Abstract
This study describes the use of a neuroplasticity-principled speech treatment approach (LSVT®LOUD) with children who have dysarthria secondary to cerebral palsy. To date, the authors have treated 25 children with mild-to-severe dysarthria, a continuum of gross and fine motor functions, and variable cognitive abilities. From this data set, two case studies are presented that represent as weak or strong responders to LSVT LOUD. These case studies demonstrate how individual and environmental features may impact immediate and lasting responses to treatment. Principles that drive activity-dependent neuroplasticity are embedded in LSVT LOUD and may contribute to positive therapeutic and acoustic outcomes. However, examination of the response patterns indicated that intensity (within and across treatment sessions) is necessary but not sufficient for change. Weak responders may require a longer treatment phase, better timing (e.g., developmentally, socially), and a more prominent desire to communicate successfully during daily activities. Strong responders appear to benefit from the intensity and saliency of treatment as well as from intrinsic and extrinsic rewards for using the trained skills for everyday communication. Finally, possibilities are presented for technological solutions designed to promote accessibility to the intensive task repetition and maintenance required to drive lasting changes.
Introduction
Between 40–50% of individuals with cerebral palsy (CP) have speech disorders (CitationBax, Tydeman, & Flodmark, 2006; CitationKennes, Rosenbaum, Hanna, Walter, Russell, Raina, et al., 2002). Speech disorders include hypernasality, breathy voice quality, monotonous speech, reduced loudness, uncontrolled rate and rhythm of the voice, disordered respiration, and disordered articulation (CitationKeesee, 1976; CitationPennington, Willcutt, & Rhee, 2005; CitationSolomon & Charron, 1998; CitationWorkinger, & Kent, 1991). Dysarthria is a term used to characterize the speech disorders associated with CP and is defined as a motor disturbance of speech involving one or more of the speech sub-systems or co-ordination among them (CitationHodge & Wellman, 1999; CitationYorkston, Beukelman, Strand, & Bell, 1999). Speech-language pathologists are trained to target all speech sub-systems either in some systematic order (e.g., respiratory then laryngeal) or in combination (e.g., laryngeal and articulation) (CitationHodge & Wellman, 1999; CitationLove, 1992; CitationStrand, 1995; CitationYorkston, Beukelman, Strand, & Bell, 1999). See CitationPennington, Miller, and Robson (2010a, p. 10) for a brief review. Those who specialize in paediatric motor speech disorders support an intensive motor training approach regardless of sub-systems targeted (CitationHodge & Wellman, 1999; CitationLove, 1992; CitationPennington et al., 2010a; CitationStrand, 1995; CitationYorkston et al., 1999). However, CitationPennington et al. (2010a) found no randomized control studies evaluating treatment effectiveness of any approach (e.g., single sub- system, multi-system, long-term, short-term), let alone intensive motor speech training in children with dysarthria secondary to CP. Their review identified a total of 10 studies that have presented Phase I (CitationRobey, 2004) documentation of treatment effects with children who have dysarthria secondary to CP (CitationPennington et al., 2010a). CitationPennington et al. (2010a) concluded that treatments targeting either speech rate, vocal loudness, or a combination of these appeared to be associated with improvements in overall voice quality, articulatory precision, and intelligibility (e.g., CitationFox & Boliek, 2012; CitationLevy, Ramig, & Camarata, 2013; CitationPennington, Miller, Robson, & Steen, 2010b; CitationPennington, Roelant, Thompson, Robson, Steen, & Miller, 2013).
This article is one of a series that describes the use of a neuroplasticity-principled speech treatment approach Lee Silverman Voice Treatment (LSVT LOUD) with children who have dysarthria secondary to CP. The first study included five participants (CitationFox & Boliek, 2012); the second included eight participants (Boliek, Fox, Namdaran, Hilstad, & Piccott, in preparation), and a total of 12 participants are in an ongoing study. In total this forms a cohort of 25 treated children having mild-to-severe dysarthria, a continuum of gross and fine motor functions, and somewhat variable cognitive abilities. This cohort included children from a wide range of family, social, and educational environments, creating a rich tapestry of challenges and opportunities during treatment. The current study reports on two case studies of children who have dysarthria secondary to CP drawn from the second of the three treatment studies listed above. The two cases selected illustrate individual and environmental features that may impact overall response to treatment. We will describe outcomes within the framework of motor-learning principles and activity-dependent neuroplasticity and provide considerations for clinical practice based upon our current experiences.
Principles of motor learning and activity-dependent neuroplasticity in voice and speech treatment
Recently there has been an explosion of literature from the neurosciences documenting structural and neurobiological changes in the brain as a result of rehabilitative-directed activities (e.g., CitationCotman & Berchtold, 2002; CitationKleim, Hogg, VandenBerg, Cooper, Bruneau, & Remple, 2004). These changes are collectively termed activity-dependent neuroplasticity (CitationKleim & Jones, 2008). For reviews, see CitationKleim, Jones, & Schallert (2003) and CitationPetzinger, Fisher, McEwen, BeelerWalsh, and Jakowec (2013). This body of research has led to the formulation of motor learning principles thought to be necessary when using rehabilitative strategies to enhance activity-dependent neuroplasticity in adults and children with neurological impairments (e.g., CitationCotman & Berchtold, 2002; CitationGarvey, Giannetti, Alter, & Lum, 2007; CitationKleim & Jones, 2008).
CitationKleim and Jones (2008) offered a comprehensive, but not exhaustive, list of principles that drive activity-dependent neuroplasticity in the context of speech rehabilitation, such as specificity of training, sufficient repetitions, and intensity of training. CitationSchertz and Gordon (2008) specifically described key treatment principles that may be essential to drive changes in both behaviour and underlying neural functioning in children with CP. Their list included: intensive task repetition, progressive challenges with increasing difficulty, presence of motivators and rewards, and task-oriented training towards functional goals. Collectively, these principles may be important to incorporate into speech and voice rehabilitation protocols for children with CP.
A speech treatment protocol developed for people with Parkinson Disease (PD), called LSVT LOUD, has been previously discussed in the literature within the framework of motor learning and activity-dependent neuroplasticity principles (CitationFox, Ramig, Ciucci, Sapir, McFarland, & Farley, 2006). The details of LSVT LOUD have been described elsewhere (CitationFox, Ebersbach, Ramig, & Sapir, 2012; CitationSapir, Ramig, & Fox, 2011). Phase IV efficacy and effectiveness (CitationRobey, 2004) has been established for use of LSVT LOUD with PD (CitationRamig, Sapir, Countryman, Pawlas, O’Brien, Hoehn, et al., 2001a; CitationRamig, Sapir, Fox, & Countryman, 2001b). In addition, some single-subject designs and case studies using LSVT LOUD with other neurological disorders have reported positive improvements for dysarthria related to ataxia (CitationSapir, Spielman, Ramig, Hinds, Countryman, Fox, et al., 2003), stroke (CitationMahler & Ramig, 2012), multiple sclerosis (CitationSapir, Pawlas, Ramig, Seeley, Fox, & Corboy, 2001) and, most recently, CP (CitationFox & Boliek, 2012; CitationLevy et al., 2013). Following is a brief elucidation of how select principles (indicated in italics below) described by CitationKleim and Jones (2008) and CitationSchertz and Gordon (2008) are integrated into the target and mode of LSVT LOUD treatment for children with CP.
Target
The trained target of LSVT LOUD is healthy vocal loudness, which is considered important for some children with spastic CP and dysarthria, due to their disordered voice characteristics. These vocal signs may be due to muscle weakness, reduced central drive, or poor co-ordination of respiratory and laryngeal sub-systems (CitationAnsel & Kent, 1992; CitationWorkinger & Kent, 1991), all of which may potentially benefit from specificity of vocal training (CitationFox & Boliek, 2012). Further, increased vocal effort has been associated with increased activity in the orofacial muscles (CitationMcHenry, 1997; CitationWohlert & Hammen, 2000), which may facilitate some improvements in articulation and velopharyngeal function (CitationMcHenry, 1997; CitationYoung, Zajac, Mayo, & Hooper, 2001).
Specificity of vocal training is addressed first through maximum performance tasks (long ah, high ah, low ah). These are considered core voice exercises geared to enhance basic respiratory-laryngeal strength and co-ordination, build endurance, and improve voice quality. These tasks also establish the level of perceived effort on the part of the child that is required to produce healthy vocal loudness. Second, vocal training is task-oriented towards functional goals. The core voice training achieved in the first half of treatment sessions is then trained into child self-selected functional phrases and speech hierarchy exercises with the goal of systematically moving improved voice function into daily communication. Functional communication goals are different for each child and tailored to his or her communication environment.
Mode
The mode of delivery of LSVT LOUD is four individual 1-hour sessions, 4 days per week for 4 weeks delivered by certified LSVT clinicians. In addition, structured homework and carryover exercises are assigned for all 28 days of treatment. illustrates the number of intensive task repetitions and time spent on motor speech practice that is systematically structured in LSVT LOUD. These values represent the absolute minimum number of repetitions completed during a treatment session. For example, if a child has very short duration maximum sustained ahs, there may be 20–25 repetitions to fill the 15 minutes spent on this task.
Table I. Minimum repetitions and time spent on treatment exercises in LSVT LOUD.
Within each exercise category of LSVT LOUD, there are progressive challenges to increase task difficulty. Depending upon the severity of the speech disorder and/or cognitive abilities of the child, these challenges may include: (a) reducing the amount of verbal and visual cueing, (b) decreasing modelling, (c) adding dual cognitive loads (e.g., mentally solving math problems and answering with the target voice), (d) adding dual motor tasks (e.g., walking while talking), (e) increasing vocal length and endurance, and (f) adding environmental distracters (e.g., increasing background noise). The goal for each session is to continually challenge the motor system by increasing task complexity while maintaining active engagement of the child.
The child's interests and communication goals are used to structure the speech exercises. For example, speech stimuli may be related to a child's love of sports or Disney characters. Role-playing communication goals such as presentations for class or talking at a family reunion also are used. In this manner, the standardized protocol is individualized to each child to ensure the speech stimuli and communication practice in therapy is motivating and rewarding. Further, the intrinsic feeling of improved voice and successful communication are used as motivation and rewards for continued practice vs external prizes (e.g., stickers). For example, the clinician might say, “That is a beautiful voice. Do you feel how that feels? That is the voice that will help other kids understand you”. After a few days of treatment, children are encouraged to label their target voice. Children often come up with descriptions such as “my powerful voice”, my “smooth and loud voice”, or “my junior high voice”. This gives the therapist and the child a personalized identifier for future external and internal cueing.
Summary of current data of LSVT LOUD in children with CP and dysarthria
Our initial study using LSVT LOUD with five children (aged 5–7) who had dysarthria secondary to spastic quadriplegia-type CP involved a multiple baseline, single-subject experimental design (CitationBarlow & Hersen, 1984; CitationWatson and Workman, 1981). The goal was to test the presence of a therapeutic effect immediately following and 6-weeks after LSVT LOUD (CitationFox & Boliek, 2012; CitationRobey, 2004). The results of that study demonstrated initial evidence of a therapeutic effect including a post- treatment parental perception of improved loudness and more confidence in oral communication. Speech-language pathologists also perceived improved vocal loudness, as well as better pitch variability, articulatory precision, and vocal quality in the children who received treatment. Several children maintained these gains six weeks following treatment.
The outcomes from that initial study formed the rationale for a second Phase I pre–post, between-groups, design to validate and expand our understanding of the nature of observed changes in the original study (CitationFox & Boliek, 2012; CitationRobey, 2004). This study (CitationBoliek et al., in preparation) involved eight children who had dysarthria secondary to spastic quadriplegia-type CP (aged 6–12) and eight age- and gender-matched typically-developing children. Reported here are preliminary findings from the second study. Perceptual ratings by parents and speech-language pathologists confirmed the positive therapeutic effects observed in the original study (CitationFox, Boliek, Namdaran, Nickerson, Gardner, Piccott, et al., 2008). In addition, significant changes were detected for increased overall intelligibility (CitationBoliek, Chan, Kaytor, Chin, & Fox, 2012a). Neurophysiological outcomes following treatment indicated better respiratory support for speech, greater common cortical drive to speech breathing muscles, and some evidence of neural microstructural changes in the corticospinal and association tracts (CitationBoliek, Fox, Norton, Gan, Archibald, Knuttila, et al., 2009; CitationBoliek & Fox, 2011; CitationBoliek, Nickerson, Lebel, & Fox, 2012b).
Taken together, the results of those two Phase I exploratory studies indicated that there is potential for LSVT LOUD to induce behavioural, physiological, and neural changes in children with CP. Group data indicated children with CP were able to generalize increases in vocal loudness to non- trained speaking tasks, but individual data revealed that not all children achieved and/or maintained improvements over time. In addition, most but not all children generalized their skills to untrained phrases or exhibited distributed effects shown by adults post-LSVT LOUD (e.g., CitationDromey, Ramig, & Johnson, 1995; CitationSmith, Ramig, Dromey, Perez, & Samandari, 1995). In an attempt to better understand prognostic variables that may indicate potential for therapeutic improvements in children with CP, two case studies, one representing the characteristics of children who exhibited weaker treatment effects and one representing those who showed strong responses to treatment, will be described in the context of motor learning and the target and mode of LSVT LOUD.
Method
Procedures
The individual treatment response patterns were evaluated for all participants from the second Phase I validation study described earlier (CitationBoliek et al., in preparation). The two paediatric case studies selected for the detailed review in the current presentation were chosen because they represented two ends of the response spectrum (weaker and stronger responders). Responsiveness to LSVT LOUD was measured by performance on untrained tasks; defined as carry over skills (e.g., spoken words, phrases, sentences not directly targeted in treatment). Performance on untrained tasks is arguably important because these tasks represent generalizability to everyday spoken communication. Responsiveness also was measured on trained tasks defined as those specifically targeted during treatment (e.g., maximum performance tasks). Changes in trained tasks indicate treatment effects on the physiological aspects of the speech mechanism. Untrained and trained treatment outcomes were assessed using both perceptual and acoustic measurements.
Participants
F1001 was a female, aged 10 years, 9 months of age at the time of treatment. She was characterized as having spastic-type, quadriplegia CP with a Gross Motor Function Classification (GMFC) score of V. Her speech assessment indicated moderate dysarthria, poor respiratory co-ordination for speech, and mild velopharyngeal incompetence. Pre-treatment perceptions by a trained speech- language pathologist indicated that F1001 exhibited: (a) a variable voice quality that became pressed at the end of phrases, (b) short breath groups, (c) mild hypernasality, and (d) mild imprecise articulation. Educational records indicated that F1001 had co-morbid learning disabilities in processing written language but had average cognitive abilities. F1001 was receiving most academic subjects in a total inclusion environment with a full-time educational aide. Her parents indicated that F1001 had friends who included her in a variety of age-appropriate activities.
M901 was a male, aged 10 years, 9 months of age at the time of treatment. He was characterized as having spastic-type, quadriplegia CP with a GMFC score of III. His speech assessment indicated moderate dysarthria and poor respiratory support for speech. Pre-treatment perceptions by a trained speech-language pathologist indicated that M901 exhibited: (a) a moderate breathy voice quality that became aphonic at the ends of phrases and sentences, (b) short breath groups, and (c) moderate imprecise articulation. Educational records indicated that M901 had co-morbid learning disabilities in processing written language but had average cognitive abilities. M901 was receiving all academic subjects in a total inclusion environment with a full-time educational aide. His parents indicated that M901 had a few friends but most social activities were with family members and adult friends of the family.
Data collection
Children were tested three times including pre-, post-, and follow-up sessions. Testing was conducted in the research laboratory using a standardized protocol. The same individualized adaptive seating was maintained across all three test sessions. During testing, children were not cued, other than to do their best (i.e., no cues to the treatment target of voice) and were tested by individuals not associated with the treatment phase of the study. The same individuals were used to test children before and after treatment. Testers did not have access to participant records or diagnosis other than the obvious physical characteristics associated with CP.
Acoustic and perceptual data were collected according to standardized protocols as detailed in CitationFox and Boliek (2012) and CitationHodge, Daniels, and Gotzke (2006) and summarized here. All acoustic signals were recorded from a unidirectional microphone placed on the child's forehead 10 cm from the mouth and held in place by a soft headband. Sound recordings were acquired using a digital audio recorder and a sampling rate of 44.5 kHz. A sound pressure level meter was used to measure a calibration tone (440 Hz) presented at 10 cm distance and recorded for each child and session. The calibration tone was then used during the analysis phase as the reference sound pressure for calculating vocal sound pressure (dB SPL).
Children were required to produce a minimum of five trials of: (a) maximum duration sustained phonation (ah), (b) highest pitch productions of ah, (c) lowest pitch productions of ah, and (d) sentence repetition (Buy bobby a puppy. The blue spot is on the key. The potato stew is in the pot.). Children also completed the standardized TOCS+ protocol for word productions (CitationHodge et al., 2006). The same word lists were used across all three testing sessions.
Data analysis
Measurements of untrained tasks included: (a) sound pressure (dB SPL) from untrained sentences, (b) f0 variability from untrained sentences, (c) auditory-perceptual analysis of loudness, loudness variability, pitch, pitch variability, voice quality, and articulatory precision on productions of untrained sentences as judged by three speech- language pathologists (as per protocol in CitationFox & Boliek, 2012), and (d) overall intelligibility derived from 10 naïve listeners using an open-set response to tokens recorded from the Test of Children's Speech Plus (TOCS+) software program (Canadian Language and Literacy Research Network; www.cllrnet.ca). Tokens were randomized across testing sessions and listeners were blind to session.
Measurements of trained tasks included: (a) sound pressure (dBSPL) for maximum sustained ahs, (b) duration for maximum sustained ahs, (c) highest fundamental frequency (fo) for high ahs, (d) lowest f0 for low ahs, (e) cycle-to-cycle variability in amplitude (shimmer) from maximum sustained ahs, (f) cycle-to-cycle variability in timing (jitter) from maximum sustained ahs, (g) harmonics-to-noise ratio from maximum sustained ahs, and (h) auditory-perceptual analysis of loudness, loudness variability, pitch, pitch variability, and voice quality on productions of maximum sustained ahs as judged by three speech-language pathologists. Judges were asked to select a preference from paired samples (pre vs post; pre vs follow-up; post vs follow-up), but were blind to session.
Inter- and intra-measurer/rater reliably calculations were done on 20% of the group data from which these cases were drawn. Inter-measurer reliabilities ranged from r = .87–.99 and intra-measurer reliabilities ranged from r = .92–.99.
Statistical analysis or determination of treatment effects
All continuous variables derived in the pre-treatment session were compared to those obtained in the post-treatment session and to those obtained in the 12-week follow-up session using paired t-tests (a priori set, p < .05). Categorical variables (listener preference) were tested for significance using Chi-square (χ2, p < .05).
Results
Twenty-one outcome variables were measured immediately post- and 12-weeks following LSVT LOUD. lists all 21 variables tested. Arrows indicate improvement (↑) or no change (→) following treatment as determined by statistical significance. Nine variables have been categorized as untrained (sentences and TOCS+ stimuli not practiced in treatment), and 12 variables have been categorized as trained (maximum performance tasks that were part of treatment). To provide specific values, selected acoustic variables are presented in and . Of the total number of variables tested F1001 improved on 7/21 and M901 improved on 12/21. Most of F1001 improvements were on trained variables, whereas M901 had more improvements on untrained variables. A test of difference between two independent proportions (7/21 and 12/21) revealed a slight trend (z = 1.55, p < .06, 1-tailed), indicating M901 exhibited more improvement than F1001. The detailed outcomes for these two cases (weak and strong) are presented below.
Figure 1. Acoustic, perceptual, and intelligibility pre-, post- and 12-weeks following treatment for F1001 and M901. Panels A and C depict sound pressure level (in dB SPL, mouth-to-microphone distance of 10 cm) derived from maximum sustained phonation (ahs) and untrained phrases, respectively. Panel B depicts durations (in sec) associated with maximum sustained phonation (ahs). Panel D depicts overall intelligibility (in %) derived from open-set responses by naïve listeners on TOCS+ stimuli. Error bars represent 1 standard deviation.
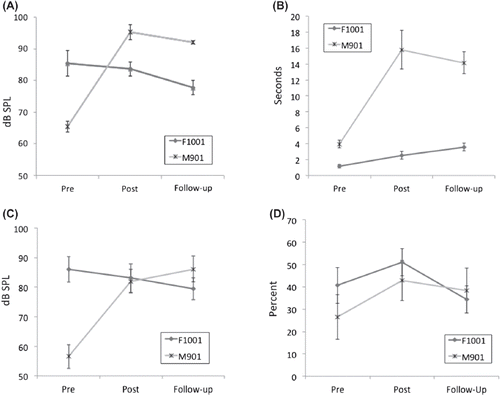
Table II. Outcomes variables for untrained and trained tasks for F1001 and M901(↑ = positive change and → = no change).
Table III. Mean acoustic measures (mean) and standard deviations (SD) of laryngeal control from pre- and post-treatment (Tx) and 12-weeks follow-up for F1001 and M901.
Case study one: Weaker treatment response
Post-treatment outcomes
F1001 was compliant and enthusiastic during the treatment phase. She completed all 16 treatment sessions, daily homework assignments, and daily carryover activities. Of the nine untreated variables, which included auditory-perceptual analysis of speech and overall intelligibility as measured by TOCS+ listeners, only one of nine improved. This improvement was a slight but statistically significant improvement (40.63–51.00%, p < .01) on overall intelligibility based on the TOCS+ data ((d)). No other untreated variables showed improvement immediately following treatment.
Compared to her pre-treatment performance, F1001 exhibited positive changes immediately following treatment on six of the 12 trained variables. (a) and (b) illustrate that F1001 was able to increase the duration of maximum sustained ahs but did not show changes in overall dB SPL for sustained phonations. However, it should be noted that her dB SPL was equivalent to her age-matched peer. Acoustic measures of shimmer, jitter, and HNR indicated significant movement towards typical values (). Listeners also preferred her vocal loudness and voice quality for sustained vowels immediately post-treatment.
Twelve-week maintenance outcomes
A maintenance schedule for 12 weeks involved one practice per day (12 ahs, 6 high ahs, 6 low ahs, twice through the 10 functional phrases and 5 minutes of a hierarchy activity). A practice DVD was given to the family as an alternative to doing practice face-to-face. At the end of 12 weeks, parents reported that the maintenance schedule was followed ∼ 60% of the time. However, no quantifiable measure of practice sessions was collected.
After the 12-week maintenance phase, F1001 showed improvement in five of nine untreated variables (). As can be seen in , listeners preferred the 12-week follow-up productions of sentences for overall loudness, pitch, pitch variability, voice quality, and articulatory precision. This was an improvement from the immediate post-treatment ratings. However, F1001 did not maintain her gains in overall intelligibility, showing a statistically significant (p < .01) drop from 51% intelligible immediately following treatment to 34% intelligible 12-weeks following treatment.
For trained tasks, F1001 maintained improvements in all six of 12 trained variables. Maximum phonation durations continued to improve ((b)). F1001 maintained her initial improvements in measures of shimmer, jitter, and HNR () and listener perception of loudness and voice quality for sustained vowels.
Case study two: Stronger treatment response
Post-treatment outcomes
As with F1001, M901 was compliant and enthusiastic during the treatment phase and completed all treatment sessions, homework assignments, and daily carryover activities. M901 showed improvements in six of the nine untreated variables (). As can be seen in (c), M901 showed a significant increase in vocal SPL when producing untrained sentences as well as improved f0 variability for the same sentences (). In addition, listeners preferred post-treated untrained sentences for loudness, loudness variability, and articulatory precision (). M901 showed a statistically significant improvement (26.56% to 42.76%, p < .01) on overall intelligibility based on the TOCS+ data, as can be seen in (d).
As can be seen in , compared to his pre-treatment performance, M901 exhibited positive changes immediately following treatment on six of 12 treated variables. (a) and (b) indicate that M901 was able to increase the vocal SPL and duration of his maximum sustained ahs. It should be noted that his vocal SPL was significantly lower than his age-matched peer. In addition, acoustic measures of maximum and minimum f0, jitter, and HNR improved.
Twelve-week maintenance outcomes
The 12-week maintenance schedule was the same as described for F1001. At the end of 12 weeks, parents reported that the maintenance schedule was followed ∼ 90% of the time. M901 used the practice DVD that had been set up in his room. He elected to practice with the DVD each morning before going to school. Again, no quantifiable measure of practice sessions was collected.
After the 12-week maintenance phase, M901 showed improvement in seven of nine untreated variables (). M901 continued to make improvements in vocal SPL and f0 variability during productions of untrained sentences ((c); ). Listeners continued to prefer 12-week post-treatment productions of untrained sentences for loudness, loudness variability, and articulatory precision with an additional preference for voice quality (). M901 maintained his gains in overall intelligibility of 38.39% at 12 weeks following treatment, which was significantly better (p < .01) than his pre-treatment level.
M901 improved his performance from pre- to 12-week follow-up on six of 12 treated variables. These included maximum phonation vocal SPL and duration ((b)) and minimum f0 as well as acoustic measures of jitter and HNR. M901 did not maintain improvement in his maximum f0; however, he gained improvement in listener perception of voice quality for sustained vowels.
Discussion
Case-study treatment outcomes in the context of motor learning principles and activity-dependent neuroplasticity
These two case studies represent a continuum of response to a speech treatment protocol that incorporates principles of motor learning known to drive activity-dependent neuroplasticity. When specifically examining response to untrained variables, F1001 was a weaker-responder immediately post-treatment improving on only one of nine untrained variables. In contrast, M901 had strong responses to the treatment protocol, as indicated by the number of gains achieved on seven of nine untrained tasks. The pattern of outcomes for these two children will be described in the context of the target and mode of delivery of LSVT LOUD. Further, comparisons to previously published work will be provided. From our experience, there are several factors that affect treatment outcomes. Not all of these factors have been systematically measured. Therefore, the following discussion is based on both data and anecdotal observations. We offer some clinical recommendations for consideration as well.
Target
Both F1001 and M901 responded to specificity of training in the context of targeting vocal effort and healthy vocal loudness. Although F1001 did not have changes in vocal SPL for sustained vowels or untrained sentences, her pre-treatment vocal SPL was not as low as M901. At conversational distances of 30 cm or greater, pre-treatment vocal SPL for F1001 would have been above ambient noise found in most environments, whereas M901 would have been well below ambient noise level. F1001 did, however, exhibit changes in the stabilization of voice as measured by improved jitter, shimmer, harmonics-to-noise ratio, and listener perceptions of loudness and voice quality during sustained vowels immediately post-treatment. In addition, her parents anecdotally reported fewer instances of shouting or whispering. Thus, the importance of the voice target for F1001 may have been for stabilizing voicing, improving quality, and utilizing healthy vocal effort during speech. In contrast, M901 exhibited changes in vocal SPL for both sustained vowels and untrained sentences. Further he demonstrated a greater number of positive changes on untrained variables following vocal training. The greater impact for M901 may have been due to his softer voice pre-treatment. Thus, severity of voice disorder may impact the degree of improvements on voice measures following specific vocal training in a protocol such as LSVT LOUD.
Training vocal effort and loudness is thought to generally scale up effort across the speech mechanism (CitationDromey et al., 1995). Both F1001 and M901 had immediate post-treatment improvements on overall intelligibility assessed by listeners on the TOCS+ words. F1001 improved overall intelligibility immediately post; however, by 12 weeks following treatment, F1001 did not maintain improvement in word-level intelligibility; however, listeners reported improved perceptions for vocal loudness, voice quality, and articulatory precision on untrained sentences. Differential maintenance effects reported for F1001 may have been due to differences between word and sentence level productions, listening tasks, listeners (i.e., naïve vs speech-language pathologists), or a combination of these. In contrast, M901 exhibited improvements in measures of perceived articulatory precision and overall intelligibility both immediately post-treatment and at 12 weeks follow-up. Thus, the increased vocal SPL he achieved through training may have had an even more robust distributive effect on articulatory movements that were detected across tasks and listeners.
Mode
Both F1001 and M901 received the same number of repetitions during their 16 hours of direct treatment and they were both compliant with homework. Therefore, we can assume the number of intensive task repetitions for trained tasks was approximately the same throughout the treatment period. As others have stressed, the number of trials and the intensity of training is directly associated with changes in motor behaviour (CitationDamiano, 2009; CitationFox et al., 2006; CitationKleim & Jones, 2008; CitationSchertz & Gordon, 2008; CitationValvano & Newell, 1998). The difference in outcomes between these two children in untrained tasks, particularly immediately post- treatment, is likely due to a complex constellation of both intrinsic and extrinsic variables. Therefore, it would seem that intensity is necessary but not sufficient in the context of motor learning.
CitationKleim et al. (2004) summarized the two phases of learning a motor skill—a fast phase that can occur within the first few days of training and a slow phase that continues across training days. The fast phase is thought to correlate with behavioural learning of a skill; the slower phase is thought to correlate with underlying neurobiological changes. It may be that the fast-phase behavioural change described by CitationKleim et al. (2004) had a longer trajectory for F1001 than it did for M901. She did not demonstrate immediate changes post-treatment, but did continue to make gains in overall voice quality and perception of articulatory precision 12 weeks after treatment ended. If treatment had progressed for a longer period of time or if 100% compliance on maintenance practice had been met, outcomes may have been more robust and long-lasting for the measure of overall intelligibility.
M901 made significant fast-phase behavioural gains in both untrained and trained tasks. In addition, he maintained these gains and even exhibited additional improvements at the end of his 12-week maintenance phase. M901 differed from F1001 in the amount of practice completed during the maintenance phase of the treatment. Thus, the treatment dose in combination with the maintenance schedule appeared to optimize and maintain changes for M901. These later-phase gains are thought to be associated with lasting change in the context of motor map re-organization (CitationAdkins, Boychuk, Remple, & Kleim, 2006; CitationKleim et al., 2004).
Although the goal of treatment is to progressively challenge the child with increasing task difficulty, there appears to be an individualized and delicate balance between tasks promoting optimal motor output and those exceeding the child's cognitive or physical ability. In our experience when tasks become too challenging (e.g., reading), some children diminished their motor output and may even refuse to participate. This was the case with our strong responder M901 who required occasional adjustments in task difficulty to re-establish active engagement in speech exercises with good vocal effort and loudness. Thus, we had to prioritize motor practice (target in this therapy) over the complexity of tasks for some days of treatment. It may be that, when a balance is achieved between maintaining active motor output and appropriate levels of challenge, children may engage more readily in intensive motor practice and increase the likelihood of transfer of their vocal effort to untrained tasks of daily communication. Even when children have severe motor speech impairments, this balance can be achieved as described in CitationFox and Boliek (2012).
The inclusion of motivators and rewards and training towards functional goals have some overlap and will be discussed together. The meaningfulness of being a successful oral communicator is clearly comprised of a complex set of personality and environmental factors. Whereas all of the children treated were primarily oral communicators, we observed a wide range of motivation to become confident and successful. Both F1001 and M901 were being educated in an inclusive environment with the support of teacher assistants, but there was a clear distinction between their communication goals. F1001 already had a moderately large peer social network and was well integrated into school and family activities. In contrast, M901 was extremely motivated to increase his circle of friends, gain the recognition of his teacher, and become a student body leader when he transitioned to middle school. In addition, he had been approached by several local agencies to become a spokesperson for various paediatric fundraising causes. Whereas grading the exact saliency of the treatment target for each child was difficult, anecdotal evaluation of treatment gains in the context of internal motivation was possible. In the case of F1001, there were no consequences for not acquiring more intelligible speech. Her friends and family were extremely accommodating, and she reported that she was content with the way things were. On the other hand, M901 was driven by his internally set goals and recognized the need to improve his overall intelligibility and communication effectiveness in order to reach those goals. His willingness to practice every day before going to school was a testament to the saliency of LSVT LOUD in his life context.
The patterns of treatment outcomes observed in the case studies presented here are similar to patterns observed in previous studies (CitationFox & Boliek, 2012; CitationLevy et al., 2013). In those studies, the target of voice was appropriate for all children based on documented voice issues, but, as shown in the present case studies, patterns of response in those studies were variable among children (CitationBoliek et al., in preparation; CitationFox & Boliek, 2012; CitationLevy et al., 2013). Intensive repetitions and treatment were tolerated well by all children in previous studies (CitationFox & Boliek, 2012; CitationLevy et al., 2013). Commensurate with the present cases, the patterns of fast-phase and later-phase changes differed between children, indicating differential effects of treatment dose and maintenance on motor learning (CitationBoliek, et al., in preparation; CitationFox & Boliek, 2012; CitationLevy et al., 2013). In our previous study (CitationFox & Boliek, 2012), two things kept children on task for the most part: (a) keeping the speech practice salient with activities and topics that were unique to each child and (b) reinforcement of the functional goal (e.g., That is the voice that will help your friends understand you). The children who were able to read easily progressed in the hierarchy. In terms of providing motivators and rewards, three of the four children from the CitationFox and Boliek (2012) study were very easy to motivate and found intrinsic satisfaction in their improved voices. In that study, a challenge for one of the children was finding salience in the voice tasks. He did not like to practice with his parents and was not engaged when talking with peers. In fact, pre-treatment reports described him as intimidated by other kids. His avoidance of peer interaction made it difficult to use successful peer interaction as an internal motivator. For the case studies presented here, the outcome measures assessed at 12-weeks following treatment indicated that both F1001 and M901 maintained or continued to make progress on treated and untreated tasks. This observation is commensurate with children treated in the current group (CitationBoliek et al., in preparation) and the previous study (CitationFox & Boliek, 2012). This preliminary evidence suggests that the maintenance phase following treatment is extremely important. Therefore, a systematic approach to establishing and quantifying post-treatment maintenance schedules is warranted.
Clinical recommendations
The following recommendations are presented for clinicians who are deciding if LSVT LOUD is an appropriate intervention for a child with dysarthria secondary to CP. These recommendations also may be useful when considering other types of speech therapy and paediatric motor speech disorders. The recommendations presented here are in no way meant to be novel, as many of these recommendations are used daily by skilled clinicians. Nevertheless, they are derived from a culmination of our data, clinical observations, and experience using LSVT LOUD with children who have dysarthria secondary to CP.
Targeting vocal loudness
The target of voice and specificity of training voice may be useful for some children with CP. We recommend that clinicians consider more than just the absolute vocal SPL measured during assessment, but also consider maintenance of normal loudness, and modulation of vocal loudness, and overall voice quality. We have many parents who tell us, “oh—he/she has no problem being loud enough when they want”, but the ability to sustain and modulate normal vocal loudness in daily communication can be a challenge. The target of treatment is to establish healthy vocal loudness, the constellation of which will differ among children. For example, establishing healthy vocal loudness with M901 involved increasing vocal loudness (i.e., he was too soft) as well as stabilizing vocal SPL, while, at the same time, improving vocal quality. For F1001, training healthy vocal loudness did not necessarily require increasing absolute vocal loudness, but rather using it as a target to improve stability of voicing and overall voice quality.
Stay the course
We also recommend clinicians have patience when waiting to observe the spread of effects from targeting vocal effort and loudness to articulation and overall intelligibility. These effects do not always happen immediately and may not appear until as late as week 3 or 4. Too often when we as clinicians fail to see immediate outcomes, we switch our treatment targets, approaches, or both. The motor learning phase length (fast and slow) takes time to evolve, especially in atypical motor systems. We caution the clinician about changing approaches too soon in an effort to allow learning to happen and, in effect, remain focused on the initial treatment goal.
Treatment intensity
Most children can handle the intensive treatment, intensive task repetitions, progressive challenges, and endurance associated with LSVT LOUD. In fact, our observation is that many children enjoy this treatment regime because it is engaging and challenging. We have observed that children are tired at the end of early treatment sessions, but improved endurance happens rather quickly. There also are days when children resist and shut-down, but with persistence and encouragement children do push through those sessions and continue to make gains on treatment targets. By pushing children beyond what is typical in traditional speech therapy, we are helping them realize they can work harder than they thought. We recommend overt acknowledgement of their hard work, effort, and improved stamina as it occurs. Recognition of a sense of accomplishment from working hard can be an important motivator and also may offer some intrinsic rewards.
Task complexity and maintenance of motor output
There is a fine balance between increasing task complexity and maintaining the child's ability to produce speech at the targeted vocal loudness. For example, if we are increasing a task in complexity to the level of diminishing motor output, we have to be able to pull back and re-focus our activities towards the therapy target of intensive motor practice. We can do this by dropping down a level in the hierarchy or briefly practicing sustained phonation to recalibrate the system.
Task saliency
Identification of the most salient tasks for speech exercises (e.g., games to play, topics to discuss, role-play to practice) is often an evolving process. Be aware of discrepancies between parent- vs child-driven communication goals. One advantage of having an intensive treatment protocol is the ability to establish day-to-day interactions with each child. Thus, the period of trial-and-error for identifying salient tasks is short. Older children often bring in their own materials and activities, thus saving the speech-language pathologist preparation time. We recommend eliminating external rewards such as stickers, tokens, or candy. Instead focus on intrinsic rewards such as, “doesn't that make you feel good?”, “did your friends understand you better?”, “did you like how your voice sounded?” In doing so, children are provided a path to greater saliency for the treatment exercises and translation to real world experiences. We have found this to be true even for younger children and children with lower cognitive abilities. The shift from extrinsic to intrinsic rewards fosters a more natural communication interaction between the clinician and child whereby; the effort it takes to be understood by another is brought into focus for the child vs a token-economy-driven performance. The shift in reinforcement can be initially uncomfortable for both the clinician and child, but the end result is a focus on treatment exercises that address the desire on the part of the child for improved communication. Intrinsic motivation also is related to the timing of treatment. As demonstrated in the two case studies the intrinsic motivation for improving speech was very different for F1001 and M901. Timing our interventions with not only biological development, but also social and emotional development may maximize treatment outcomes.
Generalizability of skills beyond the treatment room
Children who have allowed us to utilize their entire communication environment including parents, siblings, teachers, aides, and friends, appear to have a greater degree of carryover and transfer of skills to the real world. Developing homework activities that are highly meaningful in the context of everyday communication opportunities is essential. By surveying each child's day-to-day communication prospects and engaging his or her potential communication partners will enhance practice outside of the treatment setting with opportunities for ongoing practice. Each success will serve to further reinforce and calibrate the use of healthy vocal loudness in everyday settings.
Technology solutions for intensive repetition and maintenance
It is clear that intensive practice and sufficient repetitions are important to drive change. We also recognize that an intensive treatment protocol might be one of the greatest obstacles to implementation beyond the laboratory setting. A computer program designed to assist in the delivery of LSVT LOUD has been developed and tested in a study with individuals having PD (CitationHalpern, Ramig, Matos, Petska-Cable, Spielman, Pogoda, et al., 2012). The computer program, called the LSVT Companion, guides a client through the speech treatment session while collecting data on sound pressure level, pitch, and duration, all of which can be sent to the therapist. The speech clinician can set the target levels for each client as well as individualized speech exercises. The system monitors each client's performance and provides immediate feedback about goal attainment.
Use of such a system with children with CP is an attractive alternative to providing all treatment sessions in-clinic. In addition, it may be a mechanism for continued practice with clinician oversight at the conclusion of therapy. Currently, we have an ongoing study looking at the use of the LSVT Companion in 10 children with CP. The goal is to introduce the LSVT Companion as a homework tool during the 4 weeks of in-clinic therapy and for monitoring practice during the 12-week maintenance period. This will be the first study to produce objective documentation of amount and quality of practice during a treatment maintenance phase in children with CP.
Future studies
We are currently conducting an ongoing study, as noted earlier with 10 children who have dysarthria secondary to CP. We have expanded our outcome variables to include a more in-depth account of respiratory and laryngeal physiology, inter-muscular coherence of the chest wall and neuroimaging (structural and functional) (CitationCerebral Palsy International Research Foundation, Boliek, PI, 2012-14). These data will extend our understanding of changes in underlying motor control mechanisms associated with LSVT LOUD. Additional studies are important for advancing the following objectives. There is a need to continue to define prognostic variables through studies designed to treat and evaluate a wide variety of children with motor speech disorders associated with CP including those of different ages, levels of severity, and types of dysarthrias and/or apraxia of speech. Comparative studies using LSVT LOUD (voice target) with another treatment of the same intensity but different target (e.g., articulation or respiration) would be useful in assessing the relative effectiveness associated with intensity vs treatment target. Cross-over treatment designs to assess therapeutic gains (e.g., intelligibility) when two or more single-target intensive treatments are delivered sequentially will provide important insight about the relative strength of each and the additive strength of both in the context of treatment effectiveness. Finally, systematic evaluation of daily communication participation following LSVT LOUD is needed to assess generalization of treatment effects to skills of daily living.
Conclusions
We have presented a treatment approach (LSVT LOUD) in the context of motor learning and activity-dependent neuroplasticity with children who have dysarthria secondary to CP. We used case-study examples to demonstrate how individual and environmental features affected fast-phase and long-phase responses to LSVT LOUD. These cases were representative of the continuum of responses we have observed in group and individual outcome measures (CitationBoliek, et al., in preparation; CitationFox & Boliek, 2012) and as reported by others (CitationLevy et al., 2013). The key motor-learning principles embedded in both target of treatment and mode of delivery were shown to have both positive therapeutic and acoustic outcomes in these children. However, examination of the response patterns in the two cases presented indicated that intensity (dose) is necessary but not sufficient for change. Weak responders may require a longer treatment phase, better timing (e.g., developmentally, socially), and a more prominent desire to communicate successfully during daily activities. Strong responders appear to benefit from the intensity and saliency of treatment as well as from intrinsic and extrinsic rewards for using the trained skills for everyday communication.
Clearly we need more research in all paediatric motor speech treatments (CitationFox & Boliek, 2012; CitationLevy et al., 2013; CitationPennington et al., 2010a). Continued voice and speech treatment research in this population is essential for the advancement of our understanding of prognostic variables that might indicate potential therapeutic improvements along with practical implications for motor learning and activity-dependent neuroplasticity within a developmental framework.
Acknowledgements
Funding from the Stollery Children's Hospital Foundation, the Edmonton Oilers Community Foundation, and the Alberta Heritage Foundation for Medical Research supported this work. We would like to acknowledge the children and their families for their hard work and participation in these treatment research studies.
Declaration of interest: Drs. Fox and Boliek have both financial and non-financial relationships with LSVT Global. Non-financial relationships include a preference for LSVT LOUD as treatment technique which will be discussed as a part of this seminar. Dr. Fox is employee of and has ownership interest in LSVT Global, Inc. which owns the LSVT Companion system discussed in this presentation. Dr. Boliek receives lecture honorarium and travel reimbursement from LSVT Global, Inc.
References
- Adkins, D. L., Boychuk, J., Remple, M. S., & Kleim, J. A. (2006). Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. Journal of Applied Physiology, 101, 1776–1782.
- Ansel, B. M., & Kent, R. D. (1992). Acoustic-phonetic contrasts and intelligibility in the dysarthria associated with mixed cerebral palsy. Journal of Speech and Hearing Research, 35, 296–308.
- Barlow, D. H., & Hersen, M. (1984). Single case experimental designs: Strategies for studying behavior change. New York, NY: Pergamon.
- Bax, M., Tydeman, C., & Flodmark, O. (2006). Clinical and MRI correlates of cerebral palsy: The European Cerebral Palsy Study. Journal of the American Medical Association, 296, 1602–1608.
- Boliek, C. A., Chan, V., Kaytor, D., Chin, C., & Fox, C. M. (2012a). Effects of LSVT® LOUD (Lee Silverman Voice Treatment) on speech intelligibility in children with Cerebral Palsy. Paper presented at the Sixteenth Biennial Conference on Motor Speech: Santa Rosa, CA.
- Boliek, C., & Fox, C. (2011). Neural and physiological correlates of intensive voice treatment in children with dysarthria secondary to cerebral palsy: Evidence from respiratory kinematics and trunk muscle EMG. Developmental Medicine and Child Neurology, 53, 70.
- Boliek, C. A., Fox, C. M., Namdaran, N., Hilstad, J., & Piccott, C. (in preparation). Therapeutic effects of intensive voice treatment (LSVT®LOUD) for children with spastic cerebral palsy and dysarthria: A Phase I validation study. American Journal of Speech-Language Pathology,
- Boliek, C., Fox, C., Norton, J., Gan, L., Archibald, E., Knuttila, E., et al. (2009). Changes in trunk muscle activation and respiratory kinematics during speech following intensive voice treatment (LSVT LOUD) for children with spastic cerebral palsy. Movement Disorders, 24 (Suppl. 1), S450.
- Boliek, C. A., Nickerson, C., Lebel, C., & Fox, C. M. (2012b). Evidence of microstructural changes in the brain following intensive voice treatment (LSVT®LOUD) for children with spastic cerebral palsy and dysarthria. Paper presented at the Sixteenth Biennial Conference on Motor Speech: Santa Rosa, CA.
- Cerebral Palsy International Research Foundation. (2012). Neural correlates of intensive voice treatment effects on children with cerebral palsy. Principal Investigator: Boliek, C.A., Baltimore, Maryland.
- Cotman, C. W., & Berchtold, N. C. (2002). Exercise: a behavioural intervention to enhance brain health and plasticity. Trends in Neuroscience, 25, 295–301.
- Damiano, D. L. (2009). Rehabilitative therapies in cerebral palsy: the good, the not good, and the possible. Journal of Child Neurology, 24, 1200–1204.
- Dromey, C., Ramig, L., & Johnson, A. (1995). Phonatory and articulatory changes associated with increased vocal intensity in Parkinson disease: a case study. Journal of Speech and Haring Research, 38, 751–763.
- Fox, C., Boliek, C., Namdaran, N., Nickerson, C., Gardner, B., Piccott, C., et al. (2008). Intensive voice treatment (LSVT) for children with spastic cerebral palsy. Movement Disorders, 23 (Suppl. 1), S378.
- Fox, C., Ebersbach, G., Ramig, L., & Sapir, S. (2012). LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson disease. Parkinson's Disease, Article ID 391946, 12pgs.
- Fox, C. M., & Boliek, C. A. (2012). Intensive voice treatment (LSVT LOUD) for children with spastic cerebral palsy and dysarthria. Journal of Speech, Language and Hearing Research, 55, 930–945.
- Fox, C. M., Ramig, L. O., Ciucci, M. R., Sapir, S., McFarland, D. H., & Farley, B. G. (2006). The science and practice of LSVT/LOUD; neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Seminars in Speech and Language, 27, 283–99.
- Garvey, M. A., Giannetti, M. L., Alter, K. E., & Lum, K. E. (2007). Cerebral palsy: New approaches to therapy. Current Neurology and Neuroscience Reports, 7, 147–155.
- Halpern, A., Ramig, L., Matos, C., Petska-Cable, J., Spielman, J., Pogoda, J., et al. (2012). Innovative Technology for the Assisted Delivery of Intensive Voice Treatment (LSVT® LOUD) for Parkinson Disease. American Journal of Speech-Language Pathology, 21, 354–367.
- Hodge, M., Daniels, C., & Gotzke, J. (2006). A software approach to measuring speech intelligibility in young children. International Child Phonology Conference, Edmonton, AB.
- Hodge, M. M., & Wellman, L. (1999). Management of dysarthria in children. In A. J. Caruso, & E. Strand (Eds.), Clinical Management of Motor Speech Disorders in Childhood (pp. 209–280). New York: Thieme.
- Kennes, J., Rosenbaum, R., Hanna, S. E., Walter, S., Russell, D., Raina, P., et al. (2002). Health-status of school aged children with cerebral palsy: information from a population-based sample. Developmental Medicine and Child Neurology, 44, 240–247.
- Keesee, P. (1976). Abnormal postural reflex activity and voice usage deviations in cerebral palsy. Physical Therapy, 56, 1358–1360.
- Kleim, J., Jones, T., & Schallert, T. (2003). Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical Research, 11, 1757–1769.
- Kleim, J. A., Hogg, T. M., VandenBerg, P. M., Cooper, N. R., Bruneau, R., & Remple, M. (2004). Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. The Journal of Neuroscience, 24, 628–633.
- Kleim, J. A., & Jones, T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language and Hearing Research, 51, S225–239.
- Kleinow, J., Smith, A., & Ramig, L. (2001). Speech stability in idiopathic Parkinson disease: Effects of rate and loudness manipulations. Journal of Speech, Language, and Hearing Research, 44, 1041–1051.
- Levy, E. S., Ramig, L. O., & Camarata, S. M. (2013). The effects of two speech interventions on speech function in pediatric dysarthria. Journal of Medical Speech-Language Pathology, 20, 82–87.
- Love, R. J. (1992). Childhood Motor Speech Disability. Boston, MA: Allyn & Bacon.
- Mahler, L. A., & Ramig, L. O. (2012). Intensive treatment of dysarthria secondary to stroke. Clinical Linguistics and Phonetics, 26, 681–694.
- McHenry, M. (1997). The effect of increased vocal effort on estimated velopharyngeal orifice area. American Journal of Speech Language Pathology, 6, 55–61.
- Pennington, B. F., Willcutt, E., & Rhee, S. H. (2005). Analyzing comorbidity. Advances in Child Development and Behavior, 33, 263–304.
- Pennington, L., Miller, N., & Robson, S. (2010a). Speech therapy for children with dysarthria acquired before three years of age (Review). The Cochrane Collaboration and published in the Cochrane Library, 1, 1–19.
- Pennington, L., Miller, N., Robson, S., & Steen, N. (2010b). Intensive speech and language therapy for older children with cerebral palsy: A systems approach. Developmental Medicine and Child Neurology, 52, 337–344.
- Pennington, L., Roelant, E., Thompson, V., Robson, S., Steen, N., & Miller, N. (2013). Intensive dysarthria therapy for younger children with cerebral palsy. Developmental Medicine and Child Neurology, 55, 464–471.
- Petzinger, G. M., Fisher, B. E., McEwen, S., Beeler, J. A., Walsh, J. P., & Jakowec, M. W. (2013). Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurology, 12, 716–726.
- Ramig, L., Sapir, S., Fox, C., & Countryman, S. (2001b). Changes in vocal intensity following intensive voice treatment (LSVT®) in individuals with Parkinson disease: A comparison with untreated patients and normal age-matched controls. Movement Disorders, 16, 79–83.
- Ramig, L. O., Sapir, S., Countryman, S., Pawlas, A., O’Brien, C., Hoehn, M., et al. (2001a). Intensive voice treatment (LSVT) for patients with Parkinson's disease: A 2 year follow up. Journal of Neurology, Neurosurgery, and Psychiatry, 71, 493–498.
- Robey, R. R. (2004). A five-phase model for clinical-outcome research. Journal of Communication Disorders, 37, 401–411.
- Sapir, S., Pawlas, A., Ramig, L., Seeley, E., Fox, C., & Corboy, J. (2001). Effects of intensive phonatory-respiratory treatment (LSVT) on voice in individuals with multiple sclerosis. Journal of Medical Speech Language Pathology, 9, 35–45.
- Sapir, S., Ramig, L., & Fox, C. M. (2011). Intensive voice treatment in Parkinson's disease: Lee Silverman Voice Treatment. Expert Review of Neurotherapeutics, 11, 815–830.
- Sapir, S., Spielman, J., Ramig, L. O., Hinds, S. L., Countryman, S., Fox, C., et al. (2003). Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on ataxic dysarthria: A case study. American Journal of Speech-Language Pathology, 12, 387–399.
- Schertz, M., & Gordon, A. M. (2008). Changing the model: A call for a re-examination of intervention approaches and translational research in children with developmental disabilities. Developmental Medicine and Child Neurology, 51, 6–7.
- Smith, M., Ramig, L. O., Dromey, D., Perez, K., & Samandari, R. (1995). Intensive voice treatment in Parkinson's disease: Laryngostroboscopic findings. Journal of Voice, 9, 453–459.
- Solomon, N. P., & Charron, S. (1998). Speech breathing in able-bodied children and children with cerebral palsy: A review of the literature and implications for clinical intervention. American Journal of Speech-Language Pathology, 7, 61–78.
- Strand, E. A. (1995). Treatment of motor speech disorders in children. Seminars in Speech and Language, 16, 126–130.
- Valvano, J., & Newell, K. M. (1998). Practice of a precision isometric grip-force task by children with spastic cerebral palsy. Developmental Medicine & Child Neurology, 40, 464–473.
- Watson, P. J., & Workman, E. A. (1981). The non-concurrent multiple baseline across-individuals design: An extension of the traditional multiple baseline design. Journal of Behavioral Therapy and Experimental Psychiatry, 12, 257–259.
- Wohlert, A., & Hammen, V. (2000). Lip muscle activity related to speech rate and loudness. Journal of Speech, Language, and Hearing Research, 43, 1229–1239.
- Workinger, M. S., & Kent, R. D. (1991). Perceptual analysis of the dysarthrias in children with athetoid and spastic cerebral palsy. In C. A. Moore, K. M. Yorkston, and D. R. Beukelman (Eds.), Dysarthria and Apraxia of Speech (pp. 109–126). Baltimore, MD: Paul H. Brookes Publishing Co.
- Young, L. H., Zajac, D. J., Mayo, R., & Hooper, C. R. (2001). Effects of vowel height and vocal intensity on anticipatory nasal airflow in individuals with normal speech. Journal of Speech-Language-Hearing Research, 44, 52–60.
- Yorkston, K. M., Beukelman, D. R., Strand, E. A., & Bell, K. R. (1999). Management of Motor Speech Disorders in Children and Adults. Austin, TX: Pro-ed.

