Abstract
Background and purpose — Local infiltration analgesia (LIA) is well established for effective postoperative pain relief in total knee arthroplasty (TKA). To prolong the effect of LIA, infusion pumps with local intraarticular analgesia can be used. We evaluated the effect of such an infusion pump for the first 48 h postoperatively regarding pain, knee function, length of stay (LOS) in hospital, and complications.
Patients and methods — 200 patients received peroperative LIA and a continuous intraarticular elastomeric infusion pump set at 2 mL/h. The patients were randomized either to ropivacaine (7.5 mg/mL) or to NaCl (9 mg/mL) in the pump. Visual analog scale (VAS) pain (0–100 mm), analgesic consumption, side effects of medicine, range of motion (ROM), leg-raising ability, LOS, and complications during the first 3 months were recorded.
Results — On the first postoperative day, the ropivacaine group had lower VAS pain (33 vs. 40 at 12 noon and 36 vs. 43 at 8 p.m.; p = 0.02 and 0.03, respectively), but after that all recorded variables were similar between the groups. During the first 3 months, the ropivacaine group had a greater number of superficial and deep surgical wound infections (11 patients vs. 2 patients, p = 0.02). There were no other statistically significant differences between the groups.
Interpretation — Continuous intraarticular analgesia (CIAA) with ropivacaine after TKA has no relevant clinical effect on VAS pain and does not affect LOS, analgesic consumption, ROM, or leg-raising ability. There may, however, be a higher risk of wound-healing complications including deep infections.
Achievement of postoperative pain relief after TKA is demanding. Pain scores peak on the first postoperative day, when two-thirds of TKA patients report moderate-to-severe pain (CitationWang et al. 2002, CitationWylde et al. 2011). Intense postoperative pain reduces range of motion (ROM), increases analgesic consumption, and prolongs hospital stay (CitationHorlocker 2010). Periarticular local infiltration analgesia (LIA) has been introduced as a successful and safe method to diminish postoperative pain, and it promote early mobilization after TKA (CitationBusch et al. 2006, CitationVendittoli et al. 2006, CitationToftdahl et al. 2007, CitationKerr and Kohan 2008, CitationAndersen et al. 2010a, CitationEssving et al. 2010). Interestingly, however, periarticular LIA has not been shown to reduce pain or LOS, or to improve morbidity after total hip arthroplasty (THA) (CitationDobie et al. 2012). Earlier attempts have been made using intraoperative intraarticular local anesthetics to reduce postoperative pain after TKA, with limited or no effect (CitationMauerhan et al. 1997, CitationKlasen et al. 1999, CitationRitter et al. 1999, CitationBrowne et al. 2004).
Continuous infiltration analgesia is reported to be effective in other surgical procedures (CitationLiu et al. 2006) but not in THA (CitationSpecht et al. 2011). As the effect of LIA disappears within the first 24 h, attempts have been made to prolong the analgesic effect using continuous peripheral nerve blocks (CitationFischer et al. 2008) and continuous intraarticular analgesia (CIAA) (CitationZhang et al. 2011). There have, however, been controversies about the effect of CIAA after TKA. Some studies have found that it is an effective method (CitationRasmussen et al. 2004, Gomez-Cardero and Rodriguez-Merchan 2010, CitationZhang et al. 2011, Goyal et al. 2013), whereas others have not (CitationWilliams et al. 2013). We therefore evaluated the effect of CIAA with pain, knee function, length of stay (LOS) in hospital, and complications as endpoints. We conducted a randomized, double-blind study of 200 TKAs using CIAA for 48 h via an infusion pump. Ropivacaine (7.5 mg/mL; the therapy group) or NaCl (9 mg/mL; the control group) was used as continuous intraarticular infusion at 2 mL/h.
Patients and methods
The study involved 200 consecutive patients with osteoarthritis who fulfilled the inclusion criteria of having primary knee osteoarthritis and being planned for TKA (). Exclusion criteria were bilateral TKA, taking warfarin, having contraindications regarding use of any of the study medications, having dementia, or not being able to speak Swedish (due to the high level of language skills required for questionnaires and pain evaluation). The patients were operated on at Trelleborg Hospital between January 2010 and April 2011. All patients had a standard straight central skin incision, medial parapatellar arthrotomy, and preparation of femur and tibia according to the instructions of the prosthesis manufacturer. Patients received either the Triathlon knee (Stryker, UK) or the PFC knee (DePuy, UK), depending on the surgeon’s preference. 5 orthopedic surgeons who were subspecialized in knee arthroplasty performed the surgeries.
All patients received a periarticular injection of 106 mL of a mixture containing ropivacaine (200 mg; 2 mg/mL), ketorolac (30 mg; 30 mg/mL) and epinephrine (0.5 mg; 0.1 mg/mL), of which 53 mL was injected into the posterior joint capsule and 53 mL was injected around the fascia, the anterior capsule, and the lateral and medial collateral ligaments. A further 50 mL ropivacaine (2 mg/mL) was injected subcutaneously. All patients had an epidural catheter placed in the knee joint from the lateral side of the knee. The catheter was connected through an epidural flat filter (Perifix ONE; B. Braun, Melsungen, Germany) to a Homepump (I-Flow, CA), which contained 100 mL of either ropivacaine (7.5 mg/mL) or NaCl (9 mg/mL), with an infusion rate of 2 mL/h for 48 h. Randomization was done with a computer-generated list, and a dedicated nurse who was not involved in the surgery delivered the pump to each patient during surgery. All others were blind regarding the contents of the pump. The Homepump containers were prefilled by the hospital pharmacy under sterile conditions.
The standard method of anesthesia was spinal (87%), and the remaining patients received general anesthesia. All patients received a standardized premedication and postoperative analgesia consisting of paracetamol (500 mg 2 × 4), diclofenac (25 mg 1 × 3), and a patch with buprenorphine (10 µg/h) that was changed once a week for a total of 3 weeks. Additional oxycodone (5 mg) was administered as required, and registered.
VAS pain (0–100 mm, where 0 = no pain and 100 = intolerable pain) was evaluated by the patient at rest and recorded by a dedicated nurse twice daily at 12 noon and 8 p.m. for 3 days. Furthermore, LOS, additional analgesics (apart from the standardized regime), occasions with nausea and/or vomiting, and number of changes of wound dressings during the hospital stay were registered. Active ROM of the knee (goniometry) and straight leg-raising ability were measured and recorded by physiotherapy staff both preoperatively and 3 days postoperatively.
2 weeks and 3 months postoperatively, VAS pain, analgesic consumption, and wound-healing complications were recorded again. ROM was also recorded at 3 months. The randomization list was kept secret until 3 months after operation of the last patient to be included.
Statistics
Block randomization was done with a computer-generated list, which was used by a nurse to deliver the appropriate pump to each patient. Block size for randomization was 20 patients. The primary endpoint of this study was the VAS pain assessment 3 days postoperatively. We considered a difference in VAS between the groups of 10–20 mm to be a clinically relevant difference. A power analysis then estimated that 200 patients (100 in each group) would be sufficient for detection of a statistical significance of 5% with 90% power, using a 2-sided Student t-test when the VAS SD was 20 mm. Fisher’s exact test was used for binomial variable analysis. A p-value of < 0.05 was considered statistically significant. Stata version 12.0 was used for data analyses.
Ethics
The study and study registration was performed in compliance with the Helsinki Declaration, and all patients had given their informed written consent. The ethics committee of the Faculty of Medicine, Lund University, approved the study (Dnr 2009/368), which was also registered at ClinicalTrials.gov (identifier: NCT01726686).
Results
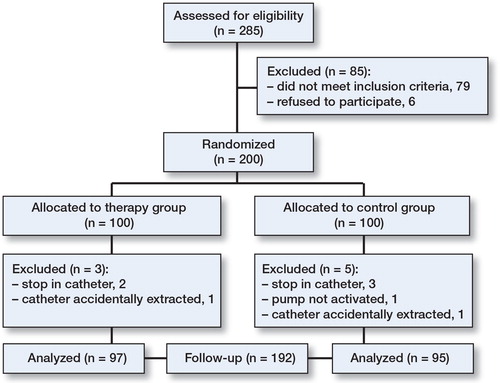
8 patients were excluded, 3 in the therapy group and 5 in the control group (). The remaining 192 patients were followed up for 3 months. Baseline data were similar between groups ().
Table 1. Patient characteristics
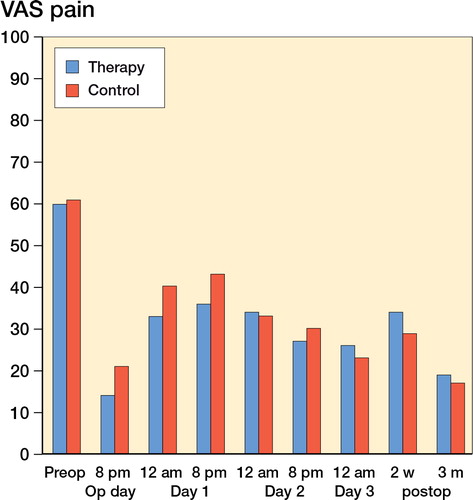
There was a statistically significant difference in VAS pain between groups at postoperative day 1 only. Mean VAS at 12 noon in the therapy group was 33, and it was 40 in the control group (p = 0.02); the corresponding values at 8 p.m. were 36 and 43 (p = 0.03) (). We also found a significant difference between the groups regarding postoperative wound infection (p = 0.02). All other recorded variables were similar between the groups ().
Table 2. Results. Data are mean (SD) or number of patients
13 patients had a superficial or deep infection of the surgical site, which was verified by bacterial culture. 11 of these patients were in the therapy group (6 superficial and 5 deep infections). The 2 corresponding patients in the control group had 1 superficial and 1 deep infection. All 7 patients with superficial infection were treated successfully with antibiotics. 4 of the 6 patients with deep infection were revised successfully by debridement and change of insert, and 2 patients were reoperated successfully with 2-stage revision of the prosthesis.
Discussion
The use of LIA in TKA is increasing, as an effective method to reduce postoperative pain (CitationBusch et al. 2006, CitationVendittoli et al. 2006, CitationToftdahl et al. 2007, CitationKerr and Kohan 2008, CitationAndersen et al. 2010a, CitationEssving et al. 2010). The effect of LIA disappears within 24 h, however, and patients receiving LIA tend to experience more pain on the second postoperative day than on the first (CitationNiemelainen et al. 2014).
The area of application of LIA can differ. CitationAndersen et al. (2008b, 2010b) found no difference between use of postoperative intraarticular LIA and use of extraarticular/intracapsular LIA. CitationDobrydnjov et al. (2011) found no difference between intraarticular and extraarticular continuous LIA at rest, while intraarticular LIA appeared to reduce pain intensity during the first exercises. Gomez-Cardero and Rodriguez-Merchan (2010) found that an analgesic infusion pump after TKA reduced postoperative pain, opioid use, and length of hospital stay without increasing the risk of complications. However, their study included only 50 patients. In a randomized, double-blind placebo-controlled trial in TKA, CitationAndersen et al. (2008a) found that high-volume ropivacaine—given first as a periarticular dose and then postoperatively as 2 intraarticular doses—reduced the pain for up to 32 h at rest and during movement. However, in our study both groups received periarticular LIA during surgery and randomization to postoperative ropivacaine or placebo did not allow us to detect any clinically relevant difference in VAS pain between the groups. To our knowledge, there has been no other prospective randomized double-blind study similar to our study.
Good pain control is essential for early mobilization after TKA. However, we consider the small difference that we found between the 2 groups regarding VAS pain on the first postoperative day to be clinically irrelevant. One limitation of our study could be that we recorded VAS pain only at rest. We chose this measure to standardize the measurement as much as possible. VAS pain during activity is very much dependent on what type of activity the patient has had, something that is difficult to control. In addition to this, intensive postoperative pain at rest is not uncommon after TKA—in contrast to THA, where patients may have more weight bearing-related pain.
Several studies have shown that there is no increased risk of infection with intraarticular catheter placement for up to 72 h (CitationBianconi et al. 2003, CitationRasmussen et al. 2004, CitationVendittoli et al. 2006). In contrast to this, we found a higher rate of superficial and deep wound infections in the therapy group. This finding is remarkable and difficult to explain, and to our knowledge it has not been found in other, similar studies. Both groups had the same type of pump, and only the contents were different. Theoretically, the pump preparation method may have introduced a risk of contamination in the therapy group, as filling a pump with 100 mL ropivacaine required five 20 mL ampoules, while the control group required only a single bag of saline to fill 10 pumps. However, pump filling was entirely done under sterile conditions in the pharmacy. One might speculate that ropivacaine could cause tissue irritation when administered continuously over 48 h. However, it has been shown that ropivacaine has an antiseptic effect (CitationBatai et al. 2002, CitationKampe et al. 2003). The finding of more infections in the ropivacaine group might be a coincidence, but it is too remarkable to be neglected and should be followed up in other studies.
In conclusion, CIAA in TKA does not appear to have any clinically relevant effect on postoperative pain and has no effect on length of hospital stay, side effects of medications, ROM, or straight leg-raising ability. To our surprise, we inexplicably found more infections in the therapy group and we have therefore discontinued the use of CIAA.
We thank Professor Jonas Ranstam, RC Syd, Clinical Sciences, Lund University, for valuable advice on statistics, and all the medical staff of the Orthopedics Department, Trelleborg Hospital, for excellent cooperation. The study was supported financially by Region Skåne and by the Erik and Angelica Sparre Foundation.
AA collected and analyzed all the data and prepared the manuscript. GF and MS designed the study and helped in writing the manuscript. All the authors recruited patients, performed the operations, and followed up the patients.
No competing interests declared.
- Andersen LO, Husted H, Otte KS, Kristensen BB, Kehlet H. High-volume infiltration analgesia in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand 2008a; 52(10): 1331-5.
- Andersen LO, Kristensen BB, Husted H, Otte KS, Kehlet H. Local anesthetics after total knee arthroplasty: intraarticular or extraarticular administration? A randomized, double-blind, placebo-controlled study. Acta Orthop 2008b; 79(6): 800-5.
- Andersen KV, Bak M, Christensen BV, Harazuk J, Pedersen NA, Soballe K. A randomized, controlled trial comparing local infiltration analgesia with epidural infusion for total knee arthroplasty. Acta Orthop 2010a; 81(5): 606-10.
- Andersen LO, Husted H, Kristensen BB, Otte KS, Gaarn-Larsen L, Kehlet H. Analgesic efficacy of intracapsular and intra-articular local anaesthesia for knee arthroplasty. Anaesthesia 2010b; 65(9): 904-12.
- Batai I, Kerenyi M, Falvai J, Szabo G. Bacterial growth in ropivacaine hydrochloride. Anesth Analg 2002; 94(3): 729-31.
- Bianconi M, Ferraro L, Traina GC, Zanoli G, Antonelli T, Guberti A et al. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 2003; 91(6): 830-5.
- Browne C, Copp S, Reden L, Pulido P, Colwell CJr.Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty 2004; 19(3): 377-80.
- Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am 2006; 88(5): 959-63.
- Dobie I, Bennett D, Spence DJ, Murray JM, Beverland DE. Periarticular local anesthesia does not improve pain or mobility after THA. Clin Orthop Relat Res 2012; 470(7): 1958-65.
- Dobrydnjov I, Anderberg C, Olsson C, Shapurova O, Angel K, Bergman S. Intraarticular vs. extraarticular ropivacaine infusion following high-dose local infiltration analgesia after total knee arthroplasty: a randomized double-blind study. Acta Orthop 2011; 82(6): 692-8.
- Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A. Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty. Acta Orthop 2010; 81(3): 354-60.
- Fischer HB, Simanski CJ, Sharp C, Bonnet F, Camu F, Neugebauer EA et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia 2008; 63(10): 1105-23.
- Gomez-Cardero P, Rodriguez-Merchan EC. Postoperative analgesia in TKA: ropivacaine continuous intraarticular infusion. Clin Orthop Relat Res 2010; 468(5): 1242-7.
- Goyal N, McKenzie J, Sharkey PF, Parvizi J, Hozack WJ, Austin MS. The 2012 Ranawat award: intraarticular analgesia after TKA reduces pain: a randomized, double-blinded, placebo-controlled, prospective study. Clin Orthop Relat Res 2013; 471(1): 64-75.
- Horlocker TT. Pain management in total joint arthroplasty: a historical review. Orthopedics 2010; 33(9 Suppl): 14-9.
- Kampe S, Poetter C, Buzello S, Wenchel HM, Paul M, Kiencke P et al. Ropivacaine 0.1% with sufentanil 1 microg/mL inhibits in vitro growth of Pseudomonas aeruginosa and does not promote multiplication of Staphylococcus aureus. Anesth Analg 2003; 97(2): 409-11.
- Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop 2008; 79(2): 174-83.
- Klasen JA, Opitz SA, Melzer C, Thiel A, Hempelmann G. Intraarticular, epidural, and intravenous analgesia after total knee arthroplasty. Acta Anaesthesiol Scand 1999; 43(10): 1021-6.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 2006; 203(6): 914-32.
- Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty 1997; 12(5): 546-52.
- Niemelainen M, Kalliovalkama J, Aho AJ, Moilanen T, Eskelinen A. Single periarticular local infiltration analgesia reduces opiate consumption until 48 hours after total knee arthroplasty. Acta Orthop 2014: 1-6. [Epub ahead of print]
- Rasmussen S, Kramhoft MU, Sperling KP , Pedersen JH. Increased flexion and reduced hospital stay with continuous intraarticular morphine and ropivacaine after primary total knee replacement: open intervention study of efficacy and safety in 154 patients. Acta Orthop Scand 2004; 75(5): 606-9.
- Ritter MA, Koehler M, Keating EM, Faris PM, Meding JB. Intra-articular morphine and/or bupivacaine after total knee replacement. J Bone Joint Surg Br 1999; 81(2): 301-3.
- Specht K, Leonhardt JS, Revald P, Mandoe H, Andresen EB, Brodersen J et al. No evidence of a clinically important effect of adding local infusion analgesia administrated through a catheter in pain treatment after total hip arthroplasty. Acta Orthop 2011; 82(3):3 15-20.
- Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop 2007; 78(2): 172-9.
- Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin M C et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am 2006; 88(2): 282-9.
- Wang H, Boctor B, Verner J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Reg Anesth Pain Med 2002; 27(2): 139-44.
- Williams D, Petruccelli D, Paul J, Piccirillo L, Winemaker M, de Beer J. Continuous infusion of bupivacaine following total knee arthroplasty: a randomized control trial pilot study. J Arthroplasty 2013; 28(3): 479-84.
- Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res 2011; 97(2): 139-44.
- Zhang S, Wang F, Lu ZD, Li YP, Zhang L, Jin QH. Effect of single-injection versus continuous local infiltration analgesia after total knee arthroplasty: a randomized, double-blind, placebo-controlled study. J Int Med Res 2011; 39(4): 1369-80.