Abstract
Background and purpose — NSAIDs are commonly used in the clinic, and there is a general perception that this does not influence healing in common types of human fractures. Still, NSAIDs impair fracture healing dramatically in animal models. These models mainly pertain to fractures of cortical bone in shafts, whereas patients more often have corticocancellous fractures in metaphyses. We therefore tested the hypothesis that the effect of an NSAID is different in shaft healing and metaphyseal healing.
Methods — 26 mice were given an osteotomy of their left femur with an intramedullary nail. 13 received injections of indomethacin, 1 mg/kg twice daily. After 17 days of healing, the femurs were analyzed with 3-point bending and microCT. 24 other mice had holes drilled in both proximal tibias, to mimic a stable metaphyseal injury. A screw was inserted in the right tibial hole only. After 7 days of indomethacin injections or control injections, screw fixation was measured with mechanical pull-out testing and the side without a screw was analyzed with microCT.
Results — In the shaft model, indomethacin led to a 35% decrease in force at failure (95% CI: 14–54). Callus size was reduced to a similar degree, as seen by microCT. Metaphyseal healing was less affected by indomethacin, as no effect on pull-out force could be seen (95% CI: –27 to 17) and there was only a small drop in new bone volume inside the drill hole. The difference in the relative effect of indomethacin between the 2 models was statistically significant (p = 0.006).
Interpretation — Indomethacin had a minimal effect on stable metaphyseal fractures, but greatly impaired healing of unstable shaft fractures. This could explain some of the differences found between animal models and clinical experience.
Should NSAIDs be used in fracture patients? Despite the fact that NSAIDs are one of the most often used drugs, their effects on bone healing are debated. There is a peculiar discrepancy between animal experiments—which show clear negative effects—and clinical experience, where healing appears to be less influenced. We propose that this discrepancy might partly be explained by differences between 2 types of fractures. Animal studies that have shown a negative effect of clinical use of NSAIDs on fracture healing were performed on the cortical bone of shafts. The clinical studies that have shown impaired fracture healing also pertain to shafts (CitationGiannoudis et al. 2000, CitationBurd et al. 2003). In contrast, most clinical fractures occur in the trabecular bone of metaphyseal regions.
Our idea that NSAIDs may have different roles in shaft healing and metaphyseal healing came from 2 observations. Firstly, we had noted that the histology of fractures of the distal radius indicated that healing was initiated by cells in the midst of the marrow (CitationAspenberg and Sandberg 2013). This is different from the standard description of shaft fracture healing, where cells are thought to be mainly derived from periosteum, the circulation, and surrounding tissue. Secondly, we noted that the anti-inflammatory TNF-α inhibitor etanercept did not show any effects in a metaphyseal injury model (CitationSandberg et al. 2012), despite the fact that previous studies had shown increased BMP-2-induced ectopic bone formation in mice (CitationEguchi et al. 2010).
We therefore hypothesized that fracture healing in the shaft and in the metaphysis would show a different response to NSAID treatment, with the NSAID being less detrimental in the metaphysis. We used 2 models, 1 for each tissue type, and compared the effect of the NSAID indomethacin in these.
Material and methods
Characterization of the screw model
We first performed a pilot study to characterize the screw pull-out model in mice (it had already been extensively validated in rats). 30 male C57Bl/6 mice, 10 weeks old, were anesthetized with isoflurane, and a 5-mm incision was made in the anterio-medial side of the right tibia, below the knee. A 0.4-mm drill hole was made approximately 0.6 mm below the epiphysis, and a screw was inserted. The screw was custom-made from Ti6A14V grade 2, thread size M 0.7 with the length of the threaded part being 0.9 mm (Rydahl Precision Components, Karlstad, Sweden). The screw head was designed to allow fitting to a pull-out device. After suturing the skin, we randomized the mice into 3 groups and they were killed on day 1, 7, or 14 after surgery. The screw pull-out force was then measured as described below.
Metaphyseal healing model
For the metaphyseal healing study, 24 male C57Bl/6 mice, also 10 weeks old, were given bilateral drill holes to the proximal tibias. The right tibia had a titanium screw inserted as described above. In the left tibia, a slightly larger cannula (0.6 mm) was used to drill the hole, which was left without any implant. After surgery, the mice were randomized to a group receiving subcutaneous injections of indomethacin, 1 mg/kg twice daily (2 mg/kg per day), or to a control group receiving equivalent volumes of phosphate-buffered saline. The first injection was given immediately after surgery. A pilot experiment without drug treatment showed a dramatic increase in pull-out force from day 1 to day 7 after surgery, but no increase from day 7 to day 14. Based on this, an exposure time of 7 days was chosen, to study the most sensitive time period. After 7 days of injections, the mice were killed and the tibias harvested. The tibias without screws were fixed overnight in 4% formalin, followed by 24 h in saline, after which they were analyzed with microCT. The tibias with a screw were tested by mechanical pull-out testing immediately after harvest, as described below.
Shaft-healing model
For the shaft-fracture experiment, we used 26 male C57Bl/6 mice that were 10 weeks old. A 10-mm incision was made in the skin laterally on the left distal femur, the patella was luxated medially, and a 0.4-mm cannula was inserted as a marrow nail into the femur through the condyles. The midfemur was then exposed and osteotomized transversely in the midshaft, with a custom-designed pair of tongs. After placing 1 suture to hold the patella in place, the skin was sutured. The animals were then randomized to 2 treatment groups with the same treatment regimens as in the metaphyseal fracture experiment, lasting until the animals were killed after 17 days. This time point was chosen after pilot experiments had shown that too many animals only had soft tissue bridging at 14 days, making mechanical test results difficult to interpret. The femurs were harvested and frozen for later analysis by microCT, followed immediately by 3-point bending tests.
All surgery, in both models, was performed under sterile conditions. The mice were anesthesized with isoflurane and the skin of the operated legs was shaved and soaked twice in chlorhexidine. Engemycin was used as antibiotic and temgesic was given to all animals for 36 h after surgery. They were given free access to food and water and were housed 2–4 in each cage, with 12 h of light and 12 h of darkness. The animals were killed with CO2 after sedation with isoflurane.
Mechanical testing
3-point bending was done using a computerized materials testing machine (100R; DDL Inc., Eden Prairie, MN). The femurs were mounted so that both metaphyses rested on a stage (distance between supporting points: 6 mm), with the ventral side pointing up. A cross-head was pointed straight down on the center of the callus, and lowered with a speed of 0.05 mm/s. The force at failure and stiffness were recorded. Stiffness was calculated as the slope of the linear portion of the load displacement curve. The bone was kept moist throughout the procedure.
For the pull-out testing of the metaphyseal screws, the tibias were excised and a holder was attached to the screw head and to the cross-head. The tibia was also attached to the machine via a suture thread around the bone, both proximal and distal to the screw. The cross-head speed was 0.01 mm/s. Force at failure was recorded.
MicroCT
All scans were performed with a Skyscan microCT system (1174; Bruker, Kontich, Belgium) using a 0.25-mm Al filter, 50 kV, in 180 degrees. Corrections were done for beam hardening and ring artifacts.
For the femurs, a pixel size of 12 µm, a rotation step of 0.4°, and frame averaging of 2 was used. The volume of interest (VOI) was defined, comprising all new-formed bone and cartilage in the central 2 mm of the callus (). This VOI was then analyzed for bone volume (BV), tissue volume (TV), and bone mineral density (BMD).
Figure 1. MicroCT images showing volume of interest (VOI) of diaphyseal and metaphyseal fractures. Median specimens from each control group, in terms of bone volume
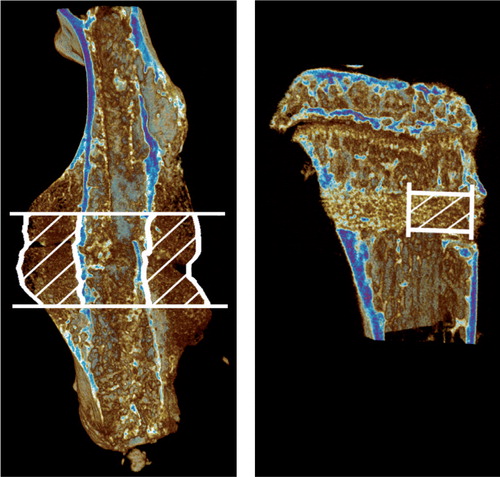
For the tibias, a pixel size of 8 µm, a rotation step of 0.5°, and frame averaging of 3 was used. The VOI was defined as a cylinder with a diameter of 0.5 mm, starting from the endosteum and following the drill hole for 1 mm (). This VOI was analyzed for BV, BMD, trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and trabecular number (Tb.N).
Statistics
The primary effect variable for both fracture experiments was force at failure, and the primary hypothesis was that indomethacin affected the force in the 2 types of fracture differently, as seen by the interaction term in a 2-way analysis of variance (ANOVA) with fracture type and drug treatment as fixed factors. We ln-transformed the data in order to study relative effects. The assumption of normal distribution was confirmed by visual inspection of the data. 95% confidence intervals (CI) for the treatment effect in the 2 models were calculated with Student’s t-test. Morphometric data were regarded as descriptive. We used the SPSS software version 20.
Ethics
All procedures were approved by the regional ethics committee (2012 85-12).
Results
Mechanical testing
Indomethacin reduced the force at failure for the shaft fractures by 34% (CI: 14 to 54, ). In contrast, there was no significant effect of indomethacin on the pull-out force for the metaphyseal screws: there was a mean increase of 5% (CI: –17 to 27). Stiffness was similarly affected (). A 2-way ANOVA with fracture type and drug treatment as fixed factors and ln-transformed force at failure as dependent variable showed significant interaction between the 2 factors (p = 0.006). This meant that indomethacin had different relative effects depending on the fracture type.
Figure 2. Force at failure with and without indomethacin in a shaft fracture model and a metaphyseal fracture model.
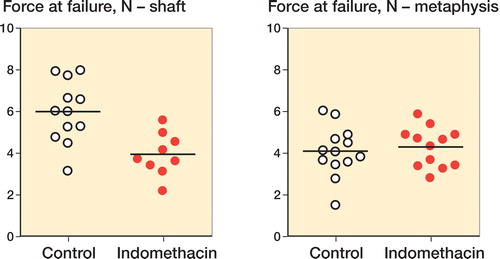
Table 1. Mechanical evaluation. Values are mean (SD)
MicroCT
Indomethacin led to a decrease in the callus bone volume of the shaft fractures, by 33% (CI: 16–50) (). Tissue volume was similarly affected (). The amount of new-formed bone in the metaphyseal drill hole was also reduced by indomethacin, although only by 12% (CI: 5–20). Qualitatively, we saw a zone of increased trabecular density where the drill hole had been. However, in 3 specimens from the indomethacin group, there were small areas within that volume where bone formation did not appear to have occurred. These samples explain much of the lower mean value compared to the control group.
Figure 3. Amount of new-formed bone in a shaft fracture model and a metaphyseal fracture model. Indomethacin led to a significant decrease in new-formed bone (by 33% in the shaft and by 12% in the metaphysis).
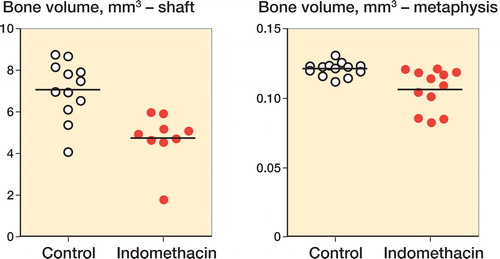
Table 2. Data from microCT. Values are mean (SD)
Samples lost
3 samples were lost in the indomethacin shaft fracture group, 2 because the stabilizing pin protruded too far distally and 1 because the femur broke at harvest. 1 sample was lost from the indomethacin metaphyseal screw group because the mouse died for unknown reasons.
Discussion
We could see a strong negative effect of the NSAID indomethacin on shaft healing after trauma, but less or no effect on metaphyseal healing. However, as the comparison was biased by a difference in stability between the 2 models, we can only be confident that the effect of indomethacin is dependent on the type of fracture.
Anti-inflammatory drugs are routinely given to fracture patients, and in general experience no strong association between such treatment and non-union has been perceived. Experimentally, however, there is a consistent strong association between dampened inflammation and impaired fracture healing (CitationKeller et al. 1987, CitationMountziaris et al. 2011). Apart from numerous animal studies, some clinical studies have also concluded that NSAIDs increase the risk of non-union in shaft fractures (CitationGiannoudis et al. 2000, CitationBurd et al. 2003, CitationHernandez et al. 2012).
The discrepancy between experimental findings and clinical experience could be explained by our results. While experimental results are almost exclusively based on shaft fractures, most clinical fractures occur in metaphyseal bone. In a meta-analysis, the effects of NSAIDs in shaft fractures showed a stronger association to non-union than did studies on spine fusion. The authors noted that studies on shafts were of poorer quality than the others, and also that the inherent differences in non-union rates of diaphyseal fractures and spine fusions are likely to indicate differences in the biology of fracture healing between the 2 locations (CitationDodwell et al. 2010).
There have only been 2 clinical trials on the effects of NSAIDs on fracture healing with an element of randomization. CitationBurd et al. (2003) performed a re-analysis of a randomized trial comparing indomethacin with radiation for use as a prophylactic against ectopic ossification after acetabular fracture. Many of the patients also had long bone fractures. These were not radiated. Non-union of the long bone fractures was more common in the indomethacin group than in the radiation or no treatment groups, with an odds ratio of 4.8 (95% CI: 1.2–19, as calculated from values in the paper). The other trial compared the early migration of knee prostheses with and without celecoxib. This was measured by radiosterometry, and in spite of a small confidence interval, no effect could be seen (CitationMeunier et al. 2009). These 2 trials concur with our animal findings.
Mice and rats are common models in skeletal research. Yet the differences to humans are many, and it is difficult to generalize from rodents to humans. If our results could be extrapolated, they would mean that patients with stable metaphyseal fractures can safely be advised to continue using NSAIDs, whereas alternative pain relief should be considered for shaft fracture patients.
We believe that one important role of inflammation in fracture healing could be recruitment of progenitor or stem cells to the fracture site. The relative amount of these cells, their proliferative ability, and their commitment to an osteogenic fate, are known—in mice—to be lower in the shaft than in the metaphysis, and also to be dependent on their location in central marrow or marrow close to a bone surface, particularly in the metaphyseal region (CitationSiclari et al. 2013). It is therefore likely that recruitment of cells from distant sources is of greater importance in a shaft fracture than it is in a metaphyseal fracture. This might explain why a drug that reduces inflammation impairs healing in the shaft more than in the metaphysis. In humans, the marrow in shaft bone is fatty—probably with an even greater scarcity of competent cells.
There is a difference in the extent of trauma in the shaft and metaphyseal models we used. The volume of the new-formed tissue in the shaft model is 2 orders of magnitude greater than in the metaphyseal model. It has been shown previously in vivo that the extent of the trauma influences the effect of indomethacin on remodeling of bone close to an injury (CitationKeller et al. 1990). A larger volume of new-formed tissue could mean that more stem cells would need to be recruited.
One limitation of this study was the choice of 2 fracture models with different mechanical stability. While the metaphyseal screw implant is stable, the diaphyseal osteotomy is not. In order to be able to say whether the difference we have seen is due to differences in location or stability, the role of stability in the response to indomethacin must be investigated further.
The mice with shaft fractures were killed 17 days after surgery, while the metaphyseal fracture mice were killed after 7 days. This difference was chosen because shaft fractures and metaphyseal fractures do not heal at the same speed. Both time points chosen included the important early inflammatory phase and these were the earliest time points at which we could see mechanical stability and measure bone strength adequately.
It is not obvious that fixation of an implanted screw can be used as a proxy for metaphyseal fracture healing. However, the pull-out force relates primarily to the amount and mechanical quality of the bone that has formed in the immediate vicinity of the screw after the insertion trauma. These quantities are closely related to what is considered to be successful fracture repair.
Pull-out force of an implanted screw was chosen as the mechanical test model in the metaphysis whereas 3-point bending was used in the diaphysis. While it would have been good to use the same test in both sites, the differences in size and shape of the bone made this difficult. In our opinion, the models used both represent the best-known methods of measuring mechanical quality—in a clinically relevant sense—of fracture healing in shaft and metaphysis.
We conclude that indomethacin had different effects on unstable diaphyseal and stable metaphyseal fracture healing, as measured by mechanical testing and microCT. Our data suggest that patients with unstable shaft fractures can be expected to have an increased risk of non-union if treated with an NSAID, while patients with stable metaphyseal fractures would be comparatively less affected, if at all. Our results also suggest that, apart from differences in stability, care should be taken when regarding results of fracture experiments performed on one type of bone tissue as being relevant for fracture healing in general.
PA and OS planned the study. OS conducted the experiments. PA and OS did the data analysis. OS initially wrote the manuscript.
No competing interests declared.
The study was funded by the Swedish Research Council, Linköping University, Östergötland County Council, and the King Gustaf V and Queen Victoria Freemason Foundation.
- Aspenberg P, Sandberg O. Distal radial fractures heal by direct woven bone formation. Acta Orthop 2013; 84 (3): 297-300.
- Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br 2003; 85 (5): 700-5.
- Dodwell ER, Latorre JG, Parisini E, Zwettler E, Chandra D, Mulpuri K, et al. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int 2010; 87 (3): 193-202.
- . Eguchi Y, Wakitani S, Imai Y, Naka Y, Hashimoto Y, Nakamura H, et al. Antitumor necrotic factor agent promotes BMP-2-induced ectopic bone formation. J Bone Miner Metab 2010; 28 (2): 157-64.
- Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br 2000; 82 (5): 655-8.
- Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop 2012; 83 (6): 653-60.
- Keller J, Bunger C, Andreassen TT, Bak B, Lucht U. Bone repair inhibited by indomethacin. Effects on bone metabolism and strength of rabbit osteotomies. Acta Orthop Scand 1987; 58 (4): 379-83.
- Keller J, Kjaersgaard-Andersen P, Bayer-Kristensen I, Melsen F. Indomethacin and bone trauma. Effects on remodeling of rabbit bone. Acta Orthop Scand 1990; 61 (1): 66-9.
- Meunier A, Aspenberg P, Good L. Celecoxib does not appear to affect prosthesis fixation in total knee replacement: A randomized study using radiostereometry in 50 patients. Acta Orthop 2009; 80 (1): 46-50.
- Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev 2011; 17 (6): 393-402.
- Sandberg O, Eliasson P, Andersson T, Agholme F, Aspenberg P. Etanercept does not impair healing in rat models of tendon or metaphyseal bone injury. Acta Orthop 2012; 83 (3): 305-10.
- Siclari VA, Zhu J, Akiyama K, Liu F, Zhang X, Chandra A, et al. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone 2013; 53 (2): 575-86

