Abstract
Genomic disorders are defined as diseases caused by rearrangements of the genome incited by a genomic architecture that conveys instability. Y-chromosome related dysfunctions such as male infertility are frequently associated with gross DNA rearrangements resulting from its peculiar genomic architecture. The Y-chromosome has evolved into a highly specialized chromosome to perform male functions, mainly spermatogenesis. Direct and inverted repeats, some of them palindromes with highly identical nucleotide sequences that can form DNA cruciform structures, characterize the genomic structure of the Y-chromosome long arm. Some particular Y chromosome genomic deletions can cause spermatogenic failure likely because of removal of one or more transcriptional units with a potential role in spermatogenesis. We describe mechanisms underlying the formation of human genomic rearrangements on autosomes and review Y-chromosome deletions associated with male infertility.
Introduction
Genomic disorders are diseases due to DNA rearrangement rather than a point mutation; i.e., a copy number variant (CNV) due to deletion or duplication rather than single nucleotide variant (SNV). The genomic rearrangement results from genomic instability incited by the local genomic architecture [Lupski Citation1998; 2009; Stankiewicz and Lupski Citation2002]. The concept of genomic disorders was delineated from experimental observations on two autosomal dominant neuropathies and two chromosomal microdeletion/microduplication syndromes: Charcot-Marie-Tooth disease type 1A (CMT1A; MIM 118220) [Lupski et al. Citation1991] and hereditary neuropathy with liability to pressure palsies (HNPP; MIM 162500) [Chance et al. Citation1993], and Smith-Magenis microdeletion syndrome (SMS; MIM 182290) and Potocki-Lupski microduplication syndrome (PTLS; MIM 610883). These diseases arise from genomic rearrangements in the proximal short arm of chromosome 17p; submicroscopic duplication of a 1.4 Mb genomic interval causes CMT1A whereas the reciprocal 1.4 Mb deletion causes HNPP; a 3.7 Mb cytogenetically visible deletion causes SMS and its reciprocal duplication causes PTLS [Bi et al. Citation2003; Chen et al. Citation1997; Potocki et al. Citation2007; Citation2000]. Genomic disorders can be either inherited or sporadic, depending on whether the rearrangement is transmitted through the germ line or occurs de novo.
The average estimates for de novo locus specific point mutations is ∼2X10−8 new mutations per locus per haploid genome; contrasting with an average estimate for de novo genomic disorder associated CNV or rearrangements of ∼10−6–10−4. The estimated 100 to 10,000X increased frequencies for de novo CNV versus single nucleotide variant are based on locus-specific data from pooled sperm PCR assays [Turner et al. Citation2008] and prevalence estimates for some particular genomic disorders [Lupski Citation2007]. Given these new mutation rates, de novo CNVs could potentially be responsible for many sporadic traits.
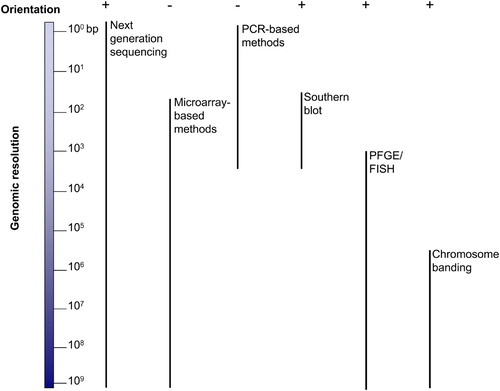
Diverse molecular techniques can be used to detect CNVs (). These methods differ in resolution, cost, and time to execute. They provide limited information regarding either genomic mapping position or orientation; therefore a multitude of techniques might be required to elucidate CNV mechanisms. The technique of choice will mainly depend on the genomic region of interest and the architectural features of that region as well as on the specific questions to be addressed.
Figure 1. Comparative graphic overview of the techniques used to resolve structural changes in the human genome. Left is shown the 3 x 109 bp haploid human genome; right is shown each technique together with a vertical bar representing its genome resolution capability. Top plus and minus represent the ability of each technique to provide orientational information. PFGE: pulsed-field gel electrophoresis; FISH: fluorescent in situ hybridization.
Mechanism for formation of genomic rearrangements
Genomic rearrangements can be generally classified as recurrent and nonrecurrent (), potentially reflecting underlying mechanisms. Recurrent rearrangements include the same genomic size interval, have breakpoints that cluster within low copy repeats (LCRs) that flank the ‘copy number’ altered region, and can have ‘hotspots’ for strand exchange within the LCR [Gu et al. Citation2008]. Nonrecurrent rearrangements are variable in size and genomic content and there is no breakpoint clustering, although breakpoints mapping in proximity to or at the LCRs are frequently found to ‘group’ at one end of the rearrangement. Repetitive sequences, such as short interspersed nuclear elements (SINEs) and long interspersed nuclear elements (LINEs), are often observed at the breakpoints, especially in deletion cases; the biological significance of that association is not yet clear [Vissers et al. Citation2009]. Breakpoint junctions for nonrecurrent rearrangements are often notable for microhomology [Conrad et al. Citation2010; Lee et al. Citation2007].
Figure 2. Representational diagram of three types of rearrangements causative for genomic disorders. Arrows (light and dark orange plus brown) represent low copy repeats (LCRs) and light blue rectangles represent a gene or genes spanning the region with altered copy number. Red horizontal bars: duplications (dup); green horizontal bars: deletion; dark blue horizontal bars triplications (trip). Recurrent rearrangements: have the same genomic size and content with breakpoints that cluster within LCRs flanking the altered region. Nonrecurrent rearrangements: are variable in size and genomic content and there is no breakpoint clustering. Nonrecurrent rearrangements with grouping: LCRs mapping close or at the breakpoints are frequently found to ‘group’ at one end. At least three different nonrecurrent rearrangements with grouping have been reported in the literature: i) associated with duplication formation; ii) associated with triplication embedded in duplications and, iii) associated with isodicentric chromosome formation. The two latter examples have been consistently associated with inverted repeats or palindromes [Carvalho et al. Citation2009; Lange et al. Citation2009].
![Figure 2. Representational diagram of three types of rearrangements causative for genomic disorders. Arrows (light and dark orange plus brown) represent low copy repeats (LCRs) and light blue rectangles represent a gene or genes spanning the region with altered copy number. Red horizontal bars: duplications (dup); green horizontal bars: deletion; dark blue horizontal bars triplications (trip). Recurrent rearrangements: have the same genomic size and content with breakpoints that cluster within LCRs flanking the altered region. Nonrecurrent rearrangements: are variable in size and genomic content and there is no breakpoint clustering. Nonrecurrent rearrangements with grouping: LCRs mapping close or at the breakpoints are frequently found to ‘group’ at one end. At least three different nonrecurrent rearrangements with grouping have been reported in the literature: i) associated with duplication formation; ii) associated with triplication embedded in duplications and, iii) associated with isodicentric chromosome formation. The two latter examples have been consistently associated with inverted repeats or palindromes [Carvalho et al. Citation2009; Lange et al. Citation2009].](/cms/asset/f914f037-5820-4183-a241-a6c8804fb6ea/iaan_a_527427_f0002_b.jpg)
Recurrent rearrangements are usually generated by non-allelic homologous recombination (NAHR; ) [Stankiewicz and Lupski Citation2002] between non-allelic but paralogous LCRs. LCRs, also called segmental duplications (SDs) [Bailey et al. Citation2002], are duplications or higher number multimer copies (e.g., 3–10) of ∼10–400 kb in length and ≥ 97% sequence identity. The LCRs likely arose by duplication of genomic segments resulting in paralogous regions [Stankiewicz and Lupski Citation2002]. SINEs and LINEs repetitive elements can also be substrates for NAHR [Shaw and Lupski Citation2004]; usually occurring between highly identical family members that can act as homologous recombination (HR) substrates. NAHR results in loss, gain, or inversion of the segment between LCRs; gene conversion can also accompany the process (; reviewed in [Gu et al. Citation2008]). Thus, the instability of the human genome can be caused by the ubiquitous presence of LCRs and repetitive sequences; i.e., local genome architecture.
Figure 3. Three main mechanisms that account for structural variations observed in association with human genomic disorders. (a) NAHR: non-allelic homologous recombination. This repair mechanism of double-strand breaks occurs by unequal crossing over using paralogous repeats (LCRs) as substrate rather than allelic homologous sequences. The resulting product will ultimately depend on the direction of the repeats involved as well as if the rearrangement occurs via an intrachromatidal, interchromatidal, or interchromosomal event (for an extensive review see [Stankiewicz and Lupski Citation2002]). This figure represents NAHR between two LCRs in the same orientation producing duplication (dup) and deletion (del) of the sequence between the repeats. Intrachromatid NAHR between inverted repeats will produce either inversion of the segment in between the LCRs or gene conversion; interchromatid NAHR (between sister chromatids) will produce either an isodicentric (idic) chromosome plus an acentric fragment or gene conversion [Lange et al. Citation2009]. (b) Non-homologous end joining (NHEJ) repair mechanism is used by eukaryotic cells to repair double-strand breaks. The NHEJ process includes detection of DSBs, molecular bridging of both broken DNA ends; modification of the ends to make them compatible and ligatable, and the final ligation step. If this latter step is not accurate it leaves an ‘information scar’ at the rejoining site as the pre-rejoining editing of the ends includes cleavage or addition of nucleotides [Gu et al. Citation2008; Hastings et al. Citation2009b]. Two rearrangements can be produced by NHEJ after two DSB events: an intrachromosomal deletion and an interchromosomal duplication. (c) Microhomology-mediated break-induced replication (MMBIR): the replication fork collapses because the fork encounters a nick on a template strand. The 5′ end of the broken molecule is recessed from the break, exposing a 3′ tail that may anneal to any single-strand DNA that shares microhomology resuming the replication by priming on another template (a nearby fork might provide the necessary ssDNA). The initial polymerase extension and replication is performed by a low processivity polymerase which may allow multiple rounds, or iterations, of disengagement and template switching until a fully processive replication is established to the end of the chromosome or replicon. As a result, complexities at the breakpoint junctions are predicted as well as rearrangements such as deletions, duplications, inversions, or chromosomal translocations depending on the genomic localization of the reinitiating fork. Absence of heterozygosity downstream of the event is predicted if the switching event occurred between homologous chromosomes rather than between sister chromatids [Hastings et al. Citation2009b].
![Figure 3. Three main mechanisms that account for structural variations observed in association with human genomic disorders. (a) NAHR: non-allelic homologous recombination. This repair mechanism of double-strand breaks occurs by unequal crossing over using paralogous repeats (LCRs) as substrate rather than allelic homologous sequences. The resulting product will ultimately depend on the direction of the repeats involved as well as if the rearrangement occurs via an intrachromatidal, interchromatidal, or interchromosomal event (for an extensive review see [Stankiewicz and Lupski Citation2002]). This figure represents NAHR between two LCRs in the same orientation producing duplication (dup) and deletion (del) of the sequence between the repeats. Intrachromatid NAHR between inverted repeats will produce either inversion of the segment in between the LCRs or gene conversion; interchromatid NAHR (between sister chromatids) will produce either an isodicentric (idic) chromosome plus an acentric fragment or gene conversion [Lange et al. Citation2009]. (b) Non-homologous end joining (NHEJ) repair mechanism is used by eukaryotic cells to repair double-strand breaks. The NHEJ process includes detection of DSBs, molecular bridging of both broken DNA ends; modification of the ends to make them compatible and ligatable, and the final ligation step. If this latter step is not accurate it leaves an ‘information scar’ at the rejoining site as the pre-rejoining editing of the ends includes cleavage or addition of nucleotides [Gu et al. Citation2008; Hastings et al. Citation2009b]. Two rearrangements can be produced by NHEJ after two DSB events: an intrachromosomal deletion and an interchromosomal duplication. (c) Microhomology-mediated break-induced replication (MMBIR): the replication fork collapses because the fork encounters a nick on a template strand. The 5′ end of the broken molecule is recessed from the break, exposing a 3′ tail that may anneal to any single-strand DNA that shares microhomology resuming the replication by priming on another template (a nearby fork might provide the necessary ssDNA). The initial polymerase extension and replication is performed by a low processivity polymerase which may allow multiple rounds, or iterations, of disengagement and template switching until a fully processive replication is established to the end of the chromosome or replicon. As a result, complexities at the breakpoint junctions are predicted as well as rearrangements such as deletions, duplications, inversions, or chromosomal translocations depending on the genomic localization of the reinitiating fork. Absence of heterozygosity downstream of the event is predicted if the switching event occurred between homologous chromosomes rather than between sister chromatids [Hastings et al. Citation2009b].](/cms/asset/b66985c6-5160-4898-ad1c-4edb8641709b/iaan_a_527427_f0003_b.jpg)
Particular features of LCRs such as size, percent DNA sequence identity, and orientation and distance between reaction/mechanism substrates are important factors influencing the ‘likelihood’ of rearrangement or instability. For example, longer LCRs seem to be able to produce larger rearrangements [Lupski Citation1998]. NAHR also seems to require a minimal size of uninterrupted DNA sequence identity, that is, a minimal efficient processing segment (MEPS) for the recombination to occur, likely representing the minimal homology requirement for HR. Experimental data suggest that MEPS in human meiosis requires between 300-500 bp in length [Gu et al. Citation2008; Stankiewicz and Lupski Citation2002], but NAHR events between human alpha-globin genes are mediated by matching fragments smaller than 50 bp in length; however these are associated with smaller sized genomic rearrangements [Lam and Jeffreys Citation2006].
Data analysis of the breakpoint sequences of the genomic disorders involving 17p12–17p11.2 documented the existence of a positional preference for strand exchange or recombination hotspots for the occurrence of the crossovers within the LCRs [Bi et al. Citation2003; Lupski Citation2004; Reiter et al. Citation1996]. Recently, an HR ‘hotspot motif’ was proposed in humans, which perhaps not surprisingly is coincident with breakpoints of rearrangements generated by NAHR, suggesting that allelic homologous recombination (AHR) hotspots can coincide with NAHR hotspots [Lindsay et al. Citation2006; Myers et al. Citation2008; Zhang et al. Citation2010a]. NAHR can be interchromosomal, intrachromosomal, or intrachromatidal depending on the location of the LCRs utilized as substrates in a particular rearrangement; interchromosomal and intrachromosomal NAHR can produce deletions and duplications whereas intrachromatidal NAHR can only produce deletions [Stankiewicz and Lupski Citation2002]. Additionally, the outcome or product resulting in duplication, deletion, inversion, or translocations, will also depend on the orientation of the LCRs [Lupski Citation1998; Ou et al. in press; Stankiewicz and Lupski Citation2002] (). Regarding the male germline, de novo NAHR mediated-recurrent genomic rearrangement deletions occur twice as often as duplication on autosomes; on the Y chromosome, for the one NAHR hotspot locus studied, the ratio of deletions to duplications for de novo events is 4:1 [Turner et al. Citation2008]. At the CMT1A/HNPP locus > 99% of neuropathy associated rearrangements are recurrent and mediated by NAHR [Zhang et al. Citation2009b; Citation2010b]. At the SMS/PTLS locus ∼70% are mediated by NAHR [Zhang et al. Citation2010a].
LCRs can also stimulate rearrangements of flanking genomic regions rather than mediate them [Carvalho et al. Citation2009; Inoue et al. Citation2002; Lee et al. Citation2007; Citation2006; Woodward et al. Citation2005]; although the mechanisms involved are different from that of NAHR. Several groups of investigators [Inoue et al. Citation2002; Lee et al. Citation2007; Citation2006; Woodward et al. 2005] observed a remarkable grouping of one of the breakpoints of the deletions and duplications that encompass the PLP1 gene at chromosome Xq22 and cause Pelizaeus-Merzbacher disease (PMD; MIM 312080). These authors showed that the genomic region surrounding PLP1 is laden with LCRs of different sizes and extents of identity that are also in different orientations; interestingly, most of the breakpoints were mapped within or flanking such LCRs. Carvalho et al. [2009] observed the same phenomena in patients carrying MECP2 duplication and statistically showed a nonrandom distribution of the ‘breakpoint grouping’ at one end that maps within or flanking the adjacent LCRs. This nonrandom association between breakpoint grouping of one end of the nonrecurrent rearrangements and LCR location suggested a possible role for these particular genome architecture features in the mechanism(s) for rearrangement formation. The mechanism(s) causing nonrecurrent rearrangements with grouping warrants further studies, but experimental evidence support a predominant role for replication-based mechanism, such as fork stalling and template switching (FoSTeS) or microhomology-mediated break induced replication (MMBIR), underlying their formation [Carvalho et al. Citation2009; Hastings et al. Citation2009a; Citation2009b; Lee et al. Citation2007].
FoSTeS was proposed to explain the phenomenology observed at the breakpoints of nonrecurrent rearrangements that cause genomic disorders. It proposes stalling of the replication fork, perhaps due to the formation of a secondary structure at the lagging strand template that might release the 3′ end of the recently formed Okazaki fragment. The disengaged 3′ end can switch to another replication fork nearby and prime DNA replication at another fork often resulting in complex rearrangements [Lee et al. Citation2007; Slack et al. Citation2006; Zhang et al. Citation2009a]. MMBIR provides molecular mechanistic details based upon experimental observations in human, bacteria (E. coli) and yeast. MMBIR proposes that a collapsed replication fork resulting in a one ended double-stranded DNA is processed to expose a 3′ end that anneals with another replication fork priming a new synthesis and initiating a break induced replication (BIR) (). Initially, the MMBIR process utilizes a poorly processive DNA polymerase which results in multiple fork collapses and template switching ultimately leading to complex rearrangements [Hastings et al. Citation2009a]. Depending on the relative position of the other replication fork on the chromosome, and the iterative nature of the process, the resulting product can be accompanied by deletions, duplications, inversions, and/or complex rearrangements.
Non-homologous end joining (NHEJ) [Lieber 2008] is another mechanism implicated in the formation of nonrecurrent rearrangements particularly when the breakpoints do not reveal microhomology but instead show small deletions or insertion of random bases at the junctions (). NHEJ is a non-homologous, non-replicative mechanism known to be responsible in somatic cells for generating the antigen-receptor diversity repertoire in the immune system and often responsible for healing double-strand breaks (DSBs) that occur during the DNA life-time [Lieber Citation2008].
Male infertility
Infertility affects approximately 1 in every 10 couples of reproductive age [de Kretser Citation1997]. Male infertility alone can account for at least 20% of cases [de Kretser Citation1997]. There are several etiological factors underlying male infertility; clinically classified as pre-testicular, such as endocrine and coital disorders; testicular, including abnormalities of sperm production; and post-testicular, such as obstructions [de Kretser Citation1997]. Sperm chromosomal alterations are highly prevalent in spermatogenic impairment. The frequency of chromosome abnormalities increases with decreasing sperm count; oligozoospermic males can carry 4.6% of chromosomal aberrations, whereas azoospermic males can have such abnormalities detected in up to 10–15% [Lidegaard et al. Citation1998; Van Assche et al. Citation1996; Yatsenko et al. Citation2010]. The great majority of observed chromosomal abnormalities are numerical or structural involving sex chromosomes [Yatsenko, et al. Citation2010]. Intriguingly, infertile males clinically diagnosed with oligoasthenoteratozoospermia (low sperm count with a high percentage of slow moving and abnormal sperm) and a normal karyotype, have a greater than ten-fold increase of diverse chromosomal abnormalities in their sperm, including diploidy, autosomal disomy, and autosomal nullisomy [Pang et al. Citation1999; Van Assche et al. Citation1996].
Genetic factors that lead to male infertility have been extensively reviewed recently [Ferlin et al. Citation2007b; O'Flynn O'Brien et al. Citation2010; Walsh et al. Citation2009]. More than 2,300 genes may play a role in male infertility [Schultz et al. Citation2003]. Thus far, there are no data reporting CNVs on autosomes as a causative infertility factor [McLachlan and O'Bryan Citation2010]. However, rearrangements within the Y chromosome, particularly on Yq11 are a frequent cause of infertility. Therefore, this review will specifically focus on what we can infer about the mechanisms underlying the formation of infertility associated rearrangements in Yq11.
Evidence for NAHR mediating rearrangements on the Y chromosome leading to male infertility
The association between male infertility and absence of genes that map to the long arm of Y chromosome was established in 1976 [Tiepolo and Zuffardi Citation1976] when 0.5% of idiopathic sub infertile individuals were found to carry cytogenetically visible deletions of the Yq11. The authors termed the missing locus ‘azoospermia factor (AZF)’. Several studies using both karyotyping and molecular techniques confirmed the association between deletions involving the Yq11 region and male infertility [Andersson et al. Citation1988; Chandley et al. Citation1989]. The use of molecular markers such as Y-chromosome locus-specific probes and sequence-tagged sites (STS) enabled the detection of small non-overlapping interstitial deletions culminating with the molecular definition of three AZF regions at Yq11 (AZFa, AZFb, and AZFc) [Ma et al. Citation1992; Reijo et al. Citation1995; Vogt et al. Citation1992; Citation1996] ().
Figure 4. Schematic representation of the Y chromosome. Black horizontal line, male-specific region of the Y chromosome (MSY). The horizontal rectangle represents the Y chromosome with its short (Yp) and long (Yq) arms. In red, pseudoautosomal regions (PAR1 and PAR2); in black, heterochromatic regions; arrows in light blue represent palindromes or inverted repeats. (a) Major deletions observed in infertile males: AZFa, P5/proximal-P1 (AZFb), P5/distal-P1, P4/distal-P1, AZFc. The green horizontal lines (above) delimit the regions according to the reference genome (adapted from [Repping et al. Citation2002; Skaletsky et al. Citation2003]); the purple arrows on top of the green bars represent the orientation and location of the LCRs shown to mediate AZF deletions through NAHR. (b) Other structural variations involving the Y chromosome. In sharp blue, regions that show length variation within worldwide populations; in light purple (below), regions that show any other kind of variation such as inversions (inv), partial deletions(del), and duplications (dup) (adapted from [Repping et al. Citation2006]). Figure not to scale.
![Figure 4. Schematic representation of the Y chromosome. Black horizontal line, male-specific region of the Y chromosome (MSY). The horizontal rectangle represents the Y chromosome with its short (Yp) and long (Yq) arms. In red, pseudoautosomal regions (PAR1 and PAR2); in black, heterochromatic regions; arrows in light blue represent palindromes or inverted repeats. (a) Major deletions observed in infertile males: AZFa, P5/proximal-P1 (AZFb), P5/distal-P1, P4/distal-P1, AZFc. The green horizontal lines (above) delimit the regions according to the reference genome (adapted from [Repping et al. Citation2002; Skaletsky et al. Citation2003]); the purple arrows on top of the green bars represent the orientation and location of the LCRs shown to mediate AZF deletions through NAHR. (b) Other structural variations involving the Y chromosome. In sharp blue, regions that show length variation within worldwide populations; in light purple (below), regions that show any other kind of variation such as inversions (inv), partial deletions(del), and duplications (dup) (adapted from [Repping et al. Citation2006]). Figure not to scale.](/cms/asset/ad58280a-8554-4693-bb9a-646d5f2ebf6f/iaan_a_527427_f0004_b.jpg)
Most frequently, males with Y chromosome microdeletions have non-obstructive idiopathic azoospermia or severe oligozoospermia, but there may be other factors underlying the disease as some rare familial cases are reported in the literature [Gatta et al. Citation2002; Krausz et al. Citation2006; Kuhnert et al. Citation2004; Luddi et al. Citation2009; Reijo et al. Citation1995]. The testicular histology of patients can be quite variable ranging from a rare complete absence of germ cells (Sertoli-cell-only syndrome, SCO), to the presence of cells arrested in different meiotic stages occasionally producing mature sperm [Reijo et al. Citation1995]. Such clinical phenotypic variation renders the genotype-phenotype correlation challenging. Each of these regions carries a number of male infertility candidate genes but, with the exception of AZFa, no point mutations leading to infertility in AZFb and AZFc have been reported. In contrast, a report based on a 10 year study of 1,997 infertile men in Italy shows the prevalence of Y chromosome microdeletions in unselected infertile males is 3.2%; this number rises to 5.5% in males with severe oligozoospermia and to 8.3% in males with nonobstructive azoospermia [Ferlin et al. Citation2007a]. Other studies report the frequency of de novo AZF deletions is 6 to 8% in severely oligospermic males [Walsh et al. Citation2009] and ∼13% in males with idiopathic azoospermia [Reijo et al. Citation1995].
Deletions encompassing the AZFa region are associated with a more severe phenotype, such as SCO syndrome and are rarely found among infertile males [Kamp et al. Citation2001]. Two widely expressed genes are the primary candidates for the infertility phenotype, USP9Y (ubiquitin-specific protease 9) and DDX3Y (DEAD/H box polypeptide). USP9Y is the only gene for which a point mutation was reported; it was predicted to produce a truncated protein that leads to azoospermia [Sun et al. Citation1999]. However, two studies reported transmission of deletions spanning USP9Y by fertile fathers, suggesting that it might not be as essential for spermatogenesis as previously suggested [Krausz et al. Citation2006; Luddi, et al. Citation2009].
Some AZFa deletions are recurrent, ∼792 kb in size, and mediated by intrachromosomal NAHR between two 10–12 kb HERV15 retroviral sequence blocks with an average identity of 94% [Blanco et al. Citation2000; Kamp et al. Citation2000; Sun et al. Citation2000] (). Two breakpoint ‘hotspots’ with absolute identity were delineated within each of the HERV15 elements and termed ID1 (1285 bp) and ID2 (1242 bp) [Hurles et al. Citation2004; Kamp et al. Citation2000]; those segments are also substrate sites for frequent gene conversion events in humans [Hurles et al. Citation2004] and reciprocal duplications without apparent clinical consequences [Bosch and Jobling Citation2003]. Intriguingly, in one population study most of the rearrangements involving AZFa, include a small ∼98 kb (maximum size) deletion (detected by PCR assay and confirmed by Southern blot) encompassing only the gene DDX3Y (or DBY) delimited by two STS markers (sY87 and 475TEL) [Foresta et al. Citation2000]. Thus far no other group has reported this ∼98 kb deletion. The same research group published a 10-year study of 3,073 infertile males in Italy in which the 98 kb deletion alteration was observed in 7 out of 11 males (63%) with AZFa deletions [Ferlin et al. Citation2007a]; at least 4 out of 7 were shown previously to be de novo [Foresta et al. Citation2000]. This apparently biased recurrence would be readily explained by NAHR if LCRs in direct orientation could be detected flanking the DDX3Y gene. We performed a BLAST search of the reference genome for the presence of LCRs flanking the segment delimited by STS markers sY87 and 475TEL; however, no LCRs were detected (our unpublished observations). In the [Ferlin et al. 2007a] study, HERV15-recurrent deletion was observed in only 3 out of 11 (27%) males.
Two scenarios can be envisioned to explain these data. First, the use of low-resolution STS markers is masking the real size of each deletion that actually have subtle differences from each other (characterizing them as nonrecurrent rather than recurrent). This scenario still does not explain why the small deletion is found more frequently than the HERV15-NAHR deletion in this population. Second, subjects studied from that specific population (e.g., Italy) might have a Y chromosome with a different AZFa structure, compared to the reference genome, including the region between markers sY87 and 475TEL (for example, the presence of a highly similar duplication in direct orientation to the region flanking the gene DDX3Y would provide the necessary substrate for deletion). The latter possibility could result in a population-specific rearrangement-prone segment. These observations illustrate the challenge of studying the mechanism for the formation of rearrangements involving the Y chromosome: i) there are many extensive regions without unique markers, an obstacle to narrowing down the rearrangement breakpoint junctions; and ii) the Y chromosome is prone to rearrangements which generates chromosomes with a genomic structure that can be very different from the current haploid human reference genome complicating the prediction of structures and simple inferences of rearrangement mechanisms.
AZFb was the last azoospermia factor to be defined in terms of deletion extent, genomic architecture, and mechanisms for formation. It was considered a distinct deletion interval on Yq11, responsible for male infertility in a fraction of idiopathic infertile males [Ferlin et al. Citation2003]. In 2002, however, Repping et al. [2002], demonstrated that the genomic structure of the AZFb segment is laden with palindromic amplicons as previously demonstrated for the nearby AZFc region (). In fact, deletions spanning AZFb are actually the outcome of homologous recombination between amplicon P5, mapped within AZFb (), and either one of the two arms of palindrome P1 (P1 proximal and distal arms) mapped within AZFc which results in variable sized deletions from 6.2 Mb to up to 7.7 Mb. Thus, what was previously defined as a discrete region, the so-called AZFb locus, turned out to be part of its genomic neighbor, AZFc. AZFb deletion removes part of the AZFc region (1.5 Mb), including two copies of the AZFc gene candidate, Deleted in AZoospermia (DAZ). The region was then renamed after the palindromes that underlie that rearrangement, P5/proximal-P1 (AZFb) and P5/distal-P1 (AZFb + AZFc) [Repping et al. Citation2002]. Breakpoint junction sequencing revealed that 64% of those rearrangements were likely generated by NAHR between palindromes or inverted repeats [Repping et al. Citation2002]. The authors defined two ‘hotspot’ regions of 933 nt in which most of the recurrent rearrangements could be clustered (30 kb of the center of P5 and 25 kb of either minipalindromes P1.2 or P1.1 that lie within the P1 proximal and distal arms, respectively). Interestingly, our analysis of that genomic interval (unpublished observations) revealed that these ‘hotspot’ regions appear to be part of a repetitive element HERVL-A1, resembling the AZFa recurrent rearrangement ‘hotspot’ that was defined within the repetitive element HERV15 [Blanco et al. Citation2000; Kamp et al. Citation2000; Sun et al. Citation2000]. Remarkably, around 18% (2/11) of the rearrangements were produced by a non-homologous mechanism, constituting the first formal example of such events in the Y chromosome [Repping et al. Citation2002].
AZFc, also known as b2/b4 deletion, represents the most frequently deleted region among infertile males; it is estimated to occur de novo in approximately 1 in 4000 males [Kuroda-Kawaguchi et al. Citation2001]. The 4.5 Mb genomic structure that includes AZFc at Yq11 consists of six families or amplicons. These LCRs are organized as inverted (including palindromes or quasi-palindromes) and direct repeats, comprising 93% of the region [Kuroda-Kawaguchi et al. Citation2001] (). The amplicon units range from 115 kb to 678 kb, with nucleotide sequence identity varying from 99.82% to 99.98%. Most of the AZFc deletions seem to be recurrent and span ∼3.5 Mb at the Yq11 chromosome (based on the reference genome). Two LCRs, b2 and b4, 229 kb each in size and with 99.9% of nucleotide identity map in direct orientation at the proximal and distal breakpoints of AZFc [Kuroda-Kawaguchi et al. Citation2001]. All of these features support homologous recombination (NAHR) as the major mechanism for formation of the AZFc deletions [Kuroda-Kawaguchiet al. 2001]. Duplications of AZFc, including the reciprocal b2/b4 and also partial duplications were reported in some studies but they are most likely non pathogenic [Repping et al. Citation2006; Giachini et al. Citation2008; Jobling Citation2008; Balaresque et al. Citation2008].
DAZ is the primary candidate gene in AZFc. It encodes an RNA binding protein that is transcribed in the adult testis and it is expressed exclusively in germ cells [Reijo et al. Citation1995]. Using a single male as a Y-reference chromosome, Saxena et al. [2000] reported the existence of at least four DAZ copies with different numbers of intragenic tandem repeats, organized in two blocks, each comprising an inverted pair of DAZ genes, all mapped to the AZFc region. DAZ has an homologous autosomal copy on chromosome 3, DAZL, that is orthologous to the fruitfly boule gene, mutations of which cause spermatogenic failure in Drosophila. Recently, DAZ was shown to promote germ cell progression and formation of haploid germ cells [Kee et al. Citation2009], consistent with its proposed role as one of the ‘azoospermia factors’. Interestingly, DAZ genes on the Y-chromosome are a recent acquisition in primates (humans and old world monkeys) [Saxena et al. Citation2000]. Mulhall et al. [1997] proposed that the DAZ cluster is preferentially involved in quantitative rather than qualitative production of sperm.
The influence of the copy number of the TSPY (testis-specific protein, Y-linked) in male infertility was first suggested based on the fact that it was isolated from human testis and showed to be also expressed in testis of chimpanzee [Arnemann et al. Citation1987; Zhang et al. Citation1992]. TSPY maps to Yp11.2 (); the distal locus consists of one repeat unit whereas the proximal locus consists of an array of tandemly repeated units of approximately 20.4 kb each with copy number variability among the population, probably due to frequent ectopic recombination events [Tyler-Smith et al. Citation1988]. In the reference genome, the two TSPY loci are in direct orientation with an overall sequence identity of 96% [Jobling et al. Citation2007], but variation of this structure is predicted as the TSPY single copy lies within an inverted repeat (IR3; ) that shows a frequent inversion polymorphism within the population [Repping et al. Citation2006]. The TSPY array ranges in size from 23 to 64 units (0.47 to 1.3 Mb), with a median of 32 units (0.65 Mb) [Repping et al. Citation2006], but with a limited variation in copy number, supporting the contention that this locus has been undergoing selective constraint to maintain a certain unit number in the human Y chromosome likely through an intra-array NAHR mechanism [Repping et al. Citation2006]. Some studies suggest an association between low copy number of the TSPY units and male infertility due to a lower sperm count [Giachini et al. Citation2009], while one study suggests that higher copy number of the TSPY units are associated with spermatogenic failure [Vodicka et al. Citation2007], and a third study claims that TSPY copy number alteration does not represent a risk for infertility [Nickkholgh et al. 2010]. Noteworthy, are apparently nonpathological 3.0 and 3.8 Mb recurrent deletions due to NAHR mediated by the TSPY single copy gene [Jobling et al. 2007] and one of the TSPY genes from within the array [Santos et al. 1998] as well as rare nonrecurrent rearrangements likely generated by non-homologous mechanisms. The recurrent deletions are accompanied by a reduction in the copy number of the TSPY genes. Those Y-chromosome structures, albeit rare, have been reported in different populations indicating that there are not any strong selective disadvantages associated with them [Jobling et al. Citation2007], but further studies are still required. TSPY is one of the gonadoblastoma candidate genes [Tsuchiya et al. Citation1995], potentially involved in the development of other types of cancers (reviewed in [Lau et al. Citation2009]).
NAHR underlies the formation of diverse Y-chromosome structures worldwide
The Y-linked loci in the male-specific region of the Y chromosome (MSY) are haploid and paternally inherited. Because of these particular characteristics, variation results in the accumulation of mutations along generations. Population geneticists have extensively studied human male lineages to trace migrations and reconstruct human history (reviewed by [Jobling and Tyler-Smith Citation2003; Underhill and Kivisild Citation2007]). The Y phylogeny is based on Y-chromosome biallelic markers (most of which are Single Nucleotide Polymorphisms, SNPs), also called unique event polymorphisms (UEPs); such markers can be combined in haplogroups that define Y lineages with specific geographic distributions around the world [Jobling and Tyler-Smith Citation2003; Underhill and Kivisild Citation2007].
The genomic architectural complexity of the MSY, due to the remarkable presence of repeats in inverted and in direct orientation, predicts that rearrangements, other than those known to lead to AZFa, AZFb, and AZFc microdeletions, may occur. Indeed, CNVs in the Y chromosome were found to be fixed in some specific haplogroups, but they cannot be regarded as reliable UEPs as they might be subject to reversal [Jobling Citation2008]. Besides the fixed CNVs, many are shown to be recurrent, likely mediated by NAHR between the palindromes and inverted repeats on Yq11.
Repping et al. [2003] reported partial deletions of the AZFc (gr/gr and b1/b3, which were named after the repeat underlying the rearrangement) as well as duplications. Using association analysis, in addition to the fact that gr/gr deletion removes 1.6 Mb of the Y chromosome including nine transcription units with testis-specific expression, they proposed that gr/gr deletion represents a higher risk for spermatogenic failure. Such association was confirmed in some studies [Ferlin et al. Citation2005; Giachini et al. Citation2008; Lynch et al. Citation2005; Visser et al. Citation2009], but not confirmed in others [Carvalho et al. Citation2006a; Citation2006b; Machev et al. Citation2004; Zhang et al. Citation2006]. In fact, the gr/gr deletion is frequently found in East Asian individuals (∼8.0%) [Zhang et al. Citation2006] and is even more frequent within the Japanese population (∼30%); for both Asian populations the gr/gr deletion was not observed to be associated with a higher infertility risk [Carvalho et al. Citation2006b; Zhang et al. Citation2006].
Another AZFc partial deletion (1.8 Mb deletion), termed b2/b3, is frequently found in Northern Eurasia. Similar to observations regarding the gr/gr deletions, studies of b2/b3 in different world populations reveals different results regarding its potential association with risk of infertility [Repping et al. Citation2004; Wu et al. Citation2007]; please see Carvalho and Santos [2005] for a detailed discussion of gr/gr partial deletions and Navarro-Costa et al. [2010] for a detailed discussion of b2/b3 partial deletions. Such apparent contradictory results might reflect genomic structural differences in Y chromosomes between individual personal genomes and among haplogroups together with population stratification in the distribution of the Y chromosome lineages worldwide [Jobling Citation2008] confounding the association studies. Indeed, extensive structural differences among haplogroups have been reported by different studies; some structures can be explained only upon consecutive NAHR events on the reference genome [Repping et al., Citation2006]. For example, Machev et al [2004] reported the coexistence of four different gr/gr deletion types within the French population, along with inversions and duplications involving the AZFc region. Repping et al. [2006] used diverse molecular assays including PFGE and fluorescent in situ hybridization (FISH) to analyze the genomic structure of 47 haplotypes that determine the major branches of the Y chromosome genealogy. By these approaches they determined that 20 out of 47 chromosomes have structural variation involving AZFc, predominantly inversions but also deletions and large duplications, including the reciprocal duplication of the 3.5 Mb b2/b4 deletion. Therefore, detailed molecular analysis of the structure of the different Y chromosome haplogroup lineages, including sequencing and individual de novo assembly, seems to be the first requirement to interpret potential biological significance of the partial deletions.
The reason why inversions are overrepresented compared to deletions and duplications is not known; one possibility is that natural selection is acting against deletion and duplications but not inversions [Repping et al. Citation2006]. If one considers only rearrangement events that occur by NAHR, it is interesting to note that deletions are estimated to be generated more frequently than duplications and inversions because deletions involving the Y chromosome can be produced either by interchromatid or intrachromatid NAHR, whereas duplications can only be produced by interchromatid NAHR and inversions can only be produced by intrachromatid NAHR. A direct calculation of the NAHR rate in Y chromosome was performed by [Turner et al. Citation2008] using a pooled sperm-based assay to measure de novo rates of reciprocal deletion and duplications of four genomic disorders including that associated with the AZFa deletion. As expected, deletions were detected 2X as frequent as duplications for three out of four genomic disorders, all three represent autosomal loci, but that rate was 4X for AZFa. In aggregate, these data, in both AZFa and AZFc, suggest that Y chromosome NAHR might favor intrachromatidal rather than interchromatidal events potentially reflecting absence of a homologue with which to pair; further studies are required to confirm and extend those observations.
NAHR is also responsible for formation of the isodicentric Y-chromosome among infertile males
Vergnaud et al. [1986] used DNA samples from 27 individuals carrying different portions of the Y chromosome to propose a ‘deletion map’ based on the hybridization of chromosome-specific probes in addition to genotype-phenotype correlations. The repeat laden nature of the Y-chromosome was already known at that time and the multiple hybridization patterns of some of those probes made the organization of the intervals particularly challenging. Later, the analysis of the entire Y chromosome sequencing data confirmed and further portrayed the structural complexities [Kuroda-Kawaguchi et al. Citation2001; Skaletsky et al. Citation2003; Tilford et al. Citation2001]. Palindromes comprise ∼25% of the MSY, ranging from 30 kb to 2.9 Mb in size and present in ≥99.9% nucleotide sequence identity [Skaletsky et al. Citation2003]. This structural complexity likely incites frequent homologous recombination events within the Y-chromosome (Y-Y recombination) [Rozen et al. Citation2003], which, in turn, maintains the high similarity between the inverted repeats as a result of gene conversion.
According to a model proposed by Lange et al. [2009] to explain isodicentric Y (idicY) chromosome formation, the ‘palindrome maintenance’ outcome is, in fact, one of the alternative products of either intrachromatid or sister chromatid homologous recombination that frequently occurs in the long arm of the Y chromosome. Such a model for chromosome rearrangements consisting of sister chromatid NAHR between inverted LCRs, had been previously proposed for iso17q formation [Barbouti et al. Citation2004; Carvalho and Lupski Citation2008]. Lange et al. [2009] identified 60 unrelated cases carrying mostly idicYp chromosomes in patients with clinical abnormalities, including spermatogenic failure, that arose through palindrome recombination or recombination mediated by heterochromatic sequences. Palindrome P5 was shown to be the most frequently involved. Nonetheless, 8 out of 9 palindromes present on the Y chromosome long arm were found to mediate at least one of the cases studied; suggesting that this mechanism is not sequence or region-specific but rather depends upon the presence of a specific genomic architecture (e.g., inverted repeats). Interestingly, the only palindrome not involved in any rearrangement reported therein (P7) is also the smallest one [Skaletsky et al. Citation2003] suggesting that the size of the inverted repeats may influence the frequency of the event, an observation consistent with NAHR features. Lange et al. [2009] identified 2.7% of males in their cohort carrying nonobstructive azoospermia associated with the presence of an idicY or isoY; for comparison, in the same group of patients AZFc deletion was observed in 7.5% of patients supporting idicY and isoY formation as important causative factors for severe spermatogenic failure [Lange et al. Citation2009].
Evidence for alternative mechanisms generating male infertility associated with Y chromosome
Most of the rearrangements reported in the Y chromosome thus far might be generated by NAHR, but other mechanisms may also play a role. How frequent they underlie rearrangements involving the Y chromosome is still not clear mainly because of the challenges of narrowing down the breakpoint junctions due to the ubiquitous presence of repeats on the Y chromosome. Using low-resolution mapping, it is apparent that AZF regions seem to have a different frequency for recurrent and nonrecurrent rearrangements; AZFa and AZFb seem more prone to nonrecurrent rearrangements than AZFc. For example, Kuroda-Kawaguchi et al. [2001] reported 48 individuals carrying AZFc deletions; only 1 individual presented a rearrangement not involving direct pairs of LCRs suggesting a non-homologous mechanism underlying that alteration. Therefore, AZFc nonrecurrent rearrangements might be infrequent (∼2%), at least for rearrangements associated with infertility. Other reported data suggest that the frequency for nonrecurrent events might be underestimated and further studies are required. For example, Balaresque et al. [2008] described five new partial nonrecurrent, likely nonhomology-driven deletions of AZFc observed in different worldwide Y-chromosome haplogroups. Repping et al. [2006] also showed inherited Y-chromosomes that could have been generated by non-homologous mechanisms; at least two observed structures (ctr P3, YCC038) have their endpoints mapped to segments that do not show similarities to each other [Repping et al. Citation2006].
Two studies may provide insights into the frequency of rearrangements not generated by NAHR on the distal Yq11. Repping et al. [2002] sequenced 9 out of 11 breakpoints of the deletions encompassing AZFb + AZFc. They used high-resolution STS-based mapping to narrow down the deletions, followed by genomic sequencing of breakpoints, which enabled them to obtain 9 out of 11 junctions. Based on those sequenced junctions they estimate that ectopic recombination (i.e., NAHR) mediated 64% (7/11) of the breakpoints with variable size of identity [25 nt (1/11), 73 nt (1/11), and 933 nt (5/11)], mapped within the same hotspot window (∼25–30 kb). Surprisingly, though, 18% (2/11) of the alterations were produced by a mechanism that does not require homology at all. In one case the junction sequence revealed three nucleotides (TAA) of microhomology and in another case the deletion junction showed a complex rearrangement including an insertion of an unknown 31 nt segment [Repping et al. Citation2002].
We performed a genomic search of this small insertion using a web-based program BLAT (http://genome.ucsc.edu/cgi-bin/hgGateway): 21 nt out of 31 nt are actually part of a HERVL-A1 retrotransposon element present 4 times within the Y chromosome long arm (one time within the deleted interval) based on the genomic reference sequence (our unpublished data). NHEJ might be the mechanism underlying this rearrangement, as insertions of free DNA, often from mitochondria or retrotransposons, are known to occur [Hastings et al. Citation2009b]. Alternatively, FoSTeS/MMBIR might also produce such complex alteration driven by microhomologies at the junctions. Indeed, we identified a microhomology of 2 nt or 3 nt (depending on which one of the four HERVL-A1 repeats we consider) at the breakpoint junction of the deletion/HERVL-A1 insertion. We were not able to identify the origin of 10 nt out of 31 nt of the segment, hampering our attempts to propose or infer a mechanism for this rearrangement based upon the breakpoint junctions of the products of recombination. Interpretation of these experimental findings in the context of the haploid reference human genome is also limited given recent findings that reveal massive repetitive sequence ‘polymorphisms’ in personal genomes [Beck et al. Citation2010; Ewing and Kazazian Citation2010; Huang et al. Citation2010; Iskow et al. Citation2010; Lupski Citation2010]. Noteworthy, the presence of LCRs flanking nonrecurrent alterations derived from replicative-mechanism is a remarkable feature observed in rearrangements involving the X-chromosome [Carvalho et al. Citation2009; Inoue et al. Citation2002; Lee et al. Citation2007]. Other nonrecurrent deletions involving AZFb + AZFc, potentially generated by non-homologous mechanisms were also reported by other groups [Costa et al. Citation2008; Ferlin et al. Citation2007a; Yang et al. Citation2008].
Recently, a paper published by Lange et al. [2009] proposes NAHR as the mechanism to explain the formation of terminal deletions (actually producing (iso)Y or (idic)Y) of varying sizes. This mechanism successfully explained most of the 85 cases analyzed by them, but 20 (24%) are not readily explained by NAHR. The breakpoints of those 20 cases map outside of LCRs and rearrangements show variable size, allowing one to classify them as nonrecurrent rearrangements. The exact mechanism by which they occur requires further analysis of the breakpoint ends at the DNA sequence level, but they may represent homology-independent mechanisms. However, without the breakpoint sequences we cannot rule out NAHR between repetitive sequences rather than LCRs.
In aggregate, non-homologous mechanisms might be responsible for as much as 18–24% (∼20%) of the rearrangements involving the long arm of the Y chromosome. This number might represent an underestimate as in some regions (e.g., AZFc) distinction between recurrent and nonrecurrent rearrangements might be difficult if the screening assay uses low-resolution STS mapping. This assay, while enough to characterize the major deletions usually observed in infertile patients, may not allow one to distinguish if the breakpoint maps within the palindromic arms or within the short spacer segments between palindromes. For example, a general classification of an AZFb + AZFc deletion as P5/distal-P1 would not enable one to distinguish a homologous rearrangement versus a non-homologous rearrangement product as they would always be classified as recurrent and flanked by a direct pair of highly identical LCRs. However, as shown by sequencing the breakpoint junctions of different P5/distal-P1 [Repping et al. Citation2002], such rearrangements can be generated by mechanisms other than NAHR. Therefore, higher-resolution mapping and sequencing of breakpoints in individual cases are required to assess the frequency of recurrent and nonrecurrent rearrangements, as well as to infer the importance of other nonhomologous-based mechanisms, underlying deletions associated with male infertility.
Conclusions
Rearrangements involving the Y chromosome, stimulated by its genomic architecture laden with LCRs in direct and inverted orientation and large palindromes, are an important cause of male infertility in addition to structural polymorphism in worldwide Y chromosomes across human populations. To assess the causes of the infertility and to predict patterns by which the rearrangements might be generated is fundamental to understanding the mechanisms for their formation. NAHR seems to be a prevalent mechanism underlying the formation of Yq11 rearrangements. Approximately 60% of men with Y chromosomal microdeletions and severe oligozoospermia or idiopathic azoospermia have AZFc deletions compared to 16% with AZFb deletions and <5% with AZFa [Walsh et al. Citation2009], likely reflecting the characteristic genomic architecture for each genomic interval. For instance, the LCR sequences underlying AZFa deletions are 10-12 kb in size, share 94% of identity, and map far apart ∼792 kb, contrasting with the sequences underlying AZFc deletions that are 229 kb in size, with 99.9% of nucleotide identity, and an ∼3.5 Mb inter-paralogue distance. The explanation for such large differences in AZF frequencies might be due to differences in efficiency of ectopic homologous recombination as it is known to positively correlate with the size of the LCRs involved and it is influenced by the physical distance between them [Lupski Citation1998; Stankiewicz and Lupski Citation2002].
The distance between LCRs b2 and b4 is supposedly 3.5-Mb in the majority of the human Y chromosomes, but about half this size in individuals with polymorphisms for partial AZFc deletions, e.g., 1.9-Mb in gr/gr deletion and 1.7-Mb in b2/b3 deletion [Repping et al. Citation2003; Citation2004]. Interestingly, it was found that the frequency of de novo complete AZFc deletion in the Y chromosomal haplogroups fixed with gr/gr or b2/b3 deletion polymorphisms was significantly higher than in other haplogroups, suggesting an association of reduced b2-b4 distance with increased frequency of b2/b4 deletion [Zhang et al. Citation2007]. In addition, a statistically significant difference was noted between AZFc deletions generated in a b2/b3 ancestral background (higher frequency) compared to those with gr/gr deletion background [Lu et al. Citation2009]. The length of the LCR substrates used by NAHR in each case is different, 0.33-Mb in b2/b3 deletion and 0.22-Mb in gr/gr deletion [Lu et al. Citation2009], suggesting that LCR size is positively correlated with the frequency of rearrangement formation. These data suggests that different Y chromosome structures (many of them fixed in the haplogroups) may show a higher propensity to undergo rearrangements, a hypothesis that is supported by other studies [Balaresque et al. Citation2008], which has profound implications for diseases involving the Y chromosome and for its evolution. The observation that non-homologous mechanism might underlie as much as 20% of the rearrangements on the Y chromosome is still to be confirmed, but surely indicates that more research is necessary on Y chromosome breakpoints and mechanism for formation.
Future studies on CNVs as potentially causative for male infertility should include the use of whole-genome screening technologies such as array-based comparative genomic hybridization (CGH) in a cohort of male patients with idiopathic infertility. We predict that new ‘candidate genes’ for infertility might be uncovered using such assays. Additionally, the fact that chromosomal abnormalities can be 8 to 10 fold higher in infertile versus fertile males suggests that smaller genomic alterations might also be found at higher frequency in the group with infertility. Somatic rearrangements might also cause Y-chromosome structural variation. Cumulative evidence supports the notion that many of us could be mosaic for structural variations [Bruder et al. Citation2008; Flores et al. Citation2007] and that somatic rearrangements ranging in size from 82-176 kb may occur in different tissues in the same individual [Piotrowski et al. Citation2008]. Mosaicism for an additional X-chromosome in patients with Klinefelter can be as high as 26% [Yatsenko et al. Citation2010] and can be also found in patients with a normal karyotype [Elghezal et al. Citation2006]. The involvement of the Y chromosome palindromes mediating the formation of idicYp and associated with sex reversal patients mosaic for 45,X cells was shown by Lange et al. [2009]; this association might be explained by the mitotic instability of cells of individuals carrying idicYp [Lange et al. Citation2009]. Whether mosaicism of structural variation due to the palindromic Y-chromosome nature can be detected in tissues of control individuals remains to be unveiled, but if positive it may provide a subject of study for mitotic Y chromosome genomic rearrangements and mechanisms underlying them.
Declaration of Interest: The authors alone are responsible for the content and writing of the paper.
Financial Disclosure: J.R.L. is a consultant for Athena Diagnostics and Ion Torrent Systems and holds multiple United States and European patents for DNA diagnostics. Furthermore, the Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from molecular diagnostic testing (Medical Genetics Laboratories).
Abbreviations
| AZF: | = | azoospermia factor |
| BIR: | = | break induced replication |
| CGH: | = | comparative genomic hybridization |
| CMT1A: | = | Charcot-Marie-Tooth disease type 1A |
| CNV: | = | copy number variant |
| DAZ: | = | deleted in azoospermia |
| DSB: | = | double-strand break |
| FISH: | = | fluorescent in situ hybridization |
| FoSTeS: | = | fork stalling and template switching |
| HNPP: | = | hereditary neuropathy with liability to pressure palsies |
| HR: | = | homologous recombination |
| LCR: | = | low copy repeat |
| LINE: | = | long interspersed nuclear element |
| MEPS: | = | minimal efficient processing segment |
| MMBIR: | = | microhomology-mediated break induced replication |
| MSY: | = | male-specific region of the Y chromosome |
| NAHR: | = | non-allelic homologous recombination |
| NHEJ: | = | non-homologous end joining |
| PFGE: | = | pulsed-field gel electrophoresis |
| PMD: | = | Pelizaeus-Merzbacher disease |
| PTLS: | = | Potocki-Lupski syndrome |
| SCO: | = | Sertoli-cell-only syndrome |
| SD: | = | segmental duplication |
| SINE: | = | short interspersed nuclear element |
| SMS: | = | Smith-Magenis syndrome |
| STS: | = | sequence-tagged site |
| TSPY: | = | testis-specific protein, Y-linked |
| UEPs: | = | unique event polymorphisms. |
| Term definitions | ||
| azoospermia: | = | undetectable presence of sperm in semen |
| severe oligozoospermia: | = | <5 million sperm/ml |
| oligozoospermia: | = | <20 million sperm/ml. |
Acknowledgments
This work was supported in part by the National Institute of Neurological Disorders and Stroke (National Institutes of Health) grant R01NS058529 to J.R.L., Texas Children's Hospital General Clinical Research Center grant M01RR00188, and Intellectual and Developmental Disabilities Research Centers grant P30HD024064.
References
- Andersson, M., Page, D.C., Pettay, D., Subrt, I., Turleau, C., de Grouchy, J., (1988) Y;autosome translocations and mosaicism in the aetiology of 45, X maleness: assignment of fertility factor to distal Yq11. Hum Genet 79:2–7.
- Arnemann, J., Epplen, J.T., Cooke, H.J., Sauermann, U., Engel, W., Schmidtke, J. (1987) A human Y-chromosomal DNA sequence expressed in testicular tissue. Nucleic Acids Res 15:8713–8724.
- Bailey, J.A., Gu, Z., Clark, R.A., Reinert, K., Samonte, R.V., Schwartz, S., (2002) Recent segmental duplications in the human genome. Science 297:1003–1007.
- Balaresque, P., Bowden, G.R., Parkin, E.J., Omran, G.A., Heyer, E., Quintana-Murci, L., (2008) Dynamic nature of the proximal AZFc region of the human Y chromosome: multiple independent deletion and duplication events revealed by microsatellite analysis. Hum Mutat 29:1171–1180.
- Barbouti, A., Stankiewicz, P., Nusbaum, C., Cuomo, C., Cook, A., Hoglund, M., (2004) The breakpoint region of the most common isochromosome, i(17q), in human neoplasia is characterized by a complex genomic architecture with large, palindromic, low-copy repeats. Am J Hum Genet 74:1–10.
- Beck, C.R., Collier, P., Macfarlane, C., Malig, M., Kidd, J.M., Eichler, E.E., (2010) LINE-1 retrotransposition activity in human genomes. Cell 141:1159–1170.
- Bi, W., Park, S.S., Shaw, C.J., Withers, M.A., Patel, P.I., Lupski, J.R. (2003) Reciprocal crossovers and a positional preference for strand exchange in recombination events resulting in deletion or duplication of chromosome 17p11.2. Am J Hum Genet 73:1302–1315.
- Blanco, P., Shlumukova, M., Sargent, C.A., Jobling, M.A., Affara, N., Hurles, M.E. (2000) Divergent outcomes of intrachromosomal recombination on the human Y chromosome: male infertility and recurrent polymorphism. J Med Genet 37:752–758.
- Bosch, E., Jobling, M.A. (2003) Duplications of the AZFa region of the human Y chromosome are mediated by homologous recombination between HERVs and are compatible with male fertility. Hum Mol Genet 12:341–347.
- Bruder, C.E., Piotrowski, A., Gijsbers, A.A., Andersson, R., Erickson, S., Diaz de Stahl, T., (2008) Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am J Hum Genet 82:763–771.
- Carvalho, C.M., Lupski, J.R. (2008) Copy number variation at the breakpoint region of isochromosome 17q. Genome Res 18:1724–1732.
- Carvalho, C.M., Santos, F.R. (2005) Human Y-chromosome variation and male dysfunction. J Mol Genet Med 1:63–75.
- Carvalho, C.M., Zhang, F., Liu, P., Patel, A., Sahoo, T., Bacino, C.A., (2009) Complex rearrangements in patients with duplications of MECP2 can occur by fork stalling and template switching. Hum Mol Genet 18:2188–2203.
- Carvalho, C.M., Zuccherato, L.W., Bastos-Rodrigues, L., Santos, F.R., Pena, S.D. (2006a) No association found between gr/gr deletions and infertility in Brazilian males. Mol Hum Reprod 12:269–273.
- Carvalho, C.M., Zuccherato, L.W., Fujisawa, M., Shirakawa, T., Ribeiro-dos-Santos, A.K., Santos, S.E., (2006b) Study of AZFc partial deletion gr/gr in fertile and infertile Japanese males. J Hum Genet 51:794–799.
- Chance, P.F., Alderson, M.K., Leppig, K.A., Lensch, M.W., Matsunami, N., Smith, B., (1993) DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell 72:143–151.
- Chandley, A.C., Gosden, J.R., Hargreave, T.B., Spowart, G., Speed, R.M., McBeath, S. (1989) Deleted Yq in the sterile son of a man with a satellited Y chromosome (Yqs). J Med Genet 26:145–153.
- Chen, K.S., Manian, P., Koeuth, T., Potocki, L., Zhao, Q., Chinault, A.C., (1997) Homologous recombination of a flanking repeat gene cluster is a mechanism for a common contiguous gene deletion syndrome. Nat Genet 17:154–163.
- Conrad, D.F., Bird, C., Blackburne, B., Lindsay, S., Mamanova, L., Lee, C., (2010) Mutation spectrum revealed by breakpoint sequencing of human germline CNVs. Nat Genet 42:385–391.
- Costa, P., Goncalves, R., Ferras, C., Fernandes, S., Fernandes, A.T., Sousa, M., (2008) Identification of new breakpoints in AZFb and AZFc. Mol Hum Reprod 14:251–258.
- de Kretser, D.M. (1997) Male infertility. Lancet 349:787–790.
- Elghezal, H., Hidar, S., Braham, R., Denguezli, W., Ajina, M., Saad, A. (2006) Chromosome abnormalities in one thousand infertile males with nonobstructive sperm disorders. Fertil Steril 86:1792–1795.
- Ewing, A.D., Kazazian, H.H. Jr., (2010) High-throughput sequencing reveals extensive variation in human-specific L1 content in individual human genomes. Genome Res 20:1262–1270.
- Ferlin, A., Arredi, B., Speltra, E., Cazzadore, C., Selice, R., Garolla, A., (2007a) Molecular and clinical characterization of Y chromosome microdeletions in infertile men: a 10-year experience in Italy. J Clin Endocrinol Metab 92:762–770.
- Ferlin, A., Moro, E., Rossi, A., Dallapiccola, B., Foresta, C. (2003) The human Y chromosome's azoospermia factor b (AZFb) region: sequence, structure, and deletion analysis in infertile men. J Med Genet 40:18–24.
- Ferlin, A., Raicu, F., Gatta, V., Zuccarello, D., Palka, G., Foresta, C. (2007b) Male infertility: role of genetic background. Reprod Biomed Online 14:734–745.
- Ferlin, A., Tessari, A., Ganz, F., Marchina, E., Barlati, S., Garolla, A., (2005) Association of partial AZFc region deletions with spermatogenic impairment and male infertility. J Med Genet 42:209–213.
- Flores, M., Morales, L., Gonzaga-Jauregui, C., Dominguez-Vidana, R., Zepeda, C., Yanez, O., (2007) Recurrent DNA inversion rearrangements in the human genome. Proc Natl Acad Sci USA 104:6099–6106.
- Foresta, C., Ferlin, A., Moro, E. (2000) Deletion and expression analysis of AZFa genes on the human Y chromosome revealed a major role for DBY in male infertility. Hum Mol Genet 9:1161–1169.
- Gatta, V., Stuppia, L., Calabrese, G., Morizio, E., Guanciali-Franchi, P., Palka, G. (2002) A new case of Yq microdeletion transmitted from a normal father to two infertile sons. J Med Genet 39:E27.
- Giachini, C., Laface, I., Guarducci, E., Balercia, G., Forti, G., Krausz, C. (2008) Partial AZFc deletions and duplications: clinical correlates in the Italian population. Hum Genet 124:399–410.
- Giachini, C., Nuti, F., Turner, D.J., Laface, I., Xue, Y., Daguin, F., (2009) TSPY1 copy number variation influences spermatogenesis and shows differences among Y lineages. J Clin Endocrinol Metab 94:4016–4022.
- Gu, W., Zhang, F., Lupski, J.R. (2008) Mechanisms for human genomic rearrangements. Pathogenetics 1:4.
- Hastings, P.J., Ira, G., Lupski, J.R. (2009a) A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet 5:e1000327.
- Hastings, P.J., Lupski, J.R., Rosenberg, S.M., Ira, G. (2009b) Mechanisms of change in gene copy number. Nat Rev Genet 10:551–564.
- Huang, C.R., Schneider, A.M., Lu, Y., Niranjan, T., Shen, P., Robinson, M.A., (2010) Mobile interspersed repeats are major structural variants in the human genome. Cell 141:1171–1182.
- Hurles, M.E., Willey, D., Matthews, L., Hussain, S.S. (2004) Origins of chromosomal rearrangement hotspots in the human genome: evidence from the AZFa deletion hotspots. Genome Biol 5:R55.
- Inoue, K., Osaka, H., Thurston, V.C., Clarke, J.T., Yoneyama, A., Rosenbarker, L., (2002) Genomic rearrangements resulting in PLP1 deletion occur by nonhomologous end joining and cause different dysmyelinating phenotypes in males and females. Am J Hum Genet 71:838–853.
- Iskow, R.C., McCabe, M.T., Mills, R.E., Torene, S., Pittard, W.S., Neuwald, A.F., (2010) Natural mutagenesis of human genomes by endogenous retrotransposons. Cell 141:1253–1261.
- Jobling, M.A. (2008) Copy number variation on the human Y chromosome. Cytogenet Genome Res 123:253–262.
- Jobling, M.A., Lo, I.C., Turner, D.J., Bowden, G.R., Lee, A.C., Xue, Y., (2007) Structural variation on the short arm of the human Y chromosome: recurrent multigene deletions encompassing Amelogenin Y. Hum Mol Genet 16:307–316.
- Jobling, M.A., Tyler-Smith, C. (2003) The human Y chromosome: an evolutionary marker comes of age. Nat Rev Genet 4:598–612.
- Kamp, C., Hirschmann, P., Voss, H., Huellen, K., Vogt, P.H. (2000) Two long homologous retroviral sequence blocks in proximal Yq11 cause AZFa microdeletions as a result of intrachromosomal recombination events. Hum Mol Genet 9:2563–2572.
- Kamp, C., Huellen, K., Fernandes, S., Sousa, M., Schlegel, P.N., Mielnik, A., (2001) High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod 7:987–994.
- Kee, K., Angeles, V.T., Flores, M., Nguyen, H.N., Reijo Pera, R.A. (2009) Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 462:222–225.
- Krausz, C., Degl'Innocenti, S., Nuti, F., Morelli, A., Felici, F., Sansone, M., (2006) Natural transmission of USP9Y gene mutations: a new perspective on the role of AZFa genes in male fertility. Hum Mol Genet 15:2673–2681.
- Kuhnert, B., Gromoll, J., Kostova, E., Tschanter, P., Luetjens, C.M., Simoni, M., (2004) Case report: natural transmission of an AZFc Y-chromosomal microdeletion from father to his sons. Hum Reprod 19:886–888.
- Kuroda-Kawaguchi, T., Skaletsky, H., Brown, L.G., Minx, P.J., Cordum, H.S., Waterston, R.H., (2001) The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet 29:279–286.
- Lam, K.W., Jeffreys, A.J. (2006) Processes of copy-number change in human DNA: the dynamics of {alpha}-globin gene deletion. Proc Natl Acad Sci USA 103:8921–8927.
- Lange, J., Skaletsky, H., van Daalen, S.K., Embry, S.L., Korver, C.M., Brown, L.G., (2009) Isodicentric Y chromosomes and sex disorders as byproducts of homologous recombination that maintains palindromes. Cell 138:855–869.
- Lau, Y.F., Li, Y., Kido, T. (2009) Gonadoblastoma locus and the TSPY gene on the human Y chromosome. Birth Defects Res C Embryo Today 87:114–122.
- Lee, J.A., Carvalho, C.M., Lupski, J.R. (2007) A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131:1235–1247.
- Lee, J.A., Inoue, K., Cheung, S.W., Shaw, C.A., Stankiewicz, P., Lupski, J.R. (2006) Role of genomic architecture in PLP1 duplication causing Pelizaeus-Merzbacher disease. Hum Mol Genet 15:2250–2265.
- Lidegaard, O., Mikkelsen, A.L., Meldgaard, M., Brondum-Nielsen, K., Lindenberg, S. (1998) Severe male infertility. Impact of genetic factors on diagnosis and counselling. Acta Obstet Gynecol Scand 77:799–803.
- Lieber, M.R. (2008) The mechanism of human nonhomologous DNA end joining. J Biol Chem 283:1–5.
- Lindsay, S.J., Khajavi, M., Lupski, J.R., Hurles, M.E. (2006) A chromosomal rearrangement hotspot can be identified from population genetic variation and is coincident with a hotspot for allelic recombination. Am J Hum Genet 79:890–902.
- Lu, C., Zhang, J., Li, Y., Xia, Y., Zhang, F., Wu, B., (2009) The b2/b3 subdeletion shows higher risk of spermatogenic failure and higher frequency of complete AZFc deletion than the gr/gr subdeletion in a Chinese population. Hum Mol Genet 18:1122–1130.
- Luddi, A., Margollicci, M., Gambera, L., Serafini, F., Cioni, M., De Leo, V., (2009) Spermatogenesis in a man with complete deletion of USP9Y. N Engl J Med 360:881–885.
- Lupski, J.R. (2010) Retrotransposition and structural variation in the human genome. Cell 141:1110–1112.
- Lupski, J.R. (2009) Genomic disorders ten years on. Genome Med 1:42.
- Lupski, J.R. (2007) Genomic rearrangements and sporadic disease. Nat Genet 39:S43–47.
- Lupski, J.R. (2004) Hotspots of homologous recombination in the human genome: not all homologous sequences are equal. Genome Biol 5:242.
- Lupski, J.R. (1998) Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet 14:417–422.
- Lupski, J.R., de Oca-Luna, R.M., Slaugenhaupt, S., Pentao, L., Guzzetta, V., Trask, B.J., (1991) DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell 66:219–232.
- Lynch, M., Cram, D.S., Reilly, A., O'Bryan, M.K., Baker, H.W., de Kretser, D.M., (2005) The Y chromosome gr/gr subdeletion is associated with male infertility. Mol Hum Reprod 11:507–512.
- Ma, K., Sharkey, A., Kirsch, S., Vogt, P., Keil, R., Hargreave, T.B., (1992) Towards the molecular localisation of the AZF locus: mapping of microdeletions in azoospermic men within 14 subintervals of interval 6 of the human Y chromosome. Hum Mol Genet 1:29–33.
- Machev, N., Saut, N., Longepied, G., Terriou, P., Navarro, A., Levy, N., (2004) Sequence family variant loss from the AZFc interval of the human Y chromosome, but not gene copy loss, is strongly associated with male infertility. J Med Genet 41:814–825.
- McLachlan, R.I., O'Bryan, M.K. (2010) Clinical Review#: State of the art for genetic testing of infertile men. J Clin Endocrinol Metab 95:1013–1024.
- Mulhall, J.P., Reijo, R., Alagappan, R., Brown, L., Page, D., Carson, R., (1997) Azoospermic men with deletion of the DAZ gene cluster are capable of completing spermatogenesis: fertilization, normal embryonic development and pregnancy occur when retrieved testicular spermatozoa are used for intracytoplasmic sperm injection. Hum Reprod 12:503–508.
- Myers, S., Freeman, C., Auton, A., Donnelly, P., McVean, G. (2008) A common sequence motif associated with recombination hot spots and genome instability in humans. Nat Genet 40:1124–1129.
- Navarro-Costa, P., Goncalves, J., Plancha, C.E. (2010) The AZFc region of the Y chromosome: at the crossroads between genetic diversity and male infertility. Hum Reprod Update 16:525–542.
- Nickkholgh, B., Noordam, M.J., Hovingh, S.E., van Pelt, A.M., van der Veen, F., Repping, S. (2010) Y chromosome TSPY copy numbers and semen quality. Fertil Steril 94:1744–1747.
- O'Flynn O'Brien, K.L., Varghese, A.C., Agarwal, A. (2010) The genetic causes of male factor infertility: a review. Fertil Steril 93:1–12.
- Ou, Z., Stankiewicz, P., Breman, A.M., Wiszniewska, J., Cooper, M.L., Shao, L., et al. Observation and prediction of recurrent human translocations mediated by NAHR between non-homologous chromosomes. Genome Res In press.
- Pang, M.G., Hoegerman, S.F., Cuticchia, A.J., Moon, S.Y., Doncel, G.F., Acosta, A.A., (1999) Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod 14:1266–1273.
- Piotrowski, A., Bruder, C.E., Andersson, R., Diaz de Stahl, T., Menzel, U., Sandgren, J., (2008) Somatic mosaicism for copy number variation in differentiated human tissues. Hum Mutat 29:1118–1124.
- Potocki, L., Bi, W., Treadwell-Deering, D., Carvalho, C.M., Eifert, A., Friedman, E.M., (2007) Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet 80:633–649.
- Potocki, L., Chen, K.S., Park, S.S., Osterholm, D.E., Withers, M.A., Kimonis, V., (2000) Molecular mechanism for duplication 17p11.2- the homologous recombination reciprocal of the Smith-Magenis microdeletion. Nat Genet 24:84–87.
- Reijo, R., Lee, T.Y., Salo, P., Alagappan, R., Brown, L.G., Rosenberg, M., (1995) Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 10:383–393.
- Reiter, L.T., Murakami, T., Koeuth, T., Pentao, L., Muzny, D.M., Gibbs, R.A., (1996) A recombination hotspot responsible for two inherited peripheral neuropathies is located near a mariner transposon-like element. Nat Genet 12:288–297.
- Repping, S., Skaletsky, H., Brown, L., van Daalen, S.K., Korver, C.M., Pyntikova, T., (2003) Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nat Genet 35:247–251.
- Repping, S., Skaletsky, H., Lange, J., Silber, S., Van Der Veen, F., Oates, R.D., (2002) Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet 71:906–922.
- Repping, S., van Daalen, S.K., Brown, L.G., Korver, C.M., Lange, J., Marszalek, J.D., (2006) High mutation rates have driven extensive structural polymorphism among human Y chromosomes. Nat Genet 38:463–467.
- Repping, S., van Daalen, S.K., Korver, C.M., Brown, L.G., Marszalek, J.D., Gianotten, J., (2004) A family of human Y chromosomes has dispersed throughout northern Eurasia despite a 1.8-Mb deletion in the azoospermia factor c region. Genomics 83:1046–1052.
- Rozen, S., Skaletsky, H., Marszalek, J.D., Minx, P.J., Cordum, H.S., Waterston, R.H., (2003) Abundant gene conversion between arms of palindromes in human and ape Y chromosomes. Nature 423:873–876.
- Santos, F.R., Pandya, A., Tyler-Smith, C. (1998) Reliability of DNA-based sex tests. Nat Genet 18:103.
- Saxena, R., de Vries, J.W., Repping, S., Alagappan, R.K., Skaletsky, H., Brown, L.G., (2000) Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics 67:256–267.
- Schultz, N., Hamra, F.K., Garbers, D.L. (2003) A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA 100:12201–12206.
- Shaw, C.J., Lupski, J.R. (2004) Implications of human genome architecture for rearrangement-based disorders: the genomic basis of disease. Hum Mol Genet 13(Spec No 1):R57–64.
- Skaletsky, H., Kuroda-Kawaguchi, T., Minx, P.J., Cordum, H.S., Hillier, L., Brown, L.G., (2003) The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423:825–837.
- Slack, A., Thornton, P.C., Magner, D.B., Rosenberg, S.M., Hastings, P.J. (2006) On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet 2:e48.
- Stankiewicz, P., Lupski, J.R. (2002) Genome architecture, rearrangements and genomic disorders. Trends Genet 18:74–82.
- Sun, C., Skaletsky, H., Birren, B., Devon, K., Tang, Z., Silber, S., (1999) An azoospermic man with a de novo point mutation in the Y-chromosomal gene USP9Y. Nat Genet 23:429–432.
- Sun, C., Skaletsky, H., Rozen, S., Gromoll, J., Nieschlag, E., Oates, R., (2000) Deletion of azoospermia factor a (AZFa) region of human Y chromosome caused by recombination between HERV15 proviruses. Hum Mol Genet 9:2291–2296.
- Tiepolo, L., Zuffardi, O. (1976) Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet 34:119–124.
- Tilford, C.A., Kuroda-Kawaguchi, T., Skaletsky, H., Rozen, S., Brown, L.G., Rosenberg, M., (2001) A physical map of the human Y chromosome. Nature 409:943–945.
- Tsuchiya, K., Reijo, R., Page, D.C., Disteche, C.M. (1995) Gonadoblastoma: molecular definition of the susceptibility region on the Y chromosome. Am J Hum Genet 57:1400–1407.
- Turner, D.J., Miretti, M., Rajan, D., Fiegler, H., Carter, N.P., Blayney, M.L., (2008) Germline rates of de novo meiotic deletions and duplications causing several genomic disorders. Nat Genet 40:90–95.
- Tyler-Smith, C., Taylor, L., Muller, U. (1988) Structure of a hypervariable tandemly repeated DNA sequence on the short arm of the human Y chromosome. J Mol Biol 203:837–848.
- Underhill, P.A., Kivisild, T. (2007) Use of Y chromosome and mitochondrial DNA population structure in tracing human migrations. Annu Rev Genet 41:539–564.
- Van Assche, E., Bonduelle, M., Tournaye, H., Joris, H., Verheyen, G., Devroey, P., (1996) Cytogenetics of infertile men. Hum Reprod 11( Suppl 4):1–24; discussion 25–26.
- Vergnaud, G., Page, D.C., Simmler, M.C., Brown, L., Rouyer, F., Noel, B., (1986) A deletion map of the human Y chromosome based on DNA hybridization. Am J Hum Genet 38:109–124.
- Visser, L., Westerveld, G.H., Korver, C.M., van Daalen, S.K., Hovingh, S.E., Rozen, S., (2009) Y chromosome gr/gr deletions are a risk factor for low semen quality. Hum Reprod 24:2667–2673.
- Vissers, L.E., Bhatt, S.S., Janssen, I.M., Xia, Z., Lalani, S.R., Pfundt, R., (2009) Rare pathogenic microdeletions and tandem duplications are microhomology-mediated and stimulated by local genomic architecture. Hum Mol Genet 18:3579–3593.
- Vodicka, R., Vrtel, R., Dusek, L., Singh, A.R., Krizova, K., Svacinova, V., (2007) TSPY gene copy number as a potential new risk factor for male infertility. Reprod Biomed Online 14:579–587.
- Vogt, P., Chandley, A.C., Hargreave, T.B., Keil, R., Ma, K., Sharkey, A. (1992) Microdeletions in interval 6 of the Y chromosome of males with idiopathic sterility point to disruption of AZF, a human spermatogenesis gene. Hum Genet 89:491–496.
- Vogt, P.H., Edelmann, A., Kirsch, S., Henegariu, O., Hirschmann, P., Kiesewetter, F., (1996) Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 5:933–943.
- Walsh, T.J., Pera, R.R., Turek, P.J. (2009) The genetics of male infertility. Semin Reprod Med 27:124–136.
- Woodward, K.J., Cundall, M., Sperle, K., Sistermans, E.A., Ross, M., Howell, G., (2005) Heterogeneous duplications in patients with Pelizaeus-Merzbacher disease suggest a mechanism of coupled homologous and nonhomologous recombination. Am J Hum Genet 77:966–987.
- Wu, B., Lu, N.X., Xia, Y.K., Gu, A.H., Lu, C.C., Wang, W., (2007) A frequent Y chromosome b2/b3 subdeletion shows strong association with male infertility in Han-Chinese population. Hum Reprod 22:1107–1113.
- Yang, Y., Ma, M.Y., Xiao, C.Y., Li, L., Li, S.W., Zhang, S.Z. (2008) Massive deletion in AZFb/b+c and azoospermia with Sertoli cell only and/or maturation arrest. Int J Androl 31:573–578.
- Yatsenko, A.N., Yatsenko, S.A., Weedin, J.W., Lawrence, A.E., Patel, A., Peacock, S., (2010) Comprehensive 5-year study of cytogenetic aberrations in 668 infertile men. J Urol 183:1636–1642.
- Zhang, F., Carvalho, C.M., Lupski, J.R. (2009a) Complex human chromosomal and genomic rearrangements. Trends Genet 25:298–307.
- Zhang, F., Khajavi, M., Connolly, A.M., Towne, C.F., Batish, S.D., Lupski, J.R. (2009b) The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat Genet 41:849–853.
- Zhang, F., Li, Z., Wen, B., Jiang, J., Shao, M., Zhao, Y., (2006) A frequent partial AZFc deletion does not render an increased risk of spermatogenic impairment in East Asians. Ann Hum Genet 70:304–313.
- Zhang, F., Lu, C., Li, Z., Xie, P., Xia, Y., Zhu, X., (2007) Partial deletions are associated with an increased risk of complete deletion in AZFc: a new insight into the role of partial AZFc deletions in male infertility. J Med Genet 44:437–444.
- Zhang, F., Potocki, L., Sampson, J.B., Liu, P., Sanchez-Valle, A., Robbins-Furman, P., (2010a) Identification of uncommon recurrent Potocki-Lupski syndrome-associated duplications and the distribution of rearrangement types and mechanisms in PTLS. Am J Hum Genet 86:462–470.
- Zhang, F., Seeman, P., Liu, P., Weterman, M.A., Gonzaga-Jauregui, C., Towne, C.F., (2010b) Mechanisms for nonrecurrent genomic rearrangements associated with CMT1A or HNPP: rare CNVs as a cause for missing heritability. Am J Hum Genet 86:892–903.
- Zhang, J.S., Yang-Feng, T.L., Muller, U., Mohandas, T.K., de Jong, P.J., Lau, Y.F. (1992) Molecular isolation and characterization of an expressed gene from the human Y chromosome. Hum Mol Genet 1:717–726.