Abstract
Background and purpose — There is solid evidence from animal experiments that parathyroid hormone (PTH) improves fracture healing. So far, only 3 papers on PTH and fracture repair in humans have been published. They suggest that PTH may enhance fracture healing, but the results do not appear to justify specific clinical recommendations. This study was carried out to determine whether teriparatide enhances fracture healing of proximal humerus fractures.
Patients and methods — 40 post-menopausal women with a proximal humerus fracture were randomized to either daily injections with 20 µg teriparatide (PTH 1-34 (Forteo)) for 4 weeks or control treatment. At randomization, the patients were asked to assess how their pain at rest and during activity (visual analog scale (VAS)) and also function (DASH score) had been prior to the fracture. At 7 weeks and again at 3 months, their current state was assessed and the tests were repeated, including radiographs. 2 radiologists performed a blind qualitative scoring of the callus at 7 weeks. Callus formation was arbitrarily classified as ”normal” or “better”.
Results — 39 patients completed the follow-up. The radiographic assessment showed a correct correlation, “better” in the teriparatide group and “normal” in the control group, in 21 of the 39 cases. There were no statistically significant differences in pain, in use of strong analgesics, or in function between the groups at the follow-up examinations.
Interpretation — There were no radiographic signs of enhanced healing or improved clinical results in the group treated with teriparatide
During the last 15 years, researchers have tried to improve fracture healing by using additional medication or growth factors such as bisphosphonates, bone morphogenetic proteins (BMPs), and parathyroid hormone (PTH). The hopes for improvement involved 2 different considerations, faster fracture healing and a reduced proportion of non-unions (CitationAspenberg 2013).
Bisphosphonates, systemic or locally administered, have been shown to improve implant fixation, but their role in fracture treatment is unclear (CitationAbtahi et al. 2012, CitationHilding and Aspenberg 2006, Citation2007). BMPs are thought to initiate fracture healing, but they have obvious side effects such as local swelling, pain, and increased risk of wound infections (CitationAro et al. 2011, CitationCarragee et al. 2011). Studies sponsored by industry have obviously not reported the adverse effects associated with the administration of BMPs in spinal fusion (CitationCarragee et al. 2011). There is also reason to believe that there is an increased risk of cancer after BMP treatment (CitationCarragee et al. 2013).
Animal studies have shown that there is accelerated fracture healing after intermittent injections with parathyroid hormone (PTH) (CitationSkripitz and Aspenberg 2004, CitationChalidis et al. 2007). So far, only 3 papers have been published concerning PTH and fracture repair in humans. 2 papers with data from the same randomized trial involving distal radius fractures showed accelerated healing and improved callus formation (CitationAspenberg et al. 2010, CitationAspenberg and Johansson 2010). The radiological analysis showed minimal improvement in the position of the healed fracture, and there was no improvement in function. The third paper compared PTH 1-84 with placebo, and showed better healing in pubic bone fractures at 2 months in the treatment group, together with less pain and better walking ability (CitationPeichl et al. 2011). However, that study had some methodological shortcomings. In conclusion, these studies have suggested that PTH may improve fracture healing in humans but the clinical importance for different diagnoses must be investigated and confirmed.
The main aim of this study was to determine whether teriparatide enhances fracture healing of proximal humerus fractures, as evaluated on radiographs at 7 weeks. Secondary aims were to compare function, pain, and the use of opioid analgesics before the fracture, at 7 weeks, and after 3 months.
Patients and methods
Between October 2010 and March 2013, post-menopausal women with a proximal humerus fracture were randomized, using sealed envelopes, to either standard after-treatment (analgesics and physiotherapy) or this also with daily injections of 20 µg teriparatide (PTH 1-34 (Forteo, Eli Lilly and Company)). The medication was initiated within 10 days of the fracture and lasted 4 weeks. The control group did not receive any placebo injections.
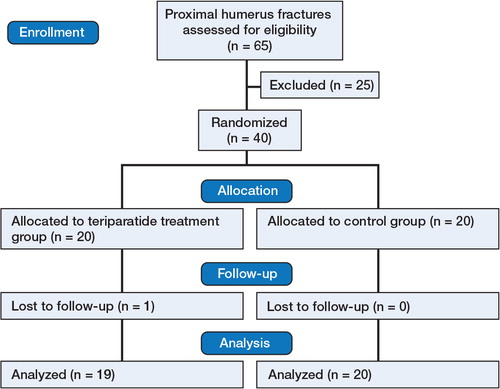
The main inclusion criterion was a fracture suitable for nonoperative treatment or fixation with osteosutures, thus making radiographic analysis of fracture healing possible. Exclusion criteria were known malignancy within 5 years prior to the fracture (except for basal cell cancer), serum calcium above reference levels, liver enzymes more than double the upper reference level, joint disease or any disease affecting bone metabolism, addiction to drugs or alcohol, medication with bisphosphonates before the fracture, mental dysfunction, inability to understand the language, or any other factors that would make informed consent impossible (Figure).
Before randomization, usually a week after the fracture had occurred, the patients attended an open visit including radiographic examination to make sure that the fracture was still suitable for nonoperative treatment. They were asked to assess how their pain at rest and during activity (visual analog scale (VAS)) and function (disabilities of the arm, shoulder and hand (DASH) score) had been before the fracture. At 7 weeks and 3 months, they were asked to assess their current state (defined at the visit as “during the past week”) and new radiographs were taken. Adverse events were recorded, together with information on current medications and those that had been terminated.
The primary outcome comprised radiographic findings of fracture healing and callus formation at 7 weeks. This assessment was done by 2 radiologists who were blind regarding the treatment, first separately and then—when the the evaluations differed—a consensus was reached between them for a final verdict. The question they had to answer (yes/no) was: “Assuming that PTH has a positive effect on fracture healing, such as increased callus formation, do you think that this patient received PTH?”
Statistics
For the radiologists to tell the right treatment in 80% of the cases (i.e. with alpha = 0.05 and a power of 80%), 26 patients would be needed. In order to increase the power, we randomized 40 patients.
We had intended to use the chi-squared test to compare the radiological assessment with the treatment given, but this was found to be unnecessary due to the obvious results. Other nonparametric data were tested with the Mann-Whitney U-test.
Ethics and registration
The study was approved by the Regional Ethics Committee in Linköping (entry number 215-09) and the Swedish Medical Products Agency (EudraCT number 2009-017320-29). It complied with the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects and was registered at ClinicalTrials.gov (identifier NCT01105832).
Results
40 post-menopausal women were randomized. 39 were treated nonoperatively and only 1 patient (in the control group) was operated on with an osteosuture. All fractures were located in the area between the anatomical and the surgical neck. The greater tubercle was also fractured in 12 patients in the PTH group and 14 patients in the control group. Since no fragments were dislocated by more than 1 cm and the angulation was less than 45 degrees, all the fractures could be classified as Neer type 1. One patient who had been randomized to the PTH group withdrew from participation since she did not want to receive the injections, thus leaving 39 patients to evaluate. The groups were similar concerning age, fracture side, diabetes, smoking habits, and osteoporosis (). The follow-up was complete except for 1 patient who did not attend the 3-month visit for personal reasons but who reported having had no complications.
Table 1. Patient characteristics (all patients were women). 13 of 23 patients who underwent screening had osteoporosis
Fracture complications
1 patient in the control group was successfully operated on 14 months after the fracture due to an exostosis at the fracture site that caused pain and a limited range of motion. 1 patient in the PTH group developed a subacromial bursitis before the 3-month visit; this was successfully treated with a subacromial injection of local anestethic and cortisone.
Safety
There were no adverse events in the control group. 6 of the 19 patients in the PTH group reported mild adverse events; 3 patients had nausea for up to 2 hours after injection during the first 1–5 days, 2 patients had episodes of sweating, and 1 patient reported having a slight headache next morning after receiving the injection in the evening.
Radiographic assessment
Before consensus was reached, the 2 radiologists had had different opinions in 8 of the 39 cases. Their final assessments were correct in 21 of 39 cases.
Pain
There were no statistically significant differences beween the groups at rest and during activity—before the fracture, at 7 weeks, and at 3 months (); nor was there a difference in the proportion of pain-free patients on those occasions (Table 3, see Supplementary data). Opiod or opiod-like analgesics were never used by 3 patients in the PTH group and 5 patients in the control group. The medication was terminated a median of 18 (0–61) days after the fracture, with no statistically significant difference between the groups. 1 patient in the control group used opiods before the fracture due to neck pain and continued to do so throughout the study.
Table 2. Levels of pain at rest and during activity before the fracture, at 7 weeks, and at 3 months (VAS). There were no statistically significant differences between the groups (Mann-Whitney U-test)
Function
Function, evaluated with DASH, did not show any statistically significant differences between the groups at follow-up (Table 4, see Supplementary data).
Discussion
This study did not show that teriparatide had any positive effect on the treatment of proximal humerus fractures, either radiographically or regarding function and pain.
The study had some weaknesses. It may have been underpowered, but there were no tendencies towards better results in either group. The length of time during which medication was given may have been too short, but an argument against this is that in a previous study, we were already able to see more callus in distal radius fractures after 5 weeks (CitationAspenberg and Johansson 2010). Furthermore, it is still not known whether a standard dose of teriparatide, 20 µg, or 40 µg is optimal. The study of distal radius fractures showed somewhat confusing results with shorter time to healing with the standard dose, while the amount of callus in the subgroup analysis was higher in the patients who received 40 µg (Aspenberg et al. 2009, CitationAspenberg and Johansson 2010). Finally, the patients were not blind as to the treatment. Placebo injection pens are not commercially available, and this study was performed without support from the manufacturer.
Despite the negative results in this study, clinicians may still expect that PTH can help in fracture treatment, given the results of animal studies. The drug is tolerated well by most patients and the side effects are mild. However, the treatment is costly and a daily injection can become a nuisance. More clinical studies addressing specific types of fractures are necessary before PTH can be recommended.
In conclusion, PTH (teriparatide) cannot be recommended as a standard treatment for proximal humerus fractures. However, the possible benefits of PTH cannot be ruled out for this group of patients if treated with a different regime, or for selected cases, or for other types of fractures.
Supplementary data
Tables 3 and 4 are available on the Acta Orthopaedica website, www.actaorthop.org, identification number 8491.
IORT_A_1073050_SM8041.pdf
Download PDF (27.6 KB)The author thanks Dr Håkan Ledin and study nurse Carmen Henriksson of the Department of Orthopaedics, Aleris Specialist Care, Motala, Sweden; study nurse Teréz Zara-Hanqvist, Linköping, Sweden; and study nurse Annette Nordström, Norrköping, Sweden, for assistance in recruiting patients and data collection. Thanks also to Dr Annicka Carlsson and Dr Georg Layth, Linköping, for assessing the radiographs.
No competing interests declared.
- Abtahi J, Tengvall P, Aspenberg P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012; 50(5): 1148–51.
- Aro HT, Govender S, Patel AD, Hernigou P, Perera de Gregorio A, Popescu GI, Golden JD, Christensen J, Valentin A. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone Joint Surg Am 2011; 93(9): 801–8.
- Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010; 25(2): 404–14.
- Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop 2010; 81(2): 234–6
- Aspenberg P. Special review: Accelerating fracture repair in humans: a reading of old experiments and recent clinical trials. Bonekey Rep 2013;2: 244. eCollection 2013
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011; 11(6): 471–91.
- Carragee EJ, Chu G, Rohatgi R, Hurwitz EL, Weiner BK, Yoon ST, Comer G, Kopjar B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 2013; 95(17): 1537–45.
- Chalidis B, Tzioupis C, Tsiridis E, Giannoudis PV. Enhancement of fracture healing with parathyroid hormone: preclinical studies and potential clinical applications. Expert Opin Investig Drugs 2007; 16(4): 441–9.
- Hilding M, Aspenberg P. Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop 2006; 77(6): 912–6.
- Hilding M, Aspenberg P. Local peroperative treatment with a bisphosphonate improves the fixation of total knee prostheses: a randomized, double-blind radiostereometric study of 50 patients. Acta Orthop 2007; 78(6): 795–9
- Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am 2011; 93(17): 1583–7.
- Skripitz R, Aspenberg P. Parathyroid hormone--a drug for orthopedic surgery? Acta Orthop Scand 2004; 75(6): 654–62. Review.