facilitating revision to other arthroplasty designsCitation27,28,43,44. Furthermore, changes in the anatomy following shoulder replacement may adversely affect the biomechanics of the shoulder. Retroversion and inclination may change the tension and the lever arm of the deltoid and the rotator cuff leading to decreased range of motion, weakness and instability whereas changes in the offset or in the humeral head size may results in impingementCitation45. It has been suggested that resurfacing arthroplasty restores the anatomyCitation44,46 but this has also been questionedCitation47,48. The disadvantage of resurfacing arthroplasty is difficulties in sizing the humeral head correctly especially in cases with flattening of the humeral head and presence of large osteophytesCitation48,49. Furthermore, it has been stated that resurfacing hemiarthroplasty is unsuitable in case of focal bone loss with less than 60% of the humeral head intactCitation44.
The outcome following resurfacing arthroplasty has been described in case seriesCitation47-53 and summarized in a review concluding that the results are at least equal to stemmed shoulder arthroplasty;Citation46 however, resurfacing arthroplasty has never been compared to other arthroplasty designs in a RCT. Furthermore, existing studies are small with few revisions and the previously reported revision rates may be imprecise. Thus, reporting of revision rate and reasons for revision using registry data is warrantedCitation27.
Total resurfacing arthroplasty is technical demanding and sufficient glenoid exposure with the humeral head intact is difficult. Total resurfacing arthroplasty is rarely used and it has only been compared to resurfacing hemiarthroplasty in two small case series without superior results Citation28,54. Thus, conventional total shoulder arthroplasty is typically preferred when a glenoid component is required.
A clinical examination including inspection, palpation, range of motion and strength is used to evaluate the integrity of the rotator cuff prior to the operation. MRI is conducted if rotator cuff pathology is suspected. Patients with combined glenohumeral osteoarthritis and symptomatic rotator cuff pathology are in most cases offered treatment with reverse shoulder arthroplasty. The design of the reverse shoulder arthroplasty changes the biomechanics enabling the shoulder to function mainly by use of the deltoid muscle with little or no dependence on the integrity of the rotator cuffCitation55. The result following reverse shoulder arthroplasty in patients with combined glenohumeral osteoarthritis and sever rotator cuff pathology has been promising and seems superior to conventional arthroplasty designsCitation55-59.
Aims of the thesis
This thesis includes 4 studies focusing on the functional outcome, shoulder-specific quality of life and risk of revision following shoulder replacement in patients with glenohumeral osteoarthritis.
The specific aims were:
To describe the process used to translate WOOS into Danish and to test the translation in a Danish population in terms of validity, reliability and responsiveness.
To evaluate patient reported outcome, revision rate and reasons for revision following resurfacing hemiarthroplasty using data from the DSR; to compare different resurfacing arthroplasty designs; and to evaluate age as a possible risk factor for a poor outcome.
To compare different arthroplasty designs with regard to patient reported outcome and risk of revision using data from the DSR.
To conduct a RCT comparing stemmed hemiarthroplasty and resurfacing hemiarthroplasty with regard to functional outcome and shoulder-specific quality of life.
Presentation of the studies – methods and results
Study I
WOOS is a patient-administrated shoulder-specific questionnaire for measurement of the quality-of-life of patients with glenohumeral osteoarthritisCitation30. It provides scores on 4 domains: 1) physical symptoms; 2) sport, recreation, and work; 3) lifestyle; 4) emotions. Each question is answered on a visual analogue scale with a possible score ranging from 0 to 100. There are 19 questions and the total score ranging from 0 to 1900. A score of 1900 signifies that the patient has an extreme decrease in the shoulder-specific quality of life, whereas a score of 0 signified that the patient has no decrease in the shoulder-specific quality of life. For simplicity of presentation, the raw scores can be converted to a percentage of a maximum score as it was done in this studyCitation30.
The translation of WOOS was done according to the recommendation be GuilleminCitation60. First 2 bilingual orthopedic surgeons with Danish as their first language, working independently, translated the original English version into Danish. In the translation process, equality of sense rather than equality of word was given priority. Then, during a conference, consensus was achieved on the first preliminary Danish version based on the 2 translations. Subsequently, 2 professional translators with English as their first language translated this version back into English. Neither of these 2 professionals had any medical knowledge and knew anything about WOOS. Finally, a committee compared the source and the final translated Danish version. The committee consisted of orthopedic surgeons with special interest in shoulder surgery. For a preliminary test, the final Danish version was tested for comprehensibility in a group of 20 consecutive patients, and no further changes were required.
We tested the Danish version of WOOS with classical test theory analyzing validity, reliability and responsiveness in a population of 20 consecutive patients diagnosed with glenohumeral osteoarthritis without symptomatic rotator cuff pathology. The patients were treated with shoulder replacement between May 2010 and April 2012 at the Department of Orthopedic Surgery, Herlev Hospital, Denmark. Patients were excluded only in case of other pathology of the upper extremity or in case of cognitive or linguistic impairment compromising the ability to complete the questionnaires.
Construct validity compares the outcome measurement tool to a gold standard when no “true value” is available. Pearson’s correlation coefficient between WOOS and CMS preoperatively was 0.62, p < 0.01 and the correlation between the change of score for WOOS and CMS was 0.73, p < 0.01. The correlation coefficient between WOOS and CMS, SF-36 and OSS at one year was 0.82, p < 0.01; 0.48, p = 0.03; and 0.82, p < 0.01 respectively. Content validity assesses whether the items measure the full range of the actual question. We found no floor and ceiling effect preoperatively or postoperatively.
Internal consistency designates the correlation between items that make up the score. We used the postoperative measurement of WOOS and found Cronbach Alpha to be 0.98. Elimination of 1 item in all 19 cases resulted in values between 0.97 and 0.98. Question 5 about grinding in the shoulder and question 6 about the affection by weather had correlations with a total score of 0.52 and 0.64 respectively. All other items had correlations with a total score of > 0.75.
The test-retest reliability was measured as the agreement between two measurements taken seven days apart and expressed as ICC. The ICC for the total WOOS was 0.96 [0.91; 0.99 CI], p < 0.01. Mean WOOS for the first and second measurement were 73 and 74 respectively with a mean difference of 1 [-4; 3 CI], p = 0.61.
Responsiveness (sensitivity) to change between before and after treatment was analyzed using: the SRM calculated as the difference between the preoperative mean score and the postoperative mean score divided by SD of the difference; and the ES calculated as the difference between the postoperative mean score and the preoperative mean score divided by the preoperative SD. We compared the results of WOOS with the results of CMS. The SRM was 1.4 and ES 2.3 for WOOS and 1.7 and 2.0 for CMS respectively suggesting good responsiveness comparable to that of CMS. 19 out of 20 patients reported improvement in WOOS. Mean WOOS was 34 preoperatively and 73 postoperatively, with a mean improvement of 39, [26; 52 CI], p < 0.01. This can be compared with a mean CMS of 26 preoperatively and 57 postoperatively, with a mean improvement of 31, [23; 40 CI], p < 0.01.
Study II
All patients with a primary operation reported to the DSR between January 2006 and December 2010 diagnosed with primary or secondary glenohumeral osteoarthritis and treated with resurfacing hemiarthroplasty were included in this study. Data reporting was checked manually. 2 double reporting and 1 reporting error were identified and excluded. 837 arthroplasties in 772 patients were eligible (65 patients were replaced bilaterally).
The Kaplan-Meier method was used to calculate and illustrate the unadjusted event rates with revision for any reason as endpoint. A Cox proportional hazard regression model was used to calculate hazard ratios as a measure for relative risk of revision with 95% CI when adjusting for additional variables. Age, sex, resurfacing arthroplasty design, previous surgery in the same shoulder (yes or no) and type of osteoarthritis (primary or secondary osteoarthritis) were included in the model. Mean differences in WOOS was adjusted for the same variables with use of general linear models when subgroups were compared.
There were 469 (56%) women and mean age was 65 years (SD 11). 715 patients had been diagnosed with primary osteoarthritis and 106 with secondary osteoarthritis. In 16 cases it were not reported if the patient were diagnosed with primary or secondary osteoarthritis. There was no further information about etiology for reported secondary osteoarthritis. 172 patients were reported as having previous surgery.
688 patients (82%) returned a complete questionnaire. Reasons for loss to follow-up are presented in . Mean WOOS was 67 (SD 26). 63 (8%) arthroplasties had been revised by the end of December 2011 with a 5 year cumulative revision rate of approximately 10% (). The most common reasons for revision were glenoid attrition and rotator cuff problem ().
Figure 6. The cumulative revision rate after resurfacing hemiarthroplasty presented with numbers at risk and 95% CI showing a 5-year revision rate of approximately 10%.
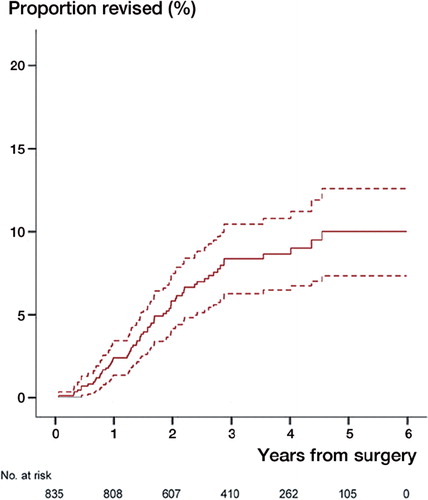
Table 1. The reasons for non-responding with number and percentage of all included arthroplasties
Table 2. The reasons for revision presented with number, percentage of all included arthroplasties and percentages of revisions
There were 543 Copeland resurfacing arthroplasties (Biomet Merck, Swindon, UK), 254 Global C.A.P. resurfacing arthroplasties (Depuy, Leeds, UK) and 40 others. There were no differences in adjusted WOOS between the 2 arthroplasty designs (mean difference 0 [0; 0 CI], p = 0.95). There were no differences in the cumulative revision rate. The adjusted risk of revision after Global C.A.P. was 1.0 [1.0; 1.0 CI], p = 0.32 with Copeland as reference.
There were 165 patients aged 55 years or younger. Mean WOOS was 55 (SD 26). Younger patients had a statistically significant inferior adjusted WOOS exceeding the minimal clinically important difference compared with older patients (mean difference 14 [9; 20 CI], p < 0.01). The adjusted risk of revision for younger patients was 1.2 [0.7; 2.4 CI], p = 0.47 with patients older than 55 years as reference. The 5 year cumulative revision rate was approximately 12% and not statistically significant different from older patients, p = 0.30 ().
Study III
We included all patients diagnosed with primary or secondary glenohumeral osteoarthritis reported to the DSR between January 2006 and December 2010. Only primary operations were included. 1209 arthroplasties in 1109 patients were eligible (100 patients were replaced bilaterally) ().
Demographic data were compared by age, sex, type of osteoarthritis (secondary or primary osteoarthritis), previous surgery in the same shoulder (yes or no) and response rate using student t-test (continues variables) and chi-square test (categorical variables). Mean differences in WOOS was adjusted for predefined potential confounders including age, sex, previous surgery in the same shoulder (yes or no) and type of osteoarthritis (primary or secondary osteoarthritis) with use of general linear models when groups were compared. The Kaplan-Meier method was used to calculate and illustrate the unadjusted revision rates and a Cox proportional hazard regression model was used to calculate hazard ratios as a measure for relative risk of revision with 95% CI when adjusting for the same predefined potential confounders.
In the analysis of patient reported outcome only patients with a complete questionnaire were included. Responders were defined as patients returning a complete questionnaire 1 year postoperatively or after a postal reminder whereas non-responders were defined as patients with an incomplete or no returned questionnaire after the postal reminder. In case of revision within 1 year postoperatively WOOS is registered as missing due to revision. In case of revision later than 1 year postoperatively WOOS are registered as usual and included in the analyses of patient reported outcome.
Inclusion of bilaterally operated patients violates the assumption that arthroplasties are independent. Nonetheless, previous studies have reported that the consequences of including bilaterally operated patients are negligible in the analysis of implant survivalCitation61-64. We do not know the consequences of including bilaterally operated patients in the analysis of WOOS. Thus we analyzed the differences in WOOS between arthroplasty designs with or without inclusion of bilaterally operated patients.
There were 113 total shoulder arthroplasties and 1096 hemiarthroplasties. 975 (81 %) patients returned a complete questionnaire. Demographic data were similar (). Mean WOOS for total shoulder arthroplasty and hemiarthroplasty were 78 (SD 25) and 66 (SD 26) respectively. Total shoulder arthroplasty had a better score, exceeding the minimal clinically important difference, when WOOS was adjusted for potential confounders (mean difference 10 [5; 15 CI], p < 0.01). The analysis without bilaterally operated patients showed similar results (mean difference 12 [6; 18 CI], p < 0.001).
Table 3. The differences in demographics between hemiarthroplasty and total shoulder arthroplasty with number and percentage (in brackets) and mean age with SD (in brackets)
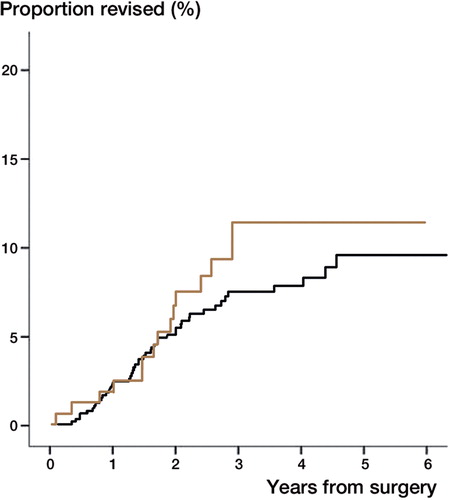
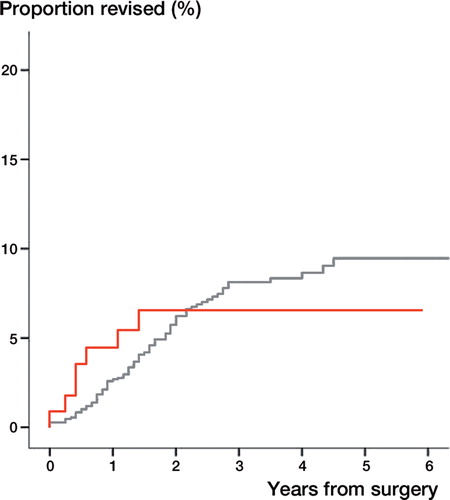
7 (6%) total shoulder arthroplasties and 79 (7%) hemiarthroplasties had been revised by the end of 2011. The unadjusted cumulative revision rates were similar (). When we compared total shoulder arthroplasty with hemiarthroplasty as reference there were no differences in risk of revision adjusted for age, sex, previous surgery in the same shoulder and type of osteoarthritis (RR = 1.1 [0.5: 2.4 CI], p = 0.80). 5 (5%) total shoulder arthroplasties and 36 (3%) hemiarthroplasties were revised within the first year. The difference between the 2 arthroplasty designs vas not statistically significant, p = 0.51.
Figure 9. The cumulative revision rate for hemiarthroplasty (red) and total shoulder arthroplasty (grey) showing no difference between arthroplasty designs, p=0.96.
There were 837 resurfacing hemiarthroplasties and 259 stemmed hemiarthroplasty. The patients treated with resurfacing hemiarthroplasty were more often male and younger and there were fever non-responders compared to stemmed hemiarthroplasty (). Mean WOOS for resurfacing hemiarthroplasty and stemmed hemiarthroplasty were 67 (SD 26) and 64 (SD 26) respectively. Resurfacing hemiarthroplasty had a better score when WOOS was adjusted for potential confounders (mean difference 5 [1; 9 CI], p = 0.02). However, the difference did not exceed the minimal clinically important difference. The analysis without bilaterally operated patients showed identical results (mean difference 5 [1; 10 CI], p < 0.001).
Table 4. The differences in demographics between resurfacing hemiarthroplasty and stemmed hemiarthroplasty with number and percentage (in brackets) and mean age with SD (in brackets).
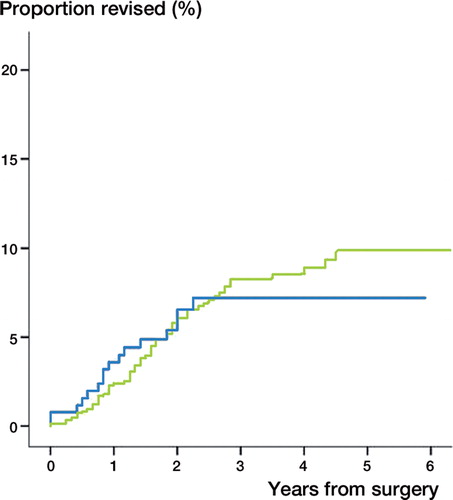
16 (6%) stemmed hemiarthroplasty and 63 (8%) resurfacing hemiarthroplasty had been revised by the end of 2011. The cumulative failure rates were similar (). When we compared resurfacing hemiarthroplasty with stemmed hemiarthroplasty as reference there were no differences in risk of revision adjusted for age, sex, previous surgery in the same shoulder and type of osteoarthritis (RR = 1.0 [0.5: 1.7 CI], p = 0.85). 12 (5%) stemmed hemiarthroplasties and 24 (3%) resurfacing hemiarthroplasties were revised within the first year. The difference between the 2 arthroplasty designs vas not statistically significant, p = 0.16.
Study IV
All patients diagnosed with glenohumeral osteoarthritis scheduled for shoulder replacement at our department were evaluated for inclusion. It was recorded if the patients have had previous surgical intervention in the same shoulder related to osteoarthritis with synovectomy, housecleaning, subacromial decompression, or a stabilizing procedure. Patients were excluded if they had any other pathological conditions affecting function of the upper extremity; a non-concentric glenoid; symptomatic rotator cuff pathology; diseases with ASA ≥3; rheumatoid arthritis; cognitive difficulties; or if less than 60 % of the native humeral head was intact.
147 patients were assessed for eligibility. 22 patients with rheumatoid arthritis, 12 patients with a non-concentric glenoid; 55 patients with symptomatic rotator cuff pathology; and 3 patients with less than 60% of the native humeral head intact were excluded. No patients were excluded due to co-morbidity with ASA ≥3 or cognitive difficulties. 15 patients refused to participate. All included patients were randomized and received the allocated treatment.
Patients were randomized to either: A re-cap hydroxyapatite coated resurfacing prosthesis with a central grooved impact-fit taper peg for cementless fixation; or a cemented stemmed modular prosthesis with the head connected to the stem by the taper locking system with the possibility of different head-stem combinations depending on the size of humerus.
All operations were performed by the same 5 specialists in shoulder surgery that normally perform shoulder replacements at our department. The surgeons had equal experience with the 2 arthroplasty designs. The operations were performed using a deltopectoral approach. All patients received identical postoperatively treatment with a sling and swathe for 2 weeks followed by a simple sling for another 2 weeks. Active range of motion was allowed at 2 weeks with protection of the subscapularis muscle. Strengthening exercises were allowed at 6 weeks.
The patients were evaluated preoperatively and postoperatively after 3 and 12 months using CMS. All patients were evaluated by one assessor with extensive experience in the use of CMS. In addition, a senior author not involved in the surgical procedure and blinded to the randomization, evaluated CMS at 1 year. Before each visit the patients completed the WOOS. Answers were given by the patient with access to supervision. The patient remained blinded to the randomization for the duration of the study. There were no cases of accidental loss of blinding.
The primary outcome, CMS, includes an assessment of pain; ADL; range of motion; and strength. There is 35 point for the subjective assessment including pain and the ability to perform ADL and 65 points for the objective assessment where 40 points are allocated to range of motion and 25 points are allocated to strength. A maximum of 100 point indicates a shoulder with no disability. We used the modified version described by Constant and colleagues in 2008Citation65. Isometric strength testing was performed using an Isobex® dynamometer (Medical Device Solutions MDS, Oberburg, Switzerland). The minimal clinically important difference has recently been defined as 10 point.66 CMS was not adjusted for age and sex. In addition, we used the pain subscale score (0–15 points) to evaluate pain-relief. The minimal clinically important difference in pain was defined as three out of 15 points.
With CMS as the primary outcome we conducted a power analysis to determine the sample size necessary to demonstrate a difference between the two groups of 10 points (standard deviation ±10.0). With a power of 0.80 and a two-sided significance level of 0.05, 16 patients in each group were required. This number was inflated by 4 patients in each group to account for loss to follow-up. The patients were randomly assigned to be treated with 1 of 2 arthroplasty designs using a random-numbers list generated with Microsoft Excel (Redmond, Washington). The assignment group for each patient were kept in sealed, opaque and consecutively numbered envelopes and revealed to the surgeon in the operating theatre just before surgery.
The initial inspection of data was performed with use of histograms and scatter plots and by comparing median and mean values and interquartile range of both CMS and WOOS. Bilateral operated patients were included and analyzed as separate cases even though it violates the assumption of independency. Independent t test was used to determine differences in scores at baseline and at 1 year between groups. ANCOVA was used to determine the change in scores from the preoperative to the postoperative measurements and to determine if there were differences between groups. Paired-samples t-test was used to compare the blinded and non-blinded evaluation. Independent t-test was used to compare the operation time. Loss to follow-up after the 3 months evaluation was included in the analysis of the 1 year follow-up using the results from the 3 months evaluation (last observation carried forward). Loss to follow-up before the 3 months evaluation was not included in the analysis of outcome. In case of cross over from resurfacing hemiarthroplasty to stemmed hemi arthroplasty intention-to-treat analysis was planned; however, there was no cross over. All analyses were performed using SPSS (version 19.0; IBM Corporation, Armonk, NY, USA) and R (version 3.0.1; R Foundation for Statistical Computing, Vienna, Austria). The level of statistical significance was set at 0.05 and all analyses were 2-tailed.
40 shoulders in 35 patients were included between September 2009 and August 2012 (5 patients were replaced bilaterally at 2 occasions regarded independent). There were 20 stemmed hemiarthroplasties and 20 resurfacing hemiarthroplasties. Of the 5 patients who had a bilateral operation, 3 had a bilateral stemmed hemiarthroplasty and two had a stemmed hemiarthroplasty on one side and a resurfacing hemiarthroplasty on the other. Mean age in entire series at time of surgery was 67 years (SD 12). There were 27 females and 13 males. The preoperative CMS and WOOS in the entire series were 24 (SD 14) and 32 (SD 18) respectively. There were no statistical significant differences in age, sex or preoperative scores except for WOOS between the 2 groups ().
Table 5. Baseline characteristics. Mean values with SD in brackets
No patients were excluded intraoperatively due to a non-concentric glenoid; symptomatic rotator cuff pathology or inadequately bone morphology interfering with the use of a resurfacing hemiarthroplasty. The mean operation time was 80 minutes (SD 15) for stemmed hemiarthroplasty and 52 minutes (SD 13) for resurfacing hemiarthroplasty with a mean of 28 [19: 37 CI], p < 0.01. 1 patient treated with resurfacing hemiarthroplasty had a temporary dysfunction of the musculocutaneus nerve. Another patient treated with resurfacing hemiarthroplasty got bullous allergic contact dermatitis eight days postoperatively probably caused by the sling and swath. It responded well to treatment with local applied corticosteroid. A superficial infection developed in 1 patient treated with stemmed hemiarthroplasty. It was treated with antibiotics for 2 weeks and resolved. No other complications were observed the first 3 months postoperatively.
1 patient from the stemmed hemiarthroplasty group died before the 3 months follow-up of reasons unrelated to the surgical intervention and 1 patient from the resurfacing hemiarthroplasty group dropped out the first postoperative day at the patient’s request. Both patients were not included in the postoperative analyses. A third patient treated with stemmed hemiarthroplasty was diagnosed with reflex dystrophy after 6 months and dropped out before the 1 year evaluation was conducted at the patient’s request. For this patient the 3 months evaluation was carried forward and used in the 1 year analyzes. There were no infections or other recorded complications and no arthroplasties had been revised at 1 year.
At 1 year CMS and WOOS in the entire series was 54 (SD 21) and 69 (SD 27) respectively. The results of resurfacing hemiarthroplasty tended to be inferior with mean differences in CMS, WOOS and subscale CMS of pain of 10, 20 and 3 respectively (). The difference in WOOS and subscale CMS of pain was statistical significant and exceeding the minimal clinically important difference whereas the difference in CMS, with the numbers available for this study, was statistical insignificant but exceeding the minimal clinically important difference. A non-blinded and a blinded assessor evaluated CMS at 1 year. There was a difference of 1 point between the 2 evaluations [-2; 4 CI], p = 0.54. Thus, we have no suspicions of observer bias.
Table 6. Outcome scores at one year. Mean values with SD in brackets.
Improvement in CMS and WOOS between the preoperative and the 1 year evaluation were seen in 34 out of 38 patients. 2 patients had a worse outcome in both CMS and WOOS at 1 year (both were treated with resurfacing hemiarthroplasty), 1 patient had a worse outcome in CMS and 1 patient had a worse outcome in WOOS (both were treated with stemmed hemiarthroplasty).
There was a significant improvement of CMS with a mean difference between the preoperative and postoperative measurement of 28 [17; 39 CI] after resurfacing hemiarthroplasty and 32 [22; 42 CI] after stemmed hemiarthroplasty. Mean improvement of WOOS was 34 [18; 49 CI] after resurfacing hemiarthroplasty and 41 [28; 53 CI] after stemmed hemiarthroplasty. The improvements were highly statistical significant, p < 0.01. The differences in mean improvement for CMS and WOOS between the arthroplasty designs was statistically insignificant (mean difference 4 [-10; 18 CI], p = 0.58 and 7 [-12; 26 CI], p = 0.48 respectively).
Discussion
Translation and validation of WOOS
Patient-reported outcomes has become popular and is increasingly used to evaluate the shoulder-specific quality of life and some authors have argued that the patient’s own perception of the results through patient reported quality of life may be the most important measure when the effects of a treatment are evaluatedCitation30.
The advantages of patient-reported outcomes are that questionnaires do not require the time of an orthopedic surgeon and they can be completed by the patient and returned by mail without attending the hospital. Thus, a questionnaire is likely to have a high patient compliance compared with radiological and clinical examinations, such as CMS. Furthermore, any influence of interobserver reliability is eliminated when questionnaires are used.
WOOS was used to evaluate the shoulder-specific quality of life. There was no validated Danish version available so WOOS was translated into Danish and transcultural adapted according to international guidelines. There was no need for substantial changes compared to the original English version. The Danish translation of WOOS had similar psychometric properties as described for the original English version in terms of validity, reliability and responsiveness.
There has been dispute about which questionnaire is most appropriate to use. The choice of questionnaire depends mainly on the diagnoses of the included patients. We chose to use WOOS since it is specific designed to evaluate patients with glenohumeral osteoarthritis; thus, WOOS is theoretically able to detect smaller changes than other more general shoulder measures. A high responsiveness has the obvious advantage that fewer subjects are required to detect clinically important differences in comparative studies. However, we found similar responsiveness of WOOS and CMS and our results can not support this hypothesis.
One limitation of the study is that we did not register the time employed in filling out WOOS. Furthermore, all the included patients were treated with shoulder replacement, and all the included patients except 1 had an improvement in WOOS between the preoperative and postoperative measurement. As a consequence we cannot justify any conclusion about the ability of WOOS to detect changes when the perceived shoulder function decreases. Nonetheless, it is recommendable to use WOOS in the evaluation of patients with glenohumeral osteoarthritis treated with shoulder replacement.
Functional outcome
The first report of resurfacing hemiarthroplasties in patients with osteoarthritis (n=30) described good functional outcome with a non-adjusted Constant Score of 58 after minimum 2 years of follow-upCitation28. Other case series have reported similar results with non-adjusted CMS between 57 and 68Citation47,49, 51-54. The RCT included in this thesis showed a CMS of 49 and 59 following resurfacing hemiarthroplasty and stemmed hemiarthroplasty respectively. With the numbers available for the RCT, the difference of 10 was statistical insignificant, although it exceeded the minimal clinically important difference. Thus, the results of resurfacing hemiarthroplasty tended to be inferior to that of stemmed hemiarthroplasty and were not as favourable as reported in previous case series.
There were no statistically significant differences between the 2 groups preoperatively with regard to CMS. Nevertheless, the differences in mean improvement between the 2 groups were rather small and statistically insignificant and differences in the preoperative score may have influenced outcome. Furthermore, patient-related factors such as previous surgery, co-morbidity, ongoing insurance case, socioeconomic status, and the severity of osteoarthritis including glenoid wear may have been unequally distributed due to the small number of patients and thus influencing outcome. Differences in preoperative scores, distribution of potential patient-related risk factors, and in the severity of osteoarthritis may also be the reason for the different findings between the present study and previous publications.
3 RCTs have compared stemmed hemiarthroplasty and total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis34, 36, 67. 1 trial67 reported a CMS of 60 following stemmed hemiarthroplasty at 1 year which is identical to the results in the RCT included in this thesis. In comparison, the results following total shoulder arthroplasty were 68. The difference of 8 was statistically insignificant. There was no difference in UCLA Shoulder rating scale with 29 and 30 following stemmed hemiarthroplasty and total shoulder arthroplasty respectively (The UCLA Shoulder rating scale has a maximum of 35 points being the bestCitation68). Another randomized control trialCitation36 reported similar CMS of 67 and 71 following stemmed hemiarthroplasty and total shoulder arthroplasty. UCLA Shoulder rating scale was 24 and 27 respectively. The third RCT3Citation4 did not include an evaluation of CMS but UCLA Shoulder rating scale was 23 and 27 following stemmed hemiarthroplasty and total shoulder arthroplasty without the difference being statistically significant. The results of the UCLA Shoulder rating scale in these 3 RCTs have been summarized in a systematic review and meta-analysisCitation39reporting a statistically significant superior outcome following total shoulder arthroplasty. The functional outcome of stemmed hemiarthroplasty and total shoulder arthroplasty have also been reported in numerous studies including larger prospective studiesCitation22, 69 and the results have been summarized in a systematic reviewCitation39 favouring total shoulder arthroplasty.
The functional outcome of resurfacing hemiarthroplasty was disappointing and tended to be inferior to that of stemmed hemiarthroplasty whereas the results following stemmed hemiarthroplasty was similar to the results reported in previous RCTs. Thus resurfacing hemiarthroplasty is a no better alternative to total shoulder arthroplasty than stemmed hemiarthroplasty. Even though it is not deducted from data included in this thesis total shoulder arthroplasty seems to be associated with a superior functional outcome compared to that of resurfacing hemiarthroplasty and stemmed hemiarthroplasty.
Patient reported shoulder-specific quality of life
WOOS was used to estimate the patient reported shoulder-specific quality of life in the RCT and in the 2 registry studies included in this thesis. Although the RCT showed a significant improvement in WOOS from 32 preoperatively to 69 at the 1-year follow-up examination in the entire series of 40 patients the result were rather disappointing compared to the results reported in a previous RCT comparing total shoulder arthroplasty and stemmed hemiarthroplastyCitation36. This study reported an improvement in WOOS from 32 preoperatively to 87 at the 2-year follow-up evaluation in the entire series of 41 patients. The reason for rather disappointing results in the RCT included in this thesis is the outcome following resurfacing hemiarthroplasty. Not only was the outcome of 59 statistically significant inferior to that of stemmed hemiarthroplasty with a mean difference of 20 but it also tended to be inferior to the results of 67 found in the registry study.
In the previously published RCT3Citation6 the results of total shoulder arthroplasty was 91; however, with the number of patients available the difference of 9 between total shoulder arthroplasty and stemmed hemiarthroplasty was statistically insignificant. The RCT included in this thesis did not evaluate total shoulder arthroplasty but data reported to the DSR showed a WOOS score of 78 following total shoulder arthroplasty which was significantly superior to that of 66 following hemiarthroplasty (resurfacing hemiarthroplasty and stemmed hemiarthroplasty). Thus, it seems like total shoulder arthroplasty is associated with a superior patient reported outcome compared to that of stemmed hemiarthroplasty and resurfacing hemiarthroplasty.
Revision rates
The revision rate of approximately 10% following resurfacing hemiarthroplasty is not as promising as reported in previous case series where up to 5% of the arthroplasties were revisedCitation28,49,50,53. A reason for the difference could be that the previous case series are small with few revisions that can make the reported revision rates imprecise. Another reason could be that previous studies were conducted in highly specialised centres with a keen interest in resurfacing arthroplasty whereas the results in this study are based on the results from the average Danish shoulder surgeon. The difference could also be caused by different patient selection due to inclusion and exclusions criteria in the previous case series.
The revision rate following resurfacing hemiarthroplasty is more in conformity with the reporting from other national shoulder registries. The Norwegian Arthroplasty Register reported cumulative revision rates of approximately 6% and 17% after 5 and 10 years respectively in 121 patients treated with resurfacing hemiarthroplastyCitation70. The latest published annual report from the New Zealand Joint Registry described that 12 (8%) out of 151 reported resurfacing hemiarthroplasties had been revised with a 3-year cumulative revision rate of approximately 10%Citation33. The latest published annual report from the National Joint Replacement Registry in Australia described that 51 (6%) out of 859 resurfacing hemiarthroplasties had been revised with a 4-year cumulative revision rate of approximately 10%Citation32. However, the results from the other registries are not adjusted for diagnosis and should be interpreted carefully.
There may be several explanations for the high revision rate following resurfacing hemiarthroplasty reported in this thesis. In Denmark approximately 30% of all arthroplasties are used in patients diagnosed with osteoarthritis but only 10% of the these patients are treated with total shoulder arthroplastyCitation29. It is likely that resurfacing hemiarthroplasty is used in patients with glenoid wear. These patients might have benefit from total shoulder replacement instead. Furthermore, resurfacing hemiarthroplasty can more easily be revised compared to other arthroplasty designs and some surgeons may choose to revise in cases with only a minor inferior outcome. These patients might not have been revised if they had a similar inferior outcome following stemmed hemiarthroplasty or total shoulder arthroplasty. Nevertheless, this is not deducted from data in this thesis and any conclusion about the reason for the high revision rate is tentative.
Revision following stemmed hemiarthroplasty and total shoulder replacement has been reported in numerous case series and few prospective studies. The results of 23 studies has been summarized in a systematic reviewCitation37 reporting that 7% total shoulder arthroplasties and 10% stemmed hemiarthroplasties were revised without the follow-up times being specified. Revision was only required in 2% of the total shoulder arthroplasties using an all-polyethylene glenoid component. Revision rates following stemmed hemiarthroplasty and total shoulder arthroplasty has also been described in a clinical multi-centre study including 690 arthroplasties (601 total shoulder arthroplasties and 89 stemmed hemiarthroplasties) with a mean follow-up time of 43 months. There was 68 (10%) reported revisions. There was a high revision rate following total shoulder arthroplasty that was related to the use of a metal-backed glenoid component that was later abandonedCitation22.
The annual report from the National Joint Replacement Registry in AustraliaCitation32 described a 4-year cumulative revision rate of 6% and 9% following total shoulder arthroplasty and stemmed hemiarthroplasty in patients with osteoarthritis. The New Zealand Joint RegistryCitation33 reported a 3-year cumulative revision rate of approximately 3% and 5% following total shoulder arthroplasty and stemmed hemiarthroplasty, respectively. The results from the New Zealand Joint Registry are not adjusted for diagnosis and should be interpreted carefully.
There were few revisions of stemmed hemiarthroplasty (n=16) and especially total shoulder arthroplasty (n=7) reported to DSR and the revision rates should be interpreted carefully; nonetheless, the revision rate is in accordance with the findings reported by other national registries and previous clinical studies. Some surgeons may find the risk of glenoid loosening and subsequently lack of acceptable revision possibilities worrying. However, total shoulder arthroplasty is most likely associated with the lowest revision rate when an all-polyethylene glenoid component is used. Furthermore, reverse total shoulder arthroplasty has now been used for more than a decade in the treatment of failed total shoulder arthroplasty with promising functional outcome and patients satisfaction despite a high need of secondary revision surgery (approximately 20%)Citation40-42.
Reasons for revision
Based on previous studies with very few revisions it has not been possible to adequately describe reasons of revisions after resurfacing hemiarthroplasty in patients diagnosed with glenohumeral osteoarthritis but previous studies of stemmed hemiarthroplasties have reported that glenoid wear (glenoid attrition) can be expected in patients treated with hemiarthroplastyCitation35,38. This is in accordance with the results from DSR where glenoid attrition was the most frequent reason of revision seen in 18 out of 837 (2%) patients treated with resurfacing hemiarthroplasty. Deep infection with need of revision was only seen in 4 (0.5%) patients. A study from the Norwegian Arthroplasty Register including all types of diagnoses reported 5 revisions due to infection in 1,526 (0.3%) hemiarthroplasties including 165 resurfacing hemiarthroplastiesCitation70. Thus deep infection seems to be a minor risk following primary surgery with resurfacing hemiarthroplasty in patients with osteoarthritis.
Overstuffing the joint is a possible reason for revision related to hemiarthroplasty. It has been described in biomechanical studies that oversizing the humeral head are changing the offset and thereby impair the rotator cuff function with additional possibility of glenoid attritionCitation24,44,71. In a previous study; radiographs showed that 4 out of 50 (8%) patients had an oversized humeral component. However, only one of these patients had clinical symptoms indicating rotator cuff problem and this case did not require revision surgeryCitation49. We still know little on why some patients fail to gain good function and pain relief after resurfacing hemiarthroplasty, and our knowledge on when the joint is mechanical overstuffed is still limited. An overstuffed joint is not an option when surgeons report on the reason for revision to DSR but the 4 major reasons for revision described in this thesis (glenoid attrition, rotator cuff problem, technical failure and pain) can all be caused by overstuffing the shoulder joint.
The use of resurfacing hemiarthroplasty in the treatment of younger patients
The definition of a young patient is disputed and limits of 50 or 55 years have previously been suggestedCitation50,52,72,73. It was decided to use the same limit of 55 years as in the 2 previously published studies of resurfacing hemiarthroplasty in young patientsCitation50,52. One of these studiesCitation50 reported a good ASES shoulder scoreCitation74 in 36 patients at the 2-year follow-up examination and only 1 revision. The other study including 26 arthroplasties reported a similar good outcome with a mean CMS of 61 and 2 revisionsCitation52.
Data from DSR showed that younger patients had a mean WOOS of 55 which was statistically significant inferior to that of older patients with a mean difference of 14. Although it seems disappointing and not as favourable as previously reported the result should be interpreted carefully. Thus it is likely that the inferior outcomes in younger patients are influenced by greater expectations to the outcome (wishes of returning to a physical demanding occupation or a high level of sport) or differences in baseline characteristics.
The 5-year cumulative revision rate was approximately 12%. With the numbers of revisions available this was not statistically significant different from that of older patients. Nonetheless, the high revision rate is worrying especially since younger patients have a long expected lifetime that may lead to an even higher revision rate at the long term.
Whether resurfacing hemiarthroplasty is the best treatment option in younger patients with glenohumeral osteoarthritis remains to be examined in comparative studies. Alternatives include physiotherapy; intraarticular injections of hyaluronate; joint preserving surgery; and treatment with other shoulder arthroplasty designs such as total shoulder arthroplasty. These treatment options should be thoroughly considered in the treatment of younger patients until the efficacy of resurfacing hemiarthroplasty has been more thoroughly documented.
Methodological considerations
A RCT represent a high level of evidence and the results can be included in meta-analyses; however, there are several potential limitations. RCTs are laborious and expensive and long-term or even mid-term follow-up results are rarely reported. Furthermore, as with the RCT included in this thesis the number of included patients is often very small with a too optimistic pre-study sample size calculation leaving the study with little statistical power. Finally, considering the few revisions following shoulder replacement it is very difficult to adequately report revision rates and reasons for revision.
The extern validity of a study can be compromised in several ways. RCTs use inclusion and exclusion criteria in an attempt to make patients as homogeneous as possible; however, this may compromise the ability to generalize the results especially to patients with co-morbidity. Furthermore, the surgeons are often more experienced and interested in the specific type of operation than the general shoulder surgeon and it is likely that RCTs provide information which can not always be generalized to other surgeonsCitation75. In the RCT included in this thesis we sought to resolve this by letting the patients be operated by the same surgeon as would have operated the patients if they had not participated in the study.
It is preferable that the results and conclusions from RCTs are supported by larger multicenter studies using prospectively collected data. This is mainly possible in countries using a national shoulder arthroplasty registry. The advantages of these registry studies include the large sample sizes leaving the study with sufficient statistical power and the possibility to adequately describe revision rates and reasons for revision. Finally, registry studies are associated with high extern validity; not only are patients rarely excluded due to age, co-morbidity and severity of osteoarthritis e.g. but the patients are also operated by a general shoulder surgeon.
The major limitation of registry studies are that there is no control over what type of implant is used; thus, a specific implant may have been selected to treat the patient for certain unknown reasons. Furthermore, information about the patient population is often sparse and as the patients are not randomly allocated there may be a different distribution of co-variables which might influence outcome.
However, in the two registry studies included in this thesis, the differences in patient reported outcome and the risk of revision were adjusted for known potential confounders (age, sex arthroplasty design, previous surgery and type of osteoarthritis).
Another limitation of the 2 registry studies included in this thesis is that there was no preoperative measurement of the patient reported outcome; thus, differences in preoperative shoulder function may have influenced the differences in WOOS between groups. Furthermore, not all revisions are captured in the DSR and it is highly likely that the reported revision rates are underestimated. Finally and very important, incorrect reporting may diminish the accuracy and reliability of the data.
Inclusion of bilaterally operated patients violates the assumption that arthroplasties are independent. Nonetheless, bilaterally operated patients were included in the analysis of WOOS in order to obtain higher statistical power. The consequences of this have not previously been described but exclusion of bilaterally operated patients did not significantly change the results and the conclusions in the registry studies.
In the 2 registry studies there was no information about WOOS in the early failures that is revised within the first year. Thus since early failures were not included in the analysis of WOOS an uneven distribution may have skewed outcome (mean WOOS would be falsely high if the percentage of early revision in one group is higher than in others). Nonetheless, we compared the percentage of early failures and found no major or statistical significant differences between arthroplasty designs.
Conclusion related to the aims of the thesis and future research
We have shown that the Danish version of WOOS, translated according to international standardized guidelines, has substantial psychometric properties at the same level as described for the original version. In clinical research it is important to define the minimal clinically important difference of the measures used. The minimal clinically important difference was not defined in the publication describing the original English version of WOOS but it has recently been suggested to be 10% of a maximum scoreCitation76. In future, consensus of this limit needs to be established. To my knowledge the responsiveness of WOOS has only been tested using preoperative and postoperative measurements with an expected improvement in WOOS; thus responsiveness of WOOS in a population that also includes patients with an expected decrease in WOOS is a subject for future research. We only tested the Danish version of WOOS with classical test theory analyzing reliability, validity and responsiveness. Modern test theory analyzing the dimensional structure of WOOS using Rasch analysis is another subject for future research. It is recommendable to use WOOS in the evaluation of patients with glenohumeral osteoarthritis treated with shoulder replacement.
The cumulative revision rate following resurfacing hemiarthroplasty was higher than reported in previous case series and the patients reported outcome was not as good as that of stemmed hemiarthroplasty and total shoulder arthroplasty reported in a previous RCT. Especially the patient reported outcome and revision rate in younger patients was worrying. Other treatment options should be considered until the efficacy of resurfacing hemiarthroplasty has been more thoroughly documented. Studies comparing shoulder replacement with physiotherapy; intraarticular injections of hyaluronate; and joint preserving surgery may be subject for future research.
Based on data from DSR the patient reported outcome (WOOS) following total shoulder arthroplasty was superior to that of hemiarthroplasty (stemmed hemiarthroplasty and resurfacing hemiarthroplasty) with a statistically significant difference exceeding the minimal clinical important difference. Furthermore, the risk of revision tended to be lowest following total shoulder arthroplasty. Based on data from this thesis, and based on existing knowledge, it seems like total shoulder arthroplasty should be preferred in the treatment of glenohumeral osteoarthritis.
The use of resurfacing hemiarthroplasty has relied on the results from case series only. The efficacy in the treatment of glenohumeral osteoarthritis has been promising; however, the RCT included in this thesis indicates that the results may be less favourable than previously reported. The limited number of patients may have influenced the results and a larger definitive RCT is needed. Nevertheless, this is laborious and small RCTs with short-term follow-up are justified since the results can be included in future meta-analyses. The results of shoulder replacement in patients with glenohumeral osteoarthritis may be influenced by factors other than arthroplasty design. Information about disease related factors (severity of osteoarthritis including glenoid wear, rotator cuff pathology), surgical related factors (bicepstenodesis, bicepsstenotomy, additional rotator cuff surgery), surgeon related factors (experience) and patient related factors (previous surgery, co-morbidity, ongoing insurance case, socioeconomic status) is sparse and subjects for future research.
Most recently stem-less shoulder arthroplasty has become available as an option in the treatment of glenohumeral osteoarthritis. There are several theoretical advantages similar to that of resurfacing arthroplasty including minimal risk of periprosthetic fracture and minimal bone resection facilitating revision to other arthroplasty designs. However, whereas total resurfacing arthroplasty is associated with difficulties in glenoid exposure with the humeral head intact this is not the case when stem-less shoulder arthroplasty is used making replacement of the glenoid possible. Stem-less shoulder arthroplasty may be the treatment of choice in the future, but the efficacy should be documented in a RCT before it is routinely used.
Summary
This thesis includes four studies focusing on the functional outcome, shoulder-specific quality of life and risk of revision following shoulder replacement in patients with glenohumeral osteoarthritis without symptomatic rotator cuff pathology.
The Danish version of WOOS, translated according to international standardized guidelines, had substantial psychometric properties comparable to the original version. It is recommendable to use WOOS in the evaluation of patients with glenohumeral osteoarthritis treated with shoulder replacement.
Data from DSR showed that the shoulder specific quality of life following total shoulder arthroplasty was superior to that of hemiarthroplasty (resurfacing hemiarthroplasty and stemmed hemiarthroplasty). The difference between stemmed hemiarthroplasty and resurfacing hemiarthroplasty was small and did not exceed the minimal clinically important difference. The revision rate following resurfacing hemiarthroplasty was surprisingly high compared with previous reports but there were no statistical significant differences in revision rate between arthroplasty designs. The shoulder specific quality of life and revision rate in patients under the age of 55 was worrying.
The use of resurfacing hemiarthroplasty has relied on the results from case series only. The efficacy in the treatment of glenohumeral osteoarthritis has been promising but the CMS found in the randomized clinical trial indicate that the functional outcome may be inferior to that of stemmed hemiarthroplasty and less favourable than previously reported. However, the limited number of patients may have influenced the results and a larger definitive RCT is needed.
Shoulder replacement is relevant and effective in the treatment of glenohumeral osteoarthritis; however, resurfacing hemiarthroplasty was associated with a poorer outcome and a higher risk of revision than previously assumed especially in patients under the age of 55. Based on data from this thesis, and based on existing knowledge, it seems like total shoulder arthroplasty should be preferred in the treatment of glenohumeral osteoarthritis. Shoulder replacement is rarely indicated in younger patients where other treatment options (e.g. physiotherapy; intraarticular injections of hyaluronate; and joint preserving surgery) should be considered until the efficacy of shoulder replacement has been more thoroughly documented.
References
- Parsons IM, Weldon EJ, III, Titelman RM, Smith KL. Glenohumeral arthritis and its management. Phys Med Rehabil Clin N Am 2004; 15: 447-74.
- Izquierdo R, Voloshin I, Edwards S, Freehill MQ, Stanwood W, Wiater JM, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg 2010; 18: 375-82.
- Iannotti JP, Williams GR. Disorders of the Shoulder: Diagnosis and Management. 2rd ed, Lippincott Williams and Wilkins, Philadelphia 2007.
- Millett PJ, Gobezie R, Boykin RE. Shoulder osteoarthritis: diagnosis and management. Am Fam Physician 2008; 78: 605-11.
- Denard PJ, Wirth MA, Orfaly RM Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am 2011; 93: 885-92.
- Petscavage JM, Ha AS, Chew FS. Current concepts of shoulder arthroplasty for radiologists: Part 1--Epidemiology, history, preoperative imaging, and hemiarthroplasty. AJR Am J Roentgenol 2012; 199: 757-67.
- Stevenson JH, Trojian T. Evaluation of shoulder pain. J Fam Pract 2002; 51: 605-11.
- Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database Syst Rev 2003: CD004258.
- Izquierdo R, Voloshin I, Edwards S, Freehill MQ, Stanwood W, Wiater JM, et al. American academy of orthopaedic surgeons clinical practice guideline on: the treatment of glenohumeral joint osteoarthritis. J Bone Joint Surg Am 2011; 93: 203-5.
- Marinko LN, Chacko JM, Dalton D, Chacko CC. The effectiveness of therapeutic exercise for painful shoulder conditions: a meta-analysis. J Shoulder Elbow Surg 2011; 20: 1351-9.
- Day RO, Graham GG. Non-steroidal anti-inflammatory drugs (NSAIDs). BMJ 2013; 346: f3195.
- Saccomanni B. Inflammation and shoulder pain--a perspective on rotator cuff disease, adhesive capsulitis, and osteoarthritis: conservative treatment. Clin Rheumatol 2009; 28: 495-500.
- Blaine T, Moskowitz R, Udell J, Skyhar M, Levin R, Friedlander J, Daley M, Altman R. Treatment of persistent shoulder pain with sodium hyaluronate: a randomized, controlled trial. A multicenter study. J Bone Joint Surg Am 2008; 90: 970-9.
- Boselli KJ, Ahmad CS, Levine WN. Treatment of glenohumeral arthrosis. Am J Sports Med 2010; 38: 2558-72.
- Weinstein DM, Bucchieri JS, Pollock RG, Flatow EL, Bigliani LU. Arthroscopic debridement of the shoulder for osteoarthritis. Arthroscopy 2000; 16: 471-6.
- Bankes MJ, Emery RJ. Pioneers of shoulder replacement: Themistocles Gluck and Jules Emile Pean. J Shoulder Elbow Surg 1995; 4: 259-62.
- Neer CS, Brown TH, Jr., McLaughlin HL. Fracture of the neck of the humerus with dislocation of the head fragment. Am J Surg 1953; 85: 252-8.
- Neer CS. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am 1974; 56: 1-13.
- Neer CS, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am 1982; 64: 319-37.
- Bishop JY, Flatow EL. Humeral head replacement versus total shoulder arthroplasty: clinical outcomes--a review. J Shoulder Elbow Surg 2005; 14: 141S-6S.
- Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br 2006; 88: 562-75.
- Edwards TB, Kadakia NR, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg 2003; 12: 207-13.
- Godeneche A, Boileau P, Favard L, Le Huec JC, Levigne C, Nove-Josserand L, Walch G, Edwards TB. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg 2002; 11: 11-8.
- Hammond G, Tibone JE, McGarry MH, Jun BJ, Lee TQ. Biomechanical comparison of anatomic humeral head resurfacing and hemiarthroplasty in functional glenohumeral positions. J Bone Joint Surg Am 2012; 94: 68-76.
- Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg 2002; 11: 130-5.
- Singh JA, Sperling J, Buchbinder R, McMaken K. Surgery for shoulder osteoarthritis: a Cochrane systematic review. J Rheumatol 2011; 38: 598-605.
- Copeland S. The continuing development of shoulder replacement: “reaching the surface”. J Bone Joint Surg Am 2006; 88: 900-5.
- Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg 2004; 13: 266-71.
- Rasmussen JV, Jakobsen J, Brorson S, Olsen BS. The Danish Shoulder Arthroplasty Registry: clinical outcome and short-term survival of 2,137 primary shoulder replacements. Acta Orthop 2012; 83: 171-3.
- Lo IK, Griffin S, Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage 2001; 9: 771-8.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011; 93: 2249-54.
- No authors listed Demographics and outcomes of shoulder arthroplasty 2012. Annual report from the Australian Joint Replacement Registry. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2012 ( Date last accessed 17 April 2013).
- No authors listed. Annual report from the New Zealand National Joint registry. http://www.cdhb.govt.nz/njr/ ( Date last accessed 17 April 2013).
- Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am 2000; 82: 26-34.
- Haines JF, Trail IA, Nuttall D, Birch A, Barrow A. The results of arthroplasty in osteoarthritis of the shoulder. J Bone Joint Surg Br 2006; 88: 496-501.
- Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am 2005; 87: 2178-85.
- Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg 2007; 16: 396-402.
- Parsons IM, Millett PJ, Warner JJ. Glenoid wear after shoulder hemiarthroplasty: quantitative radiographic analysis. Clin Orthop Relat Res 2004: 120-5.
- Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am 2005; 87: 1947-56.
- Melis B, Bonnevialle N, Neyton L, Levigne C, Favard L, Walch G, Boileau P. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg 2012; 21: 342-9.
- Ortmaier R, Resch H, Matis N, Blocher M, Auffarth A, Mayer M, Hitzl W, Tauber M. Reverse shoulder arthroplasty in revision of failed shoulder arthroplasty-outcome and follow-up. Int Orthop 2013; 37: 67-75.
- Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21: 514-22.
- Levy O, Copeland SA. Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Joint Surg Br 2001; 83: 213-21.
- Thomas SR, Sforza G, Levy O, Copeland SA. Geometrical analysis of Copeland surface replacement shoulder arthroplasty in relation to normal anatomy. J Shoulder Elbow Surg 2005; 14: 186-92.
- Williams GR, Jr., Wong KL, Pepe MD, Tan V, Silverberg D, Ramsey ML, Karduna A, Iannotti JP. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg 2001; 10: 399-409.
- Burgess DL, McGrath MS, Bonutti PM, Marker DR, Delanois RE, Mont MA. Shoulder resurfacing. J Bone Joint Surg Am 2009; 91: 1228-38.
- Mansat P, Coutie AS, Bonnevialle N, Rongieres M, Mansat M, Bonnevialle P. Resurfacing humeral prosthesis: do we really reconstruct the anatomy? J Shoulder Elbow Surg 2012.
- Mechlenburg I, Amstrup A, Klebe T, Jacobsen SS, Teichert G, Stilling M. The Copeland resurfacing humeral head implant does not restore humeral head anatomy. A retrospective study. Arch Orthop Trauma Surg 2013; 133: 615-9.
- Al-Hadithy N, Domos P, Sewell MD, Naleem A, Papanna MC, Pandit R. Cementless surface replacement arthroplasty of the shoulder for osteoarthritis: results of fifty Mark III Copeland prosthesis from an independent center with four-year mean follow-up. J Shoulder Elbow Surg 2012; 21: 1776-81.
- Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am 2008; 90: 110-7.
- Mullett H, Levy O, Raj D, Even T, Abraham R, Copeland SA. Copeland surface replacement of the shoulder. Results of an hydroxyapatite-coated cementless implant in patients over 80 years of age. J Bone Joint Surg Br 2007; 89: 1466-9.
- Raiss P, Pape G, Becker S, Rickert M,Loew M. [Cementless humeral surface replacement arthroplasty in patients less than 55 years of age]. Orthopade 2010; 39: 201-8.
- Thomas SR, Wilson AJ, Chambler A, Harding I, Thomas M. Outcome of Copeland surface replacement shoulder arthroplasty. J Shoulder Elbow Surg 2005; 14: 485-91.
- Pritchett JW. Long-term results and patient satisfaction after shoulder resurfacing. J Shoulder Elbow Surg 2011; 20: 771-7.
- Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br 2006; 88: 562-75.
- Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am 2005; 87: 1697-705.
- Jarrett CD, Brown BT, Schmidt CC. Reverse shoulder arthroplasty. Orthop Clin North Am 2013; 44: 389-408.
- Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004; 86: 388-95.
- Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007; 89: 1476-85.
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993; 46: 1417-32.
- Lie SA, Engesaeter LB, Havelin LI, Gjessing HK, Vollset SE. Dependency issues in survival analyses of 55,782 primary hip replacements from 47,355 patients. Stat Med 2004; 23: 3227-40.
- Ranstam J, Karrholm J, Pulkkinen P, Makela K, Espehaug B, Pedersen AB, Mehnert F, Furnes O. Statistical analysis of arthroplasty data. I. Introduction and background. Acta Orthop 2011; 82: 253-7.
- Robertsson O, Ranstam J. No bias of ignored bilaterality when analysing the revision risk of knee prostheses: analysis of a population based sample of 44,590 patients with 55,298 knee prostheses from the national Swedish Knee Arthroplasty Register. BMC Musculoskelet Disord 2003; 4: 1.
- Schwarzer G, Schumacher M, Maurer TB, Ochsner PE. Statistical analysis of failure times in total joint replacement. J Clin Epidemiol 2001; 54: 997-1003.
- Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg 2008; 17: 355-61.
- Kukkonen J, Kauko T, Vahlberg T, Joukainen A, Aarimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. J Shoulder Elbow Surg 2013.
- Sandow MJ, David H, Bentall SJ. Hemiarthroplasty vs total shoulder replacement for rotator cuff intact osteoarthritis: how do they fare after a decade? J Shoulder Elbow Surg 2013.
- Amstutz HC, Sew Hoy AL, Clarke IC. UCLA anatomic total shoulder arthroplasty. Clin Orthop Relat Res 1981: 7-20.
- Singh JA, Sperling JW, Cofield RH. Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg 2012; 21: 1039-44.
- Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop 2009; 80: 83-91.
- Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am 2004; 86-A: 575-80.
- Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg 2011; 20: 123-30.
- Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg 2004; 13: 604-13.
- Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg 2002; 11: 587-94.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al.. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009; 374: 1105-12.
- Polk A, Rasmussen JV, Brorson S, Olsen BS. Reliability of patient-reported functional outcome in a joint replacement registry. Acta Orthop 2013; 84(1): 12-7.