Abstract
Background and purpose — Fractures of the scaphoid are often not detected on initial plain radiographs. Conventional management of clinically suspected scaphoid fractures is cast immobilization for 2 weeks and then reassessment. Early MRI is a diagnostic alternative. We compared the cost and usefulness of the early MRI diagnostic strategy with that of conventional management.
Patients and methods — This prospective pseudo-randomized study included patients between 18 and 49 years of age who attended Bergen Accident and Emergency Department, Bergen, Norway during 1 year in 2009–2010, after sustaining an acute wrist trauma in the previous week and with a clinically suspected scaphoid fracture. 61 patients were investigated with acute MRI, while 63 patients received standard treatment as a control group. We used cost-minimization analysis to estimate the cost of the 2 patient groups.
Results — Concerning cost, there were no statistically significant differences in the total direct medical costs or in indirect costs between the groups. Concerning usefulness, patients in the MRI group without a fracture (n = 35) used a cast for fewer days (mean 1 day) than patients in the control group with no fractures (n = 52) (mean 14 days; p < 0.001). They had less than half the number of days on sick leave than patients in the control group (mean 7 days vs. 15 days; p = 0.002).
Interpretation — In a Norwegian setting, an early MRI was of value in patients with clinically suspected scaphoid fracture and normal plain radiographs.
Initial plain radiographs have a sensitivity of 65–93% for scaphoid fractures. (Tiel-van CitationBuul et al. 1993, CitationHunter et al. 1997, CitationDorsay et al. 2001). Conventional management of occult scaphoid fractures involves a temporary wrist cast, and a review in approximately 2 weeks for repeated clinical and radiographic assessment. Previous studies have shown that only 20% of patients treated with cast immobilization for clinically suspected scaphoid fractures—who initially have negative radiographs—are finally diagnosed with scaphoid fractures. Thus, 4 in 5 patients are immobilized unnecessarily (CitationHunter et al. 1997, CitationDorsay et al. 2001, CitationBrydie et al. 2003, CitationPillai and Jain. 2005, CitationJenkins et al. 2008).
Acute MRI of wrist and hand injuries has become an increasingly better tool for diagnosing or ruling out injuries that are otherwise often missed or over-treated (CitationHunter et al. 1997, CitationBrydie et al. 2003, CitationKhalid et al. 2010, CitationMcCullough et al. 2011, CitationBergh et al. 2012, CitationJorgsholm et al. 2013). Previous studies have shown that early MRI gives a reduction in immobilization time in patients with suspected scaphoid fractures, and facilitates a faster return to work (CitationKumar et al. 2005, CitationHansen et al. 2009, CitationPatel et al. 2013). There has been disagreement in previous studies about whether early MRI in clinically suspected scaphoid fractures is cost-saving compared with conventional management (CitationGooding et al. 2004, CitationBrooks et al. 2005, CitationNikken et al. 2005, CitationJenkins et al. 2008, CitationHansen et al. 2009, De CitationZwart et al. 2012, CitationPatel et al. 2013). Furthermore, direct and indirect costs vary between countries, different healthcare systems, and different institutional settings. National studies are therefore important in order to find out the relevance for a particular country.
We estimated and compared the direct, indirect, and overall costs of a diagnostic strategy involving early MRI and of the traditional conservative management in the treatment of clinically suspected scaphoid fractures with normal plain radiographs in a Norwegian setting. We also compared the benefits and disadvantages of the 2 diagnostic strategies for the patients.
Patients and methods
This prospective study was conducted at Bergen Accident and Emergency (A&E) Department, Norway from November 5, 2009 to November 4, 2010. Bergen A&E is an outpatient clinic that treats almost all minor injuries in Bergen, which is the second largest city in Norway. Annually, 100,000 patients attend the A&E, 40,000 of whom are injured.
Patients aged 18–49 years who attended the A&E within a week after sustaining an acute wrist trauma requiring radiographic examination were assessed for eligibility in the study. Only clinically suspected scaphoid fractures were included.
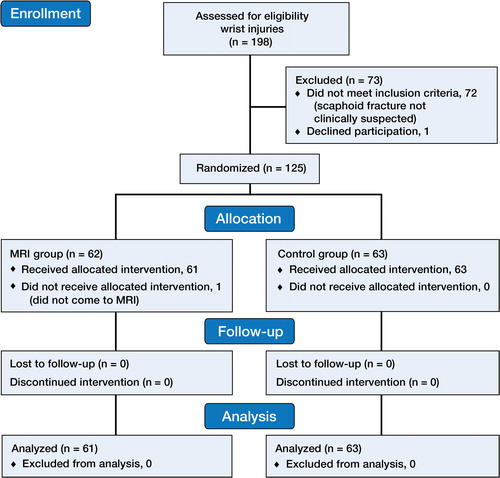
The exclusion criteria were radiographically identified fractures or dislocations, as well as patients with previous wrist fractures, rheumatoid arthritis, and contraindications for MRI such as pregnancy, metal implants, and claustrophobia ().
Patients were examined with 3 commonly used clinical tests for suspected scaphoid fractures. We constructed a clinical scaphoid score (CSS) by grading and adding the results of these tests: tenderness in the anatomical snuffbox with the wrist in ulnar deviation, tenderness over the scaphoid tubercle with the wrist slightly extended, and pain upon longitudinal compression of the thumb (CitationBergh et al. 2014). The CSS is therefore a sum of the findings from these examinations. Both the clinical examination and the interpretation of the X-rays were done by the doctors on call at the A&E, as part of their daily clinical practice.
Selection and randomization
This study was part of a larger project looking into different aspects of wrist injuries (n = 198) (CitationBergh et al. 2012, 2014). This leg of the study included 125 patients with clinical suspicion of a scaphoid fracture with a CSS of ≥ 3 and with normal plain radiographs. At the design stage of the study, CSS ≥ 3 was hypothesized to be an appropriate level for suspicion of a scaphoid fracture. Every other day for the entire study period, patients were allocated to 2 different groups based on diagnostic strategy—the early MRI group or the control group (). Thus, 61 patients received intervention with acute MRI and treatment according to the MRI results, and 63 patients were controls who received the standard treatment ().
Table 1. Demographic data and baseline characteristics of the MRI group and the control group
Follow-up of patients
The patients in the MRI group had MRI done as soon as possible (within mean 1 (0–7) day) after attending the A&E, and they had a follow-up consultation when the results of the MRI were available. They were treated according to the MRI results. The patients in the control group received conventional treatment with a below-elbow scaphoid cast for 2 weeks, after which they had a follow-up consultation with a repeat clinical examination, investigation, and treatment as with standard practice. Half of the patients in the control group had scaphoid fracture ruled out by clinical examination, with or without repeat radiographs after 2 weeks. In the remaining half of the control group, the doctor still suspected a scaphoid fracture at the 2-week assessment and MRI was requested ().
Plain radiography and MRI
We used a standard protocol for radiography with extended wrist views and a series of 4 images including the distal part of the radius and the proximal part of the metacarpals (CitationBergh et al. 2012). The MRI protocol included 4 different sequences and the MRI scans were performed in a 1.5 Tesla whole-body scanner with a wrist coil. An experienced musculoskeletal radiologist interpreted all the MRI results (CitationBergh et al. 2012). We had a preset treatment protocol to address possible pathological MRI findings; see CitationBergh et al. (2012).
Cost analysis
To our knowledge, no previous studies have demonstrated differences in patient outcome between these 2 diagnostic strategies. We assumed that there would be no difference in health outcome between the strategies, and therefore used cost-minimization analysis (CMA) (CitationRobinson 1993, Drummond 2005). The cost perspective in this Norwegian study corresponds to the expenses for the National Social Security, including healthcare provider costs and sick leave benefits combined with the expenses for employers when employees are on sick leave.
We chose a time frame that would be sufficient to capture all major health and economic consequences, including both intended effects and unintended side effects. All the records at the A&E Department were reviewed 2 years after the inclusion of patients was completed. We also obtained sick leave data from the Norwegian Labor and Welfare Service (Ny arbeids- og velferdsforvaltning, NAV) from the start of the study until 6 months after inclusion was completed.
The direct and indirect costs were calculated for each patient in each group. Cost analysis was performed by comparing the cost of management of patients in the groups. We also calculated the costs for subgroups of patients within the groups: patients with scaphoid fractures, patients with all types of wrist fractures, and patients without fractures.
Direct costs
Direct costs included costs for consultations, radiographs, MRIs, plasters, and bandages (Table 3, see supplementary data). Information about each direct cost in 2010 (in Norwegian kroner (NOK)) was obtained from the Norwegian Health Economics Administration (HELFO; Helseøkonomiforvaltningen) and from the Norwegian Directorate of Health (Helsedirektoratet). Patients’ co-payment was a part of the treatment-activities costs.
Indirect costs
Sick leave including self-certification was used as a measure of indirect costs. The employer covers 100% of an employee’s income from day 1 to 16 of the sick-leave period. The Norwegian Labor and Welfare Service (NAV) gives compensation for all loss of income up to 6 × G for the rest of the first year, where G is the Norwegian public pension base rate (on May 1, 2010, it was 9,600 euros).
We recorded whether patients were employees or self-employed, and their type of work. The patient’s type of work was divided into 3 categories: blue-collar, white-collar, and other.
We calculated the number of days of sick leave for each patient using data from our own records and from the NAV. The NAV has all the data on the sick-leave period from day 17.
We also received information from medical certificates issued by general practitioners or other medical specialists. If the patient had a partial or graded sick leave over a certain period of time, we converted this to the equivalent number of whole days on sick leave. We assumed that employees worked full-time, if nothing else was stated in the medical records. We chose not to differentiate the wages according to occupation, but used an average salary for all employees in order to be able to generalize from a relatively small group of patients. The cost analysis would otherwise be very sensitive to those few patients with either a very high salary or a very low salary.
According to Statistics Norway (SSB), we calculated the average cost of sick leave per day to be 242 euros in 2010, which corresponds to an average daily personal income including payroll tax of 14%.
Statistics
Results for continuous variables are given as mean values with 95% confidence intervals. For continuous variables, t-test was used to compare mean values. If Levene’s test showed significant differences in the variance estimates, Satherwaite’s approach was used. For the cross-tabulations, chi-square tests were used. Any p-values less than 0.05 were considered statistically significant. IBM SPSS software version 21 was used for the statistical analyses.
Ethics
The study was approved by the Norwegian Ethics Committee for Medical Research (2009/869). The patients included gave their informed consent for participation in the study.
Results
The baseline characteristics of the MRI group and the control group showed balanced groups regarding prognostic factors ().
Fractures
We diagnosed 7 scaphoid fractures in the MRI group (n = 61) and 4 in the control group (n = 63). The number of other fractures was also higher in the MRI group than in the control group (22 as opposed to 8) ().
Table 2. The different fracture locations identified. 3 patients had more than 1 fracture. None of the patients with scaphoid fractures had additional fractures
Healthcare provider resources
The numbers of consultations per patient were almost identical in the 2 groups. We found statistically significant differences between the numbers of radiographs, the numbers of MRIs, and the numbers of casts and elastic bandages used in the 2 groups (Table 3, see supplementary data).
Usefulness
When patients were diagnosed as having either scaphoid fractures or other fractures, the number of days using a cast was similar (). The total number of days using a cast was fewer for patients in the MRI group (mean 12 days) than for those in the control group (mean 17 days) (mean difference 5 (−1 to 9) days) (p = 0.05). This difference was even more obvious for the mean number of days using a cast for patients without fractures in the MRI group (1 day as opposed to 14 days in the control group; mean difference 13 (11–15) days; p < 0.001). Mean length of time for a definite diagnosis was 1 (0–7) day for patients in the MRI group and it was 18 (13–68) days for the patients in the control group who received a MRI as part of the reassessment after 2 weeks or more. About half of the patients in the control group (n = 32) had few clinical symptoms at the reassessment after 2 weeks, and they were therefore discharged without MRI. 2 patients in the MRI group (but none in the control group) were referred to an orthopedic specialist, and neither of them received operative treatment.
Table 4. Usefulness; days with plaster treatment in subgroups of patients
The number of days of absence from work was not significantly lower in the MRI group than in the control group as a whole (Table 5, see supplementary data). However, for patients with no fractures, there were significantly fewer days of sick leave in the MRI group than in the control group (a mean of 7 days as opposed to a mean of 15 days; mean difference 8 (3–13) days; p = 0.002). All 11 patients who were diagnosed with scaphoid fractures in the study were treated with plaster. 8 of these patients were employed. 5 received a sick-leave certificate and 3 did not.
Blue-collar patients had more sick leave days (mean 17 days) than white-collar patients (mean 9 days) (mean difference 8 (0–15) days; p = 0.04).
Direct costs
We did not find any statistically significant difference in the sum of all direct costs of medical activities in the 2 groups: 30,160 euros in the MRI group and 27,997 euros in the control group (Table 3, see supplementary data).
In order to detect the 7 scaphoid fractures in the MRI group and the 4 scaphoid fractures in the control group, 61 MRI group patients and 63 control patients were investigated. Thus, we calculated that the average direct cost of detecting and treating a patient with an occult scaphoid fracture was 4,308 euros in the MRI group and 6,999 euros in the control group. The average direct medical cost of detecting and treating a patient with any occult wrist fracture was 1,160 euros in the MRI group (n = 26) and more than the double—2,545 euros—in the control group (n = 11).
Indirect costs
Three-quarters of the patients in both groups were wage earners (). Only patients in paid work and on sick leave generated indirect costs. We found similar indirect costs for the 2 groups (147,404 euros for the MRI group and 186,550 euros for the control group) (Table 5, see supplementary data). However, for patients in paid work with no fracture, the indirect cost in the MRI group was less than half of that in the control group (Table 5, see supplementary data).
Overall costs
Overall cost is the sum of direct and indirect costs. The overall costs were 177,564 euros in the MRI group and 214,548 euros in the control group. Neither the difference in direct costs nor the difference in indirect costs was statistically significantly different between the MRI group and the control group. However, for the MRI group the direct costs were slightly higher while the indirect costs were slightly lower than in the control group. The difference in total costs between the MRI group and the control group was therefore marginal. The direct costs accounted for 15% of the overall cost and the indirect costs accounted for 85% of the overall cost.
Discussion
Usefulness, strengths and limitations
In this prospective pseudo-randomized controlled trial of adults with clinically suspected scaphoid fractures, patients who received early MRI used a cast for fewer days when any type of wrist fracture was ruled out than the control group (1 day as opposed to 14 days). This difference is in agreement with findings in earlier studies (CitationBrydie et al. 2003, CitationBrooks et al. 2005, CitationKumar et al. 2005, CitationHansen et al. 2009). Mean length of time for a definite diagnosis was 1 day in patients in the MRI group and 18 days for the patients in the control group who underwent MRI. About half of the patients in the control group (n = 32) had few clinical symptoms at the reassessment after 2 weeks, and were therefore discharged without MRI. Other studies have shown a similar reduction in the time until final diagnosis by using early MRI (CitationBrooks et al. 2005, CitationRaby 2001).
It could be considered a weakness of the present work that the examination procedure took the form of a pseudo-randomized controlled study rather than a blind, randomized controlled study. The patients included followed one of 2 different diagnostic strategies depending on the day of presentation. This procedure was found to be the most practical to follow in our busy accident and emergency unit. The doctor in charge knew which diagnostic strategy group the patient was allocated to (early MRI or control), but could not move the patient to the other group. We therefore consider the risk of selection bias to be small.
Another weakness of our study is that we do not know with certainty the number of occult fractures or soft tissue injuries that were present in the control group. About half of the patients in this group (n = 32) had few clinical symptoms at the reassessment after 2 weeks. MRI was therefore not requested, but theoretically, an occult wrist fracture could have become partially asymptomatic after 2 weeks of immobilization in a plaster. We checked all relevant medical records at our accident and emergency unit up to 2 years after the inclusion of patients was complete. Only 1 patient returned with signs of continued pain in the wrist. A volar wrist ganglion was diagnosed by MRI and probably caused his symptoms. This indicates that there was identical clinical outcome in these 2 groups.
The strength of our prospective study was that all patients were examined in a standardized manner with the 3 most commonly used clinical tests to identify scaphoid fractures.
Patients in the MRI group without any fracture had less than half the number of days of sick leave than patients in the control group with no fractures. Other studies have also found a reduction in time absent from work when using subacute MRI (CitationHansen et al. 2009), but this has been contradicted by others (CitationBrooks et al. 2005). Blue-collar patients had more days of sick leave than white-collar patients, as has been found in other studies (CitationVinnars et al. 2007). Of the 11 patients in our study who were diagnosed with scaphoid fractures, 5 were given a sick leave certificate and 3 were not. Other studies have also found that some patients go to work in spite of their immobilized and cast scaphoid fracture (van der Molen et al. 1999, CitationFusetti 2003, CitationVinnars et al. 2007).
Costs
Our choice of using cost-minimization analysis required that the clinical endpoint after the 2 different diagnostic strategies should be identical. Our clinical endpoint was that the fracture was healed and the patient had returned to work. An indication that this assumption is correct was that none of the patients in the 2 groups were on sick leave for longer than 2 months after their acute wrist injury. None of the patients returned to our accident and emergency unit with a late-discovered scaphoid fracture during the 2 years that followed. The principle of this cost analysis could be transferred to other countries. However, direct costs, sick-leave rules, and possible payment of expenses from the insurance companies will vary from country to country. The effect of different direct activity costs can be estimated. In our model, it is possible to use a one-way sensitive analysis of one specific parameter (e.g. MRI) to access the impact of the output of the model.
Direct costs
Despite the relatively high cost of MRI, our overall estimation of direct costs in all patients was similar in the 2 groups. This was contrary to what we had assumed. Conventional treatment was only marginally cost-saving regarding direct costs, which is in agreement with other studies (CitationGooding et al. 2004, CitationBrooks et al. 2005) yet contrasts with another study that found that the mean cost of management was marginally lower with early MRI management (CitationPatel et al. 2013).
However, when we compared the average direct costs for detection and treatment of one occult scaphoid fracture, the cost was less in the MRI group (4,308 euros) than in the control group (6,999 euros). Moreover, the average direct medical cost per occult fracture was less than half in the MRI group than in the control group when the analysis was extended to diagnosing any wrist fracture, which is supported by other authors (CitationGooding et al. 2004). This difference in costs for identification of occult fractures was probably caused by the fact that early MRI identifies more fractures and that some fractures may have been missed in the control group. More importantly, we do not know the significance of these undiscovered fractures, but in our study none of the patients returned during the subsequent 2-year period with signs of avascular necrosis, non-union, or other complications. This calculation is further complicated by the fact that a higher proportion of patients with known fractures in the MRI group would result in higher costs due to the fracture treatment. The question is really whether this cost is “unnecessary”, as the patient actually receives a complete diagnosis and treatment as opposed to follow-up consultations, further reviews, and possibly subsequent investigations. In recent years, the cost of MRI has decreased in Norway. This has resulted in more frequent use of MRI at an early stage. The next development in the years to come will probably be a small, dedicated extremity scanner suitable for wrist examination, preferably located at the accident and emergency unit, which would be even cheaper and more time-saving than a conventional whole-body scanner with a wrist coil as used in the present study (CitationDorsay et al. 2001, CitationKhalid et al. 2010).
Indirect costs
No statistically significant difference in indirect costs was found between the 2 groups. Patients in the MRI group who were employed, and who did not have any type of wrist fracture, had less than half the indirect costs of patients in the control group. This reflects that with early exclusion of pathology with MRI, the patient can be discharged and return to work. We found that the indirect costs for employees who were on sick leave represented 85% of the overall cost. This is in accordance with another study where the indirect cost accounted for 90% of the overall cost (CitationFusetti 2003).
Conclusion
By means of cost-minimization analysis, we found only a marginal difference in overall costs between the 2 groups of patients: one with early MRI for suspected occult scaphoid fracture and the other group following a conventional diagnostic strategy. However, from the point of view of usefulness, we found that patients receiving early MRI—which ruled out wrist fracture—had substantially fewer days in a cast unnecessarily and spent less than half the number of days on sick leave.
Supplementary data
Tables 3 and 5 are available at Acta’s website (www.actaorthop.org), identification number 7145.
Supplementary Material
Download PDF (33.6 KB)Conception and design of the study: THB, KS, TL, LAS, SVB, LL, SAL, and CB. Acquisition of data: THB, KS, LAS, SVB, and CB. Analysis and interpretation of data: THB, KS, TL, LAS, SVB, LL, SAL, and CB. Drafting of the article: THB. Critical revision for important intellectual content: KS, TL, LAS, SVB, LL, SAL, and CB. Final approval of the version to be submitted: THB, KS, TL, LAS, SVB, LL, SAL, and CB. Responsible for the overall content as guarantors: THB and CB.
We thank the staff of Bergen Accident and Emergency Department, including Frank van Betten and Arve Strandenes. We are grateful to our colleagues at Bergen Hand Surgery Center: Leiv Hove, Yngvar Krukhaug, Eivind Strandenes, and Rakel Gudmundsdottir. We also thank Mehdi Behzadi of the Department of Radiology, Stavanger University Hospital, Stavanger, Norway for interpretation of the MRIs. Research grants were received from the University of Bergen and the Norwegian Research Council.
No competing interests declared.
- Bergh TH , Lindau T , Bernardshaw SV , Behzadi M , Soldal LA , Steen K , Brudvik C . A new definition of wrist sprain necessary after findings in a prospective MRI study. Injury 2012; 43(10): 1732-42.
- Bergh TH , Lindau T , Soldal LA , Bernardshaw SV , Behzadi M , Steen K , Brudvik C . Clinical scaphoid score (CSS) to identify scaphoid fracture with MRI in patients with normal x-ray after a wrist trauma. Emerg Med J 2014; 31(8): 659-64.
- Brooks S , Cicuttini FM , Lim S , Taylor D , Stuckey SL , Wluka AE . Cost effectiveness of adding magnetic resonance imaging to the usual management of suspected scaphoid fractures. Br J Sports Med 2005; 39(2):7 5-9.
- Brydie A , Raby N . Early MRI in the management of clinical scaphoid fracture. Br J Radiol 2003; 76(905): 296-300.
- De Zwart AD , Beeres FJ , Ring D , Kingma LM , Coerkamp EG , Meylaerts SA , Rhemrev SJ . MRI as a reference standard for suspected scaphoid fractures. Br J Radiol 2012; 85(1016): 1098-101.
- Dorsay TA , Major NM , Helms CA . Cost-effectiveness of immediate MR imaging versus traditional follow-up for revealing radiographically occult scaphoid fractures. AJR Am J Roentgenol 2001; 177(6): 1257-63.
- Drummond M . Methods for the economic evaluation of health care programmes. 3rd ed. Oxford; New York: Oxford University Press, 2005.
- Fusetti C . Direct and indirect costs in the conservative management of undisplaced scaphoid fractures Eur J Orthop Surg Traumatol. 2003; 13: 241-4.
- Gooding A , Coates M , Rothwell A , Accident Compensation Corporation. Cost analysis of traditional follow-up protocol versus MRI for radiographically occult scaphoid fractures: a pilot study for the Accident Compensation Corporation. N Z Med J 2004; 117(1201): U1049.
- Hansen TB , Petersen RB , Barckman J , Uhre P , Larsen K . Cost-effectiveness ofMRI in managing suspected scaphoid fractures. J Hand Surg Eur Vol 2009; 34(5): 627-30.
- Hunter JC , Escobedo EM , Wilson AJ , Hanel DP , Zink-Brody GC , Mann FA . MR imaging of clinically suspected scaphoid fractures. AJR Am J Roentgenol 1997; 168(5): 1287-93.
- Jenkins PJ , Slade K , Huntley JS , Robinson CM . A comparative analysis of the accuracy, diagnostic uncertainty and cost of imaging modalities in suspected scaphoid fractures. Injury 2008; 39(7): 768-74.
- Jorgsholm P , Thomsen NO , Besjakov J , Abrahamsson SO , Bjorkman A . The benefit of magnetic resonance imaging for patients with posttraumatic radial wrist tenderness. J Hand Surg Am 2013; 38(1): 29-33.
- Khalid M , Jummani ZR , Kanagaraj K , Hussain A , Robinson D , Walker R . Role of MRI in the diagnosis of clinically suspected scaphoid fracture: analysis of 611 consecutive cases and literature review. Emerg Med J 2010; 27(4):2 66-9.
- Kumar S , O’Connor A , Despois M , Galloway H . Use of early magnetic resonance imaging in the diagnosis of occult scaphoid fractures: the CAST Study (Canberra Area Scaphoid Trial). N Z Med J 2005; 118(1209): U1296.
- McCullough NP , Smith FW , Cooper JG . Early MRI in the management of the clinical scaphoid fracture. Eur J Emerg Med 2011; 18(3): 133-6.
- Nikken JJ , Oei EH , Ginai AZ , Krestin GP , Verhaar JA , van Vugt AB , Hunink MG . Acute wrist trauma: value of a short dedicated extremity MR imaging examination in prediction of need for treatment. Radiology 2005; 234(1): 116-24.
- Patel NK , Davies N , Mirza Z , Watson M . Cost and clinical effectiveness of MRI in occult scaphoid fractures: a randomised controlled trial. Emerg Med J 2013; 30(3): 202-7.
- Pillai A , Jain M . Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med 2005; 12(2): 47-51.
- Raby N . Magnetic resonance imaging of suspected scaphoid fractures using a low field dedicated extremity MR system. Clin Radiol 2001; 56(4):3 16-20.
- Robinson R . Costs and cost-minimisation analysis. BMJ 1993; 307(6906): 726-8.
- Tiel-van Buul MM , van Beek EJ , Borm JJ , Gubler FM , Broekhuizen AH , van Royen EA . The value of radiographs and bone scintigraphy in suspected scaphoid fracture. A statistical analysis. J Hand Surg Br 1993; 18(3): 403-6.
- van der Molen AB , Groothoff JW , Visser GJ , Robinson PH , Eisma WH . Time off work due scaphoid fractures and other carpal injuries in The Netherlands in the period 1990 to 1993. J Hand Surg Br 1999; 24(2): 193-8.
- Vinnars B , Ekenstam FA , Gerdin B . Comparison of direct and indirect costs of internal fixation and cast treatment in acute scaphoid fractures: a randomized trial involving 52 patients. Acta Orthop 2007; 78(5): 672-9.