Abstract
The effectiveness and safety of tamsulosin and terazosin for patients with benign prostatic hyperplasia (BPH) was evaluated by literature review. PubMed, Embase, the Cochrane Library, Chinese biomedicine literature database (CBM), reference lists of reports, and reviews were searched for randomized controlled trials (RCTs), or quasi-RCTs of tamsulosin versus terazosin in BPH. Twelve studies involving 2,816 men were included. Outcomes included international prostate symptom score (IPSS), quality of life (QOL), maximum urinary flow rate (Qmax), average urinary flow rate (Qave), residual volume, prostate volume, and adverse effect (dizziness, severe hypotension, dry mouth). Relative risk was calculated for dichotomous data. Sensitivity analyses assessed the influence of baseline symptom severity. We found that tamsulosin is better than terazosin when assessed by IPSS (weighted mean difference (WMD)=−1.24 95% CI [− 1.98, −0.51], there was no significant difference between the two groups in QOL (WMD=0.04 95% CI [−0.16, 0.24]), Qmax (WMD=−0.38 95% CI [−1.18, 0.41]), Qave (WMD=−0.39 95% CI [− 0.84, 0.06]), residual volume (WMD=−4.32 95% CI [−10.96, 2.33]), and prostate volume (WMD=−0.28 95% CI [− 3.37, 2.81]). Fewer patients receiving tamsulosin experienced dizziness (relative risk (RR) −0.38 95% CI [0.30, 0.48]), severe hypotension (RR=0.16 95% CI [0.04, 0.68]), and dry mouth (RR=0.14 95% CI [0.03, 0.77]), compared with patients receiving terazosin. Many of the high quality RCTs showed beneficial effects of tamsulosin in terms of improving IPSS. However, whether tamsulosin proves more efficacious than terazosin in long term therapy requires confirmation by additional large sample, high quality trials.
| Abbreviations | ||
| BPH: | = | benign prostatic hyperplasia |
| CBM: | = | Chinese biomedicine literature database |
| RCTs: | = | randomized controlled trials |
| IPSS: | = | international prostate symptom score |
| QOL: | = | quality of life |
| Qmax: | = | maximum urinary flow rate |
| Qave: | = | average urinary flow rate |
| WMD: | = | weighted mean difference |
| CI: | = | confidence interval |
| RR: | = | relative risk |
| TUR: | = | transurethral resection |
| LUTS: | = | lower urinary tract symptoms |
INTRODUCTION
Benign prostatic hyperplasia (BPH) describes the histological basis of a diagnosis of prostatic enlargement leading to a bladder outflow obstruction that presents as a lower urinary tract obstruction [Bosch et al. Citation1995]. Benign growth of the prostate gland is accompanied by a significant increase in the rate of proliferation of epithelial cells in the hyperplastic acini [Barry et al. Citation1998]. Clinically, it is characterized by lower urinary tract symptoms (urinary frequency, urgency, a weak and intermittent stream, needing to strain, a sense of incomplete emptying, and nocturia), and can lead to complications, including acute urinary retention.
BPH is a chronic condition that increases in both incidence and prevalence with age. It is associated with progressive lower urinary tract symptoms and affects nearly three out of four men during the seventh decade of life. It was estimated that by 2006, approximately 115 million men in the 50 plus age bracket would suffer from BPH. As an age-related disease, the prevalence of BPH is likely to increase substantially during this century. Although not life-threatening, treatment costs are projected to rise to nearly 10 billion dollars and options have been presented [Ravish et al. Citation2007]. The symptomatic mechanisms and complications of BPH remain unclear, although obstruction of the bladder outlet is an important factor [Oishi et al. Citation1998]. The best documented risk factors are increasing age and functioning testes [Jacobsen et al. Citation1996]. Community and practice based studies suggest that men with lower urinary tract symptoms can expect a slow progression of the increased severity of symptoms [Barry et al. Citation1997]. However, symptoms can wax and wane without treatment. In men with symptoms of BPH, rates of acute urinary retention range from 1–2% a year [Jacobsen et al. Citation1997].
A greater awareness of BPH among patients and healthcare professionals is likely to increase the number of people presenting for treatment. As life-expectancy increases an effective treatment for BPH is required. The standard treatment for symptomatic BPH remains transurethral resection (TUR), but carries risks of morbidity and mortality, particularly in elderly men. Moreover, most patients with BPH reportedly choose less aggressive interventions than TUR, rather than just watchful waiting [McConnell et al. Citation1998]. Drug therapy represents an important alternative to TUR for patients with mild-to-moderate symptoms or for those who are unable or unwilling to undergo surgery because of the risk of both morbidity and mortality. Recently, several investigations have shown increased numbers of α1-adrenergic receptors in hypertrophic prostatic tissue than in normal tissue [Kyprianou et al. Citation1996]. The use of α 1-adrenoreceptor antagonists to relax the smooth muscles of the bladder neck and prostate to decrease bladder outlet resistance and facilitate urinary flow without affecting detrusor smooth muscle contractility is a reasonably well established treatment modality for symptomatic BPH [Nordling and Hald Citation1997]. The high incidence of adverse reactions (particularly orthostatic hypotension) associated with the first-generation nonselective α 1/ α 2-antagonists, e.g. phenoxybenzamine, often lead to cessation of treatment [Kirby et al. Citation1987]. Short acting α 1-selective adrenoceptor antagonists, e.g. prazosin and alfuzosin, were then introduced [Lepor et al. Citation1992]. The preference has been for longer acting and more uroselective α 1-adrenoceptor antagonists [Brawer et al. Citation1993]. Prazosin [Roehrborn et al. Citation1995], terazosin [Chapple et al. Citation1994], doxazosin [Jardin et al. Citation1994], and alfuzosin [Kunisawa et al. Citation1985], α 1-adrenoreceptor antagonists have been used to treat BPH and increase urinary outflow and lessen symptoms. However, their use is associated with adverse effects that included dizziness, severe hypotension and dry mouth. Dose titration is used to reduce these adverse effects, but approximately 10% of patients discontinue medication because of adverse reactions [Yamada et al. Citation1994].
Recent pharmacological studies of the α 1-adreno-receptor have revealed three subtypes, α 1A, α 1B and α 1D [Lepor Citation1990]. The α 1A-adrenoreceptor subtype is present in the greatest concentration in human prostate and is responsible for prostate smooth muscle contraction [Hieble et al. Citation1985]. By contrast, the α 1B-adrenoreceptor subtype is involved in smooth muscle contraction of large human arteries [Ford et al. Citation1994]. Therefore, a drug with a relatively high affinity particularly for the α 1A-adrenoreceptor could be prostate specific. Tamsulosin is a new selective α 1A-adrenoreceptor subtype antagonist; it has been reported that the affinity of tamsulosin for α 1A-receptors is seven to 38 times greater than that for α 1B-receptors [Hieble et al. Citation1995]. Further, patients treated with tamsulosin rarely experience complications such as dizziness, severe hypotension and dry mouth. Therefore, a systematic literature review was carried out to determine the safety and efficiency of tamsulosin versus terazosin for patients with BPH.
RESULTS AND DISCUSSION
We identified 68 potentially eligible trials and subsequently excluded 56 trials. This identified 12 randomized controlled trials totaling 2,816 patients. The patient characteristic baselines were comparable as shown in . [Suzuki et al. Citation2001; Okada et al. Citation2000; Na et al. Citation1998; Lee and Lee Citation1997; Narayan et al. Citation2005; Tsujii Citation2000; Zhong et al. Citation2000; Hou et al. Citation1999; Jing Citation1999; Xu et al. Citation2000; Na et al. Citation1996; Lu Citation1998]. These 12 trials all reported adverse effects including dizziness, severe hypotension, dry mouth. Similarity of the comparator groups at baseline was ensured by stratified randomization based on age at entry and severity of disease.
TABLE 1 Quality Assessment and Characteristics of Included Studies.
Meta-Analysis
Efficacy
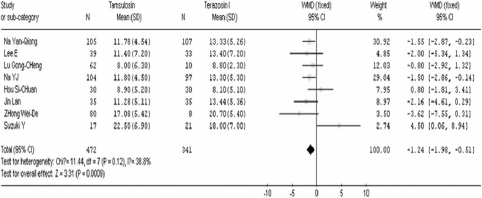
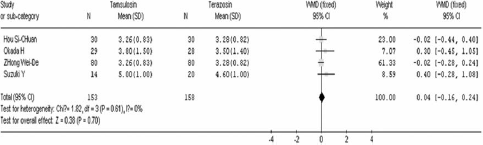
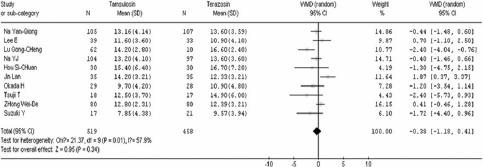
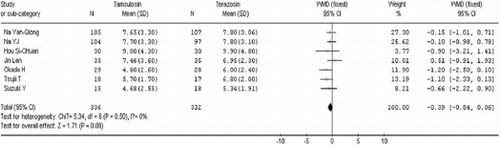
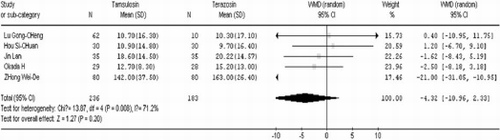
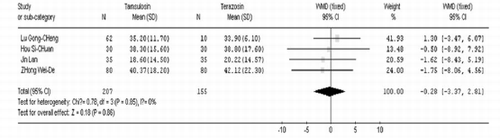
After 4 weeks of treatment, tamsulosin demonstrated a significant advantage when compared to terazosin in terms of IPSS (WMD=−1.24 95% CI [− 1.98, −0.51]. In contrast there was no significant difference between the two groups in QOL (WMD=0.04 95% CI [− 0.16, 0.24]), Qmax (WMD=−0.38 95% CI [−1.18, 0.41]), Qave (WMD=−0.39 95% CI [− 0.84, 0.06]), residual volume (WMD=−4.32 95% CI [−10.96, 2.33]), and prostate volume (WMD=−0.28 95% CI [− 3.37, 2.81]). These results are shown in .
Adverse Effects
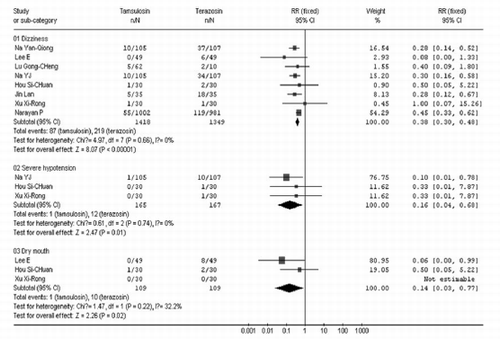
As shown in , fewer patients receiving tamsulosin experienced dizziness (RR=0.38 95% CI [0.30, 0.48]), severe hypotension (RR=0.16 95% CI [0.04, 0.68]), and dry mouth (RR=0.14 95% CI [0.03, 0.77]), compared with patients receiving terazosin. A random effects model was used because the residual volume was heterogeneous among the trials (P=0.008 <0.1, I2=71.2% > 50%). There was no significant difference between the two groups (WMD=−4.32 95% CI [—10.96, 2.33]) as shown in . Studies from both developing as well as developed countries. Sixty-eight prospective controlled trials related to the question of the safety and efficiency of tamsulosin versus terazosin for benign prostatic hyperplasia were identified. However, of these, only twelve randomized studies satisfied the inclusion criteria. The twelve studies varied in size of the study population from 35 to 1,983 patients, with a total of 2,816 subjects.
The studies identified in this systematic review were of varied quality. Two were multi-center randomized controlled trials. Nine of the twelve included trials were of relative higher quality (‘B’) and three trials were of low quality (‘C’).
All of the studies included adequate randomization yet only Lee and Lee [1997] referred to double blinding. Thus, high performance bias and measuring bias may be confounding factors. All of the studies indicated dropouts, yet an intention-to-treat analysis was omitted.
Of the 12 studies included, only four indicated allocated concealment. Selective bias could be introduced if the recruiter allocation assignment is modified. The dose-effect and time-effect relationship may not be clear. Together, along with the small number of participants in most studies as well as their quality, a robust conclusion may not be drawn. Further studies that consider these apparent caveats are warranted.
As α1-adrenergic receptor subtypes are also found in the human vas deferens and prostate gland, activation of these receptors also play a key role in the control of both the emission and expulsion phases of ejaculation. Theoretically, impairment results in ejaculatory dysfunction [Grasso et al. Citation2006]. Tamsulosin is associated with ejaculatory dysfunction, although this is possibly a central effect. In comparison the incidence of ejaculatory dysfunction or erectile dysfunction with the other a-blockers appears negligible [McVary Citation2005]. Future systematic review should adhere to recommendations designed to enhance the conduct, reporting and evaluation of retroactive ejaculation, and erectile function/ potency of these therapeutics.
Targeting α 1-adrenoceptors that maintain vascular tone, may affect the reduction of blood pressure, orthostasis, asthenia and light-headedness. The lack of need for initial dose titration of tamsulosin to limit these symptoms may be clinically advantageous compared with terazosin. Confirmation of the efficacy and dose of tamsulosin and that it is well tolerated in a more extensive large sample multi-center randomized controlled trial may show that selective α 1A-adrenoceptor antagonists provide suitable alternatives in α 1-adrenoceptor antagonists therapy. This alternative may be well-suited for the treatment of patients with mild to moderate symptomatic BPH, and in those awaiting surgery or unable to undergo surgery.
METHODS
Search Strategy
Pubmed (1966–2007), Embase (1974–2007), the Cochrane Library (2007 issue 4), Chinese biomedicine literature database (1978–2007), Chinese technological periodical full-text database (1989–2007.9), and Chinese periodical full-text database (1994–2007.9) were searched for randomized controlled trials comparing tamsulosin with terazosin. We manually searched key Chinese periodicals using search engines such as Google™ to search related references and references included in the studies. Hand searching of the reference lists of included studies and reviews was undertaken and contact was made with experts in the field; unpublished studies were not sought. No limits based on language were imposed.
The results of the above search strategy were independently screened by two reviewers, to confirm fulfillment of inclusion criteria and discard studies that were not applicable. Disagreements were resolved in consultation with a third expert party.
Inclusion Criteria
Inclusion criteria included randomized controlled trials with patients having mild to moderate benign prostatic hyperplasia with lower urinary tract symptoms (LUTS). Benign prostatic hyperplasia was diagnosed from the history, symptoms and physical examination where heart, liver and kidney function were normal. They could not have an indication for prostatectomy based on their clinical findings.
Exclusion Criteria
Patients with BPH who underwent prior prostatectomy, thermotherapy, anti-androgen therapy and catheterization because of urinary retention, those with other lower urinary tract disorders including prostatic cancer, neurogenic bladder, bladder stone and lower urinary tract infection, or those having cardiac, renal or hepatic insufficiency or dementia were excluded. Patients were not permitted to take other medications that could influence the outcome of the study, such as α /β-adrenoceptor agonists and antagonists, anticholinergics, antiandrogens, and steroid 5-alpha-reductase inhibitor.
Outcome Measures
Outcomes included international prostate symptom score (IPSS), quality of life (QOL), maximum urinary flow rate (Qmax), average urinary flow rate (Qave), residual volume, prostate volume, and adverse effect (dizziness, severe hypotension, dry mouth).
Statistical Analysis
The data was analyzed using Review Manager (version 4.2.10) and extracted and pooled data for summary estimates. The results were expressed for dichotomous outcomes (e.g., dizziness, severe hypotension, dry mouth) as relative risk (RR) with 95% confidence intervals (CIs) and continuous outcomes as weighted mean difference or standard mean difference (e.g., IPSS, QOL, Qmax, Qave, residual volume, prostate volume). The χ2 statistic was used to assess heterogeneity between trials and the I2 statistic to assess the extent of inconsistency. A fixed effect model was used to calculate summary estimates and their corresponding 95% CI. If there was significant heterogeneity, the results were confirmed using a random effects statistical model. Subgroup analyses were intended to explore important clinical differences that may be expected to alter the magnitude of treatment effect. Adverse effects were tabulated and assessed with descriptive techniques, because they were likely to be different for the various agents used.
ACKNOWLEDGMENTS
We thank Professor KeHu Yang, the Centre of Evidence Based Medicine, Lanzhou University, for his statistical consultation, and all those who willingly participated in this study.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Barry, M. J., Adolfsson, J. and Batista, J. E. (1998) Committee 6: measuring the symptoms and health impact of benign prostatic hyperplasia and its treatments. Health Publication:265–321.
- Barry, M. J., Fowler, F. J., Bin, L., Pitts, J. C., Harris, C. J. and Mulley, A. G. (1997) The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol 157:10–15.
- Bosch, J. L., Hop, W. C., Kirkels, W. J. and Schroder, F. H. (1995) Natural history of benign prostatic hyperplasia: appropriate case definition and estimation of its prevalence in the community. Urology 46(Suppl A):34–40.
- Brawer, M. K., Adams, G. and Epstein, H. (1993) Terazosin in the treatment of benign prostatic hyperplasia. Arch Fam Med 2:929–935.
- Chapple, C. R., Carter, P. and Christmas, T. J. (1994) A three month double-blind placebo controlled study of doxazosin as treatment for benign prostatic bladder outflow obstruction. Br J Urol 74:50–56.
- Ford, A. P., Williams, T. J., Blue, D. R. and Clarke, D. E. (1994) a1-adrenoreceptor classification: sharpening Occam's razor. Trends Pharmacol Sci 15:167–170.
- Grasso, M., Fortuna, F., Lania, C. and Blanco, S. (2006) Ejaculatory disorders and α1-adrenoceptor antagonists therapy: clinical and experimental researches. J Transl Med 4:31.
- Hieble, J. P., Bylund, D. B. and Clarke, D. E. (1995) Recommendation for nomenclature of αα 1-adrenoreceptor: Consensus update. Pharmacol Rev 47:267–270.
- Hieble, J. P., Caine, M. and Zalaznik, E. (1985) In vitro characterization of the a-adrenoreceptors in human prostate. Eur J Pharmacol 107:111–117.
- Hou, S. C., Dong, S. G. and Wang, X. S. (1999) Clinical study of Tamsulosin treatment of benign prostatic hyperplasia. J Modern Urol 4:254–259.
- Jacobsen, S. J., Girman, C. J., Guess, H. A., Rhodes, T., Oesterling, J. E. and Lieber, M. M. (1996) Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 155:595–600.
- Jacobsen, S., Jacobson, D. and Girman, C. (1997) Natural history of prostatism: risk factors for acute urinary retention. J Urol 158:4817.
- Jardin, A., Bensadoun, H. and Delauche-Cavallier, M. C. (1994) Long term treatment of benign prostatic hyperplasia with alfuzosin: a 24–30 month survey. Br J Urol 74:579–584.
- Jing, L. (1999) Comparison effectiveness two selective a-blocker receptor blocker treatment of benign prostatic hyperplasia. Journal of Jiangsu Pharmacy 7(1):312–316.
- Kirby, R. S., Coppinger, S. W. C., Corcoran, M. O., Chapple, C. R., Flannagan, M. and Milroy, E. J. G. (1987) Prazosin in the treatment of prostatic obstruction: a placebo-control study. Br J Urol 60:136–142.
- Kunisawa, Y., Kawabe, K., Niijima, T., Honda, K. and Takenaka, T. (1985) A pharmacological study of a-adrenergic receptor subtypes in smooth muscle of human urinary bladder and prostatic urethra. J Urol 134:396–398.
- Kyprianou, N., Tu, H. and Jacobs, S. C. (1996) Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum. Pathol 27:668–675.
- Lee, E. and Lee, C. (1997) Clinical comparisons of selective and non-selective α 1A-adrenoreceptor antagonists in benign prostatic hyper-plasia: studies on tamsulosin in a fixed dose and terazosin in increasing doses. Br J Urol 80(4):606–611.
- Lepor, H. (1990) Role of a1-adrenergic blockers in the treatment of benign prostatic hyperplasia. Prostate 3(Suppl):75–84.
- Lepor, H., Auerbach, S. and Puras-Baez, A. (1992) A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 148:1467–1474.
- Lu, G. C. (1998) Preliminary experience of Tamsulosin treatment of benign prostatic hyperplasia. Journal of Clinical Urology 13(1):46–47.
- McConnell, J., Bruskewitz, R. and Walsh, P. (1998) The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med 338:557–563.
- McVary, K. T. (2005) Sexual function and a-blockers. Rev Urol 7(Suppl. 8):S3–S11.
- Narayan, P. and Lepor, H. (2001) Long-term, open-label, phase III multicenter study of tamsulosin in benign prostatic hyperplasia. Urology 57:466–470.
- Narayan, P., O'Leary, M. P. and Davidai, G. (2005) Early efficacy of tamsulosin versus terazosin in the treatment of men with benign prostatic hyperplasia: a randomized, open-label trial. The Journal of Applied Research 5:237–245.
- Na, Y. J., Guo, Y. L. and Gu, F. L. (1998) Clinical comparisons of selective and non-selective α 1A-adrenoceptor antagonists for bladder outlet obstruction associated with benign prostatic hyperplasia: studies ontamsulosin and terazosin in Chinese patients. The Chinese Tamsulosin Study Group. J Med 29(5–6):289–304.
- Na, Y. Q., Wang, D. and Jing, J. (1996) Selective a-blocker receptor blocker treatment of benign prostatic hyperplasia. Journal of Chinese Urology 6(1 7):327–329.
- Nordling, J. and Hald, T. (1997) BPH versus LUTS: reflections on lower urinary tract symptoms in search of a definition of clinical benign prostatic hyperplasia. European Urology Update Series 6:54–60.
- Oishi, K., Boyle, P. and Barry, M. J. (1998) Committee 1: epidemiology and natural history of benign prostatic hyperplasia. Health Publication: 23–59.
- Okada, H., Kamidono, S., Yoshioka, T., Okuyama, A., Ozono, S., Hirao, Y., Okajima, E., Yamamoto, K., Kishimoto, T., Park, Y. and Kurita, T. (2000) A comparative study of terazosin and tamsulosin for symptomatic benign prostatic hyperplasia in Japanese patients. BJU Int 85(6):676–681.
- Ravish, I. R., Nerli, R. B. and Amarkhed, S. S. (2007) Finasteride to evaluate the efficacy of dutasteride in the management of patients with lower urinary tract symptoms and enlarged prostate. Arch Androl 53:17–20.
- Roehrborn, C. G., Oesterling, J. E. and Arbor, A. (1995) Hytrin community assessment trial (HYCAT): evaluation of the clinical effectiveness of terazosin versus placebo in the treatment of patients with symptomatic benign prostatic hyperplasia. J Urol 153(Suppl):272 abstr 175.
- Suzuki, Y., Katoh, T., Isurugi, K., Obara, W., Omori, S., Goto, Y., Fujioka, T. and Numasato, S. (2001) The efficacy and safety of terazosin and tamsulosin in patients with urinary disturbance accompanying prostatic hypertrophy. Hinyokika Kiyo 47(1):15–21.
- Tsujii, T. (2000) Comparison of prazosin, terazosin and tamsulosin in the treatment of symptomatic benign prostatic hyperplasia: a short-term open, randomized multicenter study. International Journal of Urology 7:199–205.
- Xu, X. R., Zhong, W. D. and Wang, L. S. (2000) Effectiveness analysis tamsulosin of and terazosin treatment of benign prostatic hyper-plasia. Journal of Practical Medicine 16(10):816–817.
- Yamada, S., Tanaka, C., Kimura, R. and Kawabe, K. (1994) a1-adrenoreceptors in human prostate: characterization and binding characteristics of alpha-1-antagonists. Life Sci 54:1845–1854.
- Zhong, W. D., Cai, Y. B. and Hu, J. B. (2000) Efficacy-cost analysis of a1-blocker receptor blocker treatment of benign prostatic hyperplasia. Academic Journal of Guangdong College of Pharmacy 6:123–128.