Abstract
Sperm progressive motility has been reported to be one of the key factors influencing in vitro fertilization rates. However, recent studies have shown that sperm DNA fragmentation is a more robust predictor of assisted reproductive outcomes including reduced fertilization rates, embryo quality, and pregnancy rates. This study aimed to compare the usefulness of sperm progressive motility and DNA damage as predictive tools of in vitro fertilization rates. Here, 136 couples provided 1,767 eggs with an overall fertilization rate of 64.2%. The fertilization rate in vitro correlated with both sperm progressive motility (r2 = 0.236; P = 0.002) and DNA fragmentation (r2 = −0.318; P < 0.001). The relative risk of a poor fertilization rate was 9.5 times higher in sperm of men with high DNA fragmentation (>40%) compared with 2.6 times in sperm with poor motility (<40%). Further, sperm DNA fragmentation gave a higher specificity (93.3%) in predicting the fertilization rate than progressive motility (77.8%). Finally, the odds ratio to determine fertilization rate (>70%) was 4.81 (1.89–12.65) using progressive motility compared with 24.18 (5.21–154.51) using DNA fragmentation. This study shows that fertilization rates are directly dependent upon both sperm progressive motility and DNA fragmentation, but sperm DNA fragmentation is a much stronger test.
Introduction
One in six couples experiences fertility problems during their reproductive lives so assisted reproductive technologies (ART) have a major role in modern society. The success of ART varies depending on a range of male and female factors, but adequate structure and function of male and female gametes is essential in all cases [Varghese et al. Citation2009]. Sperm progressive motility is essential for the sperm to penetrate the zona pellucida both in vivo and in vitro, and thus this has been considered one of the most important factors in determining fertilization rates [Chiu et al. Citation1987; Donnelly et al. Citation1998; Turner Citation2006]. Fertilization is comprised of two major steps: the interaction between the sperm and the oocyte, and fusion of male and female gametes to form a pronucleus [Wassarman Citation1999]. Vigorous sperm motility is known to facilitate fertilization by enabling the sperm to penetrate the cumulus cell, corona radiate, and finally the zona pellucida [Liu et al. Citation1991]. Reduced motility is observed in infertile men; often associated with increased mitochondrial abnormalities [Folger et al. Citation1993; Kao et al. Citation1998] and structural deformities in the flagella [Baccetti et al. Citation1993; Chemes et al. Citation1998]. The aetiology of impaired motility is related to an increase in oxidative factors in the seminal plasma [Urata et al. Citation2001; Kao et al. Citation2008], increased age [Kidd et al. Citation2001], and electromagnetic radiation [Yan et al. Citation2007].
Recently the limitations of conventional WHO parameters such as motility to predict ART success have been highlighted [Lefievre et al. Citation2007; Lewis Citation2007]. Through many qualitative studies, sperm DNA damage has been reported to be a more robust biomarker. It has associations with all ART outcomes, but specifically with fertilization [Donnelly et al. Citation1998; Sun et al. Citation1997; Esterhuizen et al. Citation2000; Host et al. Citation2000; Tomlinson et al. Citation2001; Benchaib et al. Citation2003; Citation2007; Henkel et al. Citation2003; Citation2004; Saleh et al. Citation2003; Chohan et al. Citation2004; Gandini et al. Citation2004; Huang et al. Citation2005; Payne et al. Citation2005; Borini et al. Citation2006; Muriel et al. Citation2006; Bakos et al. Citation2007; Bungum et al. Citation2007; Frydman et al. Citation2008; Lin et al. Citation2008]. In contrast, a smaller number of studies report that sperm DNA damage does not affect fertilization rates [Tomlinson et al. Citation2001; Henkel et al. Citation2003; Chohan et al. Citation2004; Gandini et al. Citation2004; Benchaib et al. Citation2007; Bungum et al. Citation2007; Frydman et al. Citation2008; Lin et al. Citation2008]. Thus, there is a controversy in the literature as to the impact of sperm DNA damage on this early fertility check point. To compare the power of motility with DNA damage as predictors of IVF fertilization, we determined both parameters in the same semen samples and correlated them with fertilization rates in vitro.
Results
Association of semen parameters with sperm DNA damage and fertilization rates
An overall fertilization rate of 64.2% was observed using the 1,767 eggs included in the study. Of the conventional semen parameters: volume, concentration, and morphology, and male and female age, there was no correlation with fertilization rate (). In contrast, there was a positive correlation with progressive motility (r2 = 0.236; P = 0.002). There was also a negative correlation between sperm DNA fragmentation and sperm progressive motility (r2 = −0.214; P = 0.005) but no association was found with any other semen parameter and sperm DNA damage. In the univalent and multivalent analyses, semen parameters (volume, concentration, and normal morphology) showed no statistical significance when predicting fertilization rate ().
Table 1. Comparison Between Semen Parameters, Age, and Sperm DNA Fragmentation with Fertilization Rate.
Table 2. The Effect of Standard Semen Parameters and DNA Fragmentation on Fertilization Rate.
Relationship between progressive motility and sperm DNA fragmentation with fertilization rate
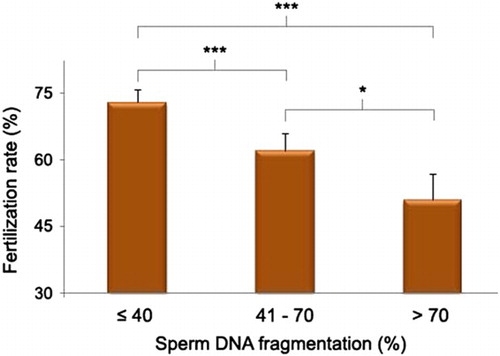
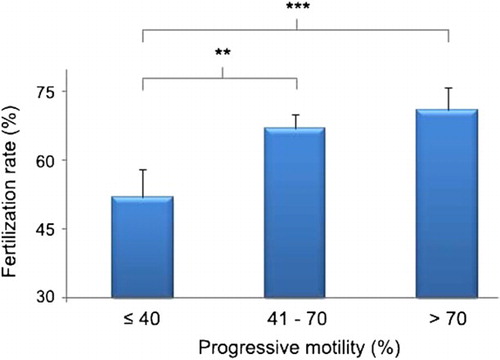
A significant negative correlation was observed between fertilization rate and sperm DNA measured in both native semen (r2 = −0.318; P < 0.001) and DGC (Density Gradient Centrifugation) sperm (r2 = −0.261; P <0.001). When the fertilization rate was categorized into two categories (<70% and ≥70%) there was a significant difference in mean progressive motility (49.24 ± 3.14 vs. 57.81 ± 1.22; P = 0.006), sperm DNA damage measured in the native semen (56.60 ± 4.43 vs. 47.03 ± 1.73; P = 0.010), and the DGC sperm (41.00 ± 4.16 vs. 33.02 ± 1.51; P = 0.044) between the two categories. When progressive motility was divided into three categories, high and moderate motility groups had good fertilization rates 71% and 67%, respectively, while low motility was associated with a lower fertilization rate of 52% (). As sperm DNA fragmentation in native semen increased, the fertilization rate was reduced; low (73%), moderate (62%) and high DNA damage (51%) fertilization rate (). The relationship was also observed for DGC sperm. The Chi-square analysis showed a significant association among the three categories of fertilization rate with both progressive motility and sperm DNA fragmentation (χ2 = 16.06 and 35.68, respectively), with four degrees of freedom. In the univalent model, abnormal motility and high sperm DNA damage in both native and DGC sperm showed a significant decrease in fertilization rate (). The association became stronger when DNA fragmentation was included in the multivalent analysis. However, when sperm progressive motility and DNA damage were included in the model, the odds ratio to obtain a good fertilization rate (>70%) when DNA damage <40% was 6.01 (CI: 1.57–24.78) and for DNA damage >70% was 2.03 (CI: 1.38–11.22), was significant.
Clinical significance of progressive motility and sperm DNA fragmentation to determine in vitro fertilization success
The odds ratio to determine fertilization using sperm motility was 4.81 (1.89–12.65) while the odds ratio was 24.18 (5.21–154.51) using sperm DNA fragmentation. Sperm of men with high DNA fragmentation and low motility results in 9.5 times and 2.6 times the increased relative risk of lower fertilization (<40%), when compared with low DNA damage and poor motility. Similarly, sperm DNA damage showed a higher specificity (93.3%) in predicting fertilization rates than progressive motility (77.8%). Sperm with high progressive motility and low sperm DNA damage had a 96.0% probability of resulting in >70% fertilization rate ().
Table 3. Prognostic Value of Progressive Motility and Sperm DNA Fragmentation to Determine Fertilization Rate in vitro.
Discussion
Evaluation of semen parameters is still the gold standard in diagnosing male infertility. These conventional tests are also used to choose IVF or ICSI as the best treatment for each couple [Repping et al. Citation2002; van Weert et al. Citation2004]. In addition to determining male fertility prior to ART, semen analysis is also performed to determine the chance of a spontaneous pregnancy [Wald Citation2008]. However, despite its continued widespread use, this conventional method of infertility diagnosis has limited use as it lacks the predictive power to determine male infertility or assisted reproductive outcomes (reviewed by [Tomlinson et al. Citation1999; Lewis Citation2007]). Microscopic analysis may remain the first stage of diagnosis but molecular testing is needed to provide a more robust prognostic tool in ART [Lopes et al. Citation1998; Tomlinson et al. Citation2001].
The most important finding in this study is that we report DNA fragmentation measured by the alkaline comet assay has a stronger prognostic ability to predict in vitro fertilization rate than progressive motility. The existing data regarding the relationship between sperm DNA fragmentation and fertilization rates are conflicting [Morris et al. Citation2002; Chohan et al. Citation2004; Henkel et al. Citation2004; Borini et al. Citation2006; Bakos et al. Citation2007; Bungum et al. Citation2007; Lin et al. Citation2008]. Our data show a strong relationship between sperm DNA fragmentation and fertilization rates in IVF both in native semen (r2 = −0.318; P < 0.001) and DGC sperm (r2 = −0.261; P < 0.001). When sperm DNA fragmentation is >40%, we found a significant negative relationship with fertilization rate. Again, this is in agreement with Benchaib et al. [2003] although they used a threshold value of 10% when measuring damage by the TUNEL assay. Our higher threshold is due to the sensitivity of the alkaline comet assay where all double and single strand breaks are measured throughout the entirety of relaxed chromatin. This is in contrast to other assays where perhaps only peripheral DNA damage is measurable. The correlation between sperm DNA damage and fertilization rates is consistent with when the sperm DNA fragmentation is low, the oocytes are able to repair the DNA damage [Sakkas et al. Citation1996; Ahmadi and Ng Citation1999]. However, their ability to repair is limited as the level of sperm DNA fragmentation increases. It is also postulated that with DNA fragmentation sperm may fail to decondense and thus not be able to develop to the pronuclear stage resulting in fertilization failure [Sakkas et al. Citation1996].
Some studies have shown relationships between semen parameters and sperm DNA damage [Tomlinson et al. Citation2001; Larson-Cook et al. Citation2003; Virro et al. Citation2004]. However, our study supports those of Frydman et al. [2008] and Greco et al. [2005] in showing few correlations between conventional semen parameters and sperm DNA fragmentation as we observed a significant negative correlation between sperm DNA and only one conventional parameter; that of sperm motility.
The present study has shown that IVF outcomes are not correlated with semen volume, sperm concentration, or morphology. However, the chance of fertilization increased with the progressive motile population of sperm. Several studies have shown fertilization failure when sperm motility is less than 30% and a reduction of fertilization rate in semen with motility < 50% [van Uem et al. Citation1985; Hirsch et al. Citation1986]. Similarly, our results show a good fertilization rate in semen with both good (>70%) and moderate (40–70%) motility but not with low motility <40% (). Our findings (also reported in another study from our group [Simon et al. Citation2010; Citation2011]) conflict with several older studies that report a decrease in fertilization rate with decrease in sperm concentration, percentage motility, or normal morphology [Battin et al. Citation1985; Hirsch et al. Citation1986; Matson et al. Citation1986]. Mahadevan and Trouson [1984] showed an influence of sperm concentration on fertilization rate when the count fell below a level of 10 million/ml, whereas variations in sperm concentration above this level had no influence on fertilization rate. However, in support of this study, sperm motility alone was shown to be the only semen parameter to influence fertilization rate [Amann Citation1989; Bongso et al. Citation1989; Donnelly et al. Citation1998]. Further, Battin et al. [1985] showed sperm motility after swim-up was also associated with the rate of fertilization. Abnormalities of sperm motility include flagellar abnormalities, deficient mitochondrial metabolism, failure of sperm recognition of the zona pellucida, and an inability to complete sperm-oocyte fusion [Kao et al. Citation2008]. Any or all of these could be the cause of reduced fertilization. Our study supports the reports by Bartoov et al. [1993] and Robinson et al. [1994] that the rate of fertilization is unaffected by an increase in abnormal morphology of the sperm.
The odds ratio to obtain a good (>70%) fertilization rate was higher using sperm DNA fragmentation then the conventional sperm motility (24.18 vs. 4.81, respectively). Although both progressive motility and DNA damage were significant to determine the fertilization rate, measurement of sperm DNA damage showed greater sensitivity (66%) and specificity (93%) in predicting good fertilization rate than progressive motility (sensitivity 58% and specificity 78%). Men with high DNA fragmentation had a higher relative risk i.e., 9.5 times, a fertilization rate of ≤40%. The strong prognostic value of sperm DNA fragmentation was also supported with a high positive predictive value (97.44%). In semen samples having both high motility and low sperm DNA fragmentation, the probability of obtaining a good fertilization rate was >96.0%. In conclusion, measurement of sperm DNA fragmentation by the alkaline comet assay has a markedly greater relative risk and specificity than the conventional measurement of sperm progressive motility in predicting in vitro fertilization rates.
Materials and Methods
This project was approved by the Office for Research Ethics Committees in Northern Ireland and the Royal Group of Hospitals Trust Clinical Governance Committee. The study was conducted at the Regional Fertility Centre, Royal Jubilee Maternity Services, Belfast, Northern Ireland, UK during the period April, 2008 to December, 2009. Sperm samples for research were obtained after written consent from all patients. A total of 240 couples attending for IVF were recruited and of these, 136 couples were included in this study as they met the following criteria: a) a minimum of five oocytes were retrieved and b) female partners were <40 years. Couples with failed fertilization and men with antisperm antibodies in their semen were excluded from the study.
Semen analysis and sperm preparation
Semen samples were collected by masturbation from men after 2–5 d of recommended abstinence, on the day of IVF treatment. Semen analysis was performed within 1 h of ejaculation, following a period of incubation at 37°C to allow for liquefaction. Sperm motility was measured by light microscopy, also according to WHO [1992] criteria. Semen was subjected to density gradient centrifugation (DGC) using a two-step discontinuous Puresperm gradient (90%–45%; Hunter Scientific Limited, UK). For each semen sample with a normozoospermic profile the whole sample was layered on top of 2 ml (90%) and 4 ml (45%) gradient and centrifuged at 250 x g for 20 min. For semen samples with less than normal WHO parameters, 1 ml of semen was layered on top of 1 ml (90%) and 1 ml (45%) gradient and centrifuged at 100 x g for 20 min. The resulting sperm pellets were washed twice with culture media (Vitrolife G5 sequential media series, Vitrolife Inc, Goteborg, Sweden) and concentrated by centrifugation at 250 and 100 x g, respectively, for 10 min or resuspended in fresh culture media (2 ml). After DGC, sperm motility was measured again. Hence, two populations of sperm were used to measure both motility and DNA damage: the whole population (native semen) for each patient and that with the best fertilizing potential as used for clinical treatment (DGC).
IVF treatment
All IVF cycles were performed according to routine procedures. Briefly, ovulation induction was achieved with recombinant FSH following a long protocol of pituitary desensitization with a GnRH analogue. HCG was administered when there were at least three follicles of diameter >17 mm, 36 h before oocyte retrieval. Mature, metaphase II oocytes obtained by vaginal ultrasound-guided aspiration were cultured in media Vitrolife G5 sequential media series (Vitrolife Inc, Goteborg, Sweden) at 37°C with 6% CO2 in air. One or two embryos were transferred into the uterine cavity after an additional 24-48 h. Luteal phase support was provided by vaginally administered progesterone.
Alkaline comet assay
Sperm DNA damage was assessed using an alkaline single cell gel electrophoresis (comet) assay as previously modified by our group [Hughes et al. Citation1997; Donnelly et al. Citation1999]. Our previous study has reported an intra-assay coefficient variation of 6% for this assay [Hughes et al. Citation1997].
Statistical analysis
Data was analyzed using the Statistical Package for the Social Sciences (SPSS 15) for Windows (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard error. The amount of fragmented DNA migrated in the comet tail was expressed as percentage of damage for native semen or DGC sperm. Sperm DNA damage was categorized into three groups: low DNA damage (0–40%), moderate DNA damage (41–70%), and high DNA damage (71–100%) [Simon et al. Citation2010]. The fertilization rate for each couple was calculated as the percentage of oocytes fertilized. Fertilization rates were categorized into two groups (≤70% and >70%) and three groups, poor (0–40%), moderate (41–70%), and good (71–100%) [Ola et al. Citation2001]. Similarly, progressive motility was categorized into three groups: poor (0–40%), moderate (41–70%), and good (71–100%) motility [Bonde et al. Citation1998; Tesarik et al. Citation2006]. All tests were two-sided with a probability value of less than 0.05 to be regarded as significant.
Spearman's Rank correlation coefficient was used to analyze the relationship between semen parameters with sperm DNA fragmentation. Logistic regression was used to evaluate the effect of semen parameters, male age, or female age on fertilization rate. Duncan's test for multi-group comparison was performed to analyze each category of fertilization rate with semen parameters and age. Pearson correlation was used to find any association between the variables: progressive motility, sperm DNA fragmentation, and fertilization rate. Finally, Chi-square analysis was performed comparing each of the three variables and their categories separately. We evaluated the effect of sperm DNA fragmentation on fertilization rate, in cases where all the standard semen parameters were normal and also where one semen parameter was abnormal. Each semen parameter was categorized into a normal or an abnormal category according to WHO [1992] guidelines. Univalent and multivalent analyzes were performed using sperm DNA damage as fixed variable and the odds ratios to obtain a fertilization rate (>70%) were determined. The odds ratios and their 95% CI, specificity, sensitivity, positive and negative predictive power, and relative risk in predicting good fertilization rates (>70%) was then estimated for sperm progressive motility compared to DNA damage.
Acknowledgment
We gratefully acknowledge Hamilton Thorne Biosciences for funding LS in his doctoral studies.
Declaration of Interest: The authors report no conflict of interest.
References
- Ahmadi, A. and Ng, S.C. (1999) Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool 284:696–704.
- Amann, R.P. (1989) Can the fertility potential of a seminal sample be predicted accurately? J Androl 10:89–98.
- Baccetti, B., Burrini, A.G., Capitani, S., Collodel, G., Moretti, E., Piomboni, P. and Renieri, T. (1993) Notulae seminologicae 2. The “short tail” and “stump” defect in human spermatozoa. Andrologia 25:331–335.
- Bakos, H.W., Thompson, J.G., Feil, D. and Lane, M. (2007) Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl 31(5):518–526.
- Bartoov, B., Eltes, F., Pansky, M., Lederman, H., Caspi, E. and Soffer, Y. (1993) Estimating fertility potential via semen analysis data. Hum Reprod 8:65–70.
- Battin, D., Vargyas, J.M., Sato, F., Brown, F. and Marrs, R.P. (1985) The correlation between in vitro fertilization of human oocytes and semen profiles. Fert Steril 44:835–838.
- Benchaib, M., Braun, V., Lornage, J., Hadj, S., Salle, B., Lejeune, H. and Guearin, J.F. (2003) Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique Hum Reprod 18(5):1023–1028.
- Benchaib, M., Lornage, J., Mazoyer, C., Lejeune, H., Salle, B. and Guerin, J.F. (2007) Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril 87(1):93–101.
- Bonde, J.P.E., Ernst, E., Jensen, T.K., Hjollund, N.H.I., Kolstad, H., Henriksen, T.B., (1998) Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 352:1172–1177.
- Bongso, T.A., Ng, S.C, Mok, H., Lim, M.N., Teo, H.L, Wong, P.C, and Ratnam, S.S. (1989) Effect of sperm motility on human in vitro fertilization. Arch Androl 22:185–90.
- Borini, A., Tarozzi, N., Bizzaro, D., Bonu, M.A., Fava, L., Flamigni, C. and Coticchio, G. (2006) Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod 21(11):2876–2881.
- Bungum, M., Humaidan, P., Axmon, A., Spano, M., Bungum, L., Erenpreiss, J. and Giwercman, A. (2007) Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 22:174–179.
- Chemes, H.E., Olmedo, S.B., Carrere, C., Oses, R., Carizza, C., Leisner, M. and Blaquier, J. (1998) Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod 13:2521–2526.
- Chiu, T.T.Y., Tam, P.P.L., Mao, K.R. and Lam, Y.M. (1987) The relationship of semen factors and the outcome of in vitro fertilization of human oocytes. J Hong Kong Med Assoc 39(1):27–31.
- Chohan, K.R., Griffin, J.T., Lafromboise, M., De Jonge, C.J. and Carrell, D.T. (2004) Sperm DNA damage relationship with embryo quality and pregnancy outcome in IVF patients. Fertil Steril 82(2):S55–S56.
- Donnelly, E.T., Lewis, S.E.M., McNally, J.A. and Thompson, W. (1998) In vitro fertilization and pregnancy rates: the influence of sperm motility and morphology on IVF outcome. Fertil Steril 70(2):305–314.
- Donnelly, E.T., McClure, N. and Lewis, S.E. (1999) The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 14:505–512.
- Esterhuizen, A.D., Franken, D.R., Lourens, J.G.H., Prinsloo, E. and Van Rooyen, L.H. (2000) Sperm chromatin packing as an indicator of in-vitro fertilization rate. Hum Reprod 15(3):657–661.
- Folger, T., Bertheussen, K., Lindal, S., Torbergsen, T. and Oian, P. (1993) Mitochondrial disease and reduced sperm motility. Hum Reprod 8(11):1863–1868.
- Frydman, N., Prisant, N., Hesters, L., Frydman, R., Tachdjian, G., Cohen-Bacrie, P. and Fanchin, R. (2008) Adequate ovarian follicular status does not prevent the decrease in pregnancy rates associated with high sperm DNA fragmentation. Fertil Steril 89(1): 93–98.
- Gandini, L., Lombardo, F., Paoli, D., Caruso, F., Eleuteri, P., Leter, G., (2004) Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod 19(6):1409–1417.
- Greco, E., Scarselli, F., Iacobelli, M., Rienzi, L., Ubaldi, F., Ferrero, S., (2005) Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 20:226–230.
- Henkel, R., Hajimohammad, M., Stalf, T., Hoogendijk, C., Mehnert, C., Menkveld, R., (2004) Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril 81(4):965–972.
- Henkel, R., Kierspel, E., Hajimohammad, M., Stalf, T., Hoogendijk, C., Mehnert, C., (2003) DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod BioMed Online 7(4):477–484.
- Hirsch, I., Gibbons, W.E., Lipshultz, L.I., Rossavik, K.K., Young, R.L., Poindexter, A.N., (1986) In vitro fertilization in couples with male factor infertility. Fert Steril 45:659–664.
- Host, E., Lindenberg, S. and Smidt-Jensen, S. (2000) The role of DNA strand breaks in human spermatozoa used for IVF and ICSI. Acta Obstet Gynecol Scand 79:559–563.
- Huang, C.C., Lin, D.P.C., Tsao, H.M., Cheng, T.C., Liu, C.H. and Lee, M.S. (2005) Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril 84(1):130–140.
- Hughes, C.M., Lewis, S.E.M., McKelvey-Martin, V. and Thompson, W. (1997) Reproducibility of human sperm DNA measurements using a single cell gel electrophoresis assay. Mutat Res 374:261–268.
- Kao, S.H., Chao, H.T. and Wei, Y.H. (1998) Multiple deletions of mitochondrial DNA are associated with the decline of motility and fertility of human spermatozoa. Mol Hum Reprod 4:657–666.
- Kao, S.H., Chao, H.T., Chen, H.W., Hwang, T.I.S., Liao, T.L. and Wei, Y.H. (2008) Increase of oxidative stress in human sperm with lower motility. Fertil Steril 89:1183–1190.
- Kidd, S.A., Eskenazi, B. and Wyrobek, A.J. (2001) Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 75:237–248.
- Larson-Cook, K.L., Brannian, J.D., Hansen, K.A., Kasperson, K.M., Aamold, E.T. and Evenson, D.P. (2003) Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril 80:895–902.
- Lefievre, L., Bedu-Addo, K., Conner, S.J., Machado-Oliveira, G.S., Chen, Y., Kirkman-Brown, (2007) Counting sperm does not add up any more: time for a new equation? Reprod 133(4):675–684.
- Lewis, S. (2007) Is sperm evaluation useful in predicting human fertility? Reproduction 134:1–11.
- Lin, H.H., Lee, R.K., Li, S.H., Lu, C.H., Sun, F.J. and Hwu, Y.M. (2008) Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 90(2):352–359.
- Liu, D.Y., Clarke, G.N. and Baker, H.W. (1991) Relationship between sperm motility assessed with the Hamilton-Thorn motility analyzer and fertilization rates in vitro. J Androl 12(4):231–239.
- Lopes, S., Sun, J.G., Jurisicova, A., Meriano, J. and Casper, R.F. (1998) Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 69:528–532.
- Mahadevan, M.M. and Trounson, A.O. (1984) The influence of seminal characteristics on the success rate of human in vitro fertilization. Fert steril 42:400–405.
- Matson, P.L., Turner, S.R., Yovich, J.M., Tuvik, A.I. and Yovich, J.L. (1986) Oligospermic infertility treated by in vitro fertilization. Aust NZ J Obstet Gynec 26:84–87.
- Morris, I.D., Iiott, S., Dixon, L. and Brison, D.R. (2002) The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod 17(4):990–998.
- Muriel, L., Garrido, N., Fernández, J.L., Remohí, J., Pellicer, A., De los Santos, M.J. and Meseguer, M. (2006) Value of the sperm DNA fragmentation level, measured by the sperm chromatin dispersion (SCD) test, in the IVF and ICSI outcome. Fertil Steril 85:371–383.
- Ola, B., Afnan, M., Sharif, K., Papaioannou, S., Hammadieh, N. and Barratt, C.L.R. (2001) Should ICSI be the treatment of choice for all cases of in-vitro conception? Hum Reprod 16(12):2485–2490.
- Payne, J.F., Raburn, D.J., Couchman, G.M., Price, T.M., Jamison, M.G. and Walmer, D.K. (2005) Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril 84:356–364.
- Repping, S., van Weert, J.M., Mol, B.W., de Vries, J.W. and van der Veen, F. (2002) Use of the total motile sperm count to predict total fertilization failure in in vitro fertilization. Fertil Steril 78:22–28.
- Robinson, J.N., Lockwood, G.M., Dokras, A., Egan, D.M., Nicholson, S.C., Ross, C. and Barlow, D.H. (1994) Does isolated teratozoospermia affect performance in in vitro fertilization and embryo transfer? Hum Reprod 9:870–874.
- Sakkas, D., Urner, F., Bianchi, P.G., Bizzaro, D., Wagner, I., Jaquenoud, N., (1996) Sperm chromatin anomalies can influence decondensation after intracytoplasmic sperm injection. Hum Reprod 11:837–843.
- Saleh, R.A., Agarwal, A., Nada, E.A., El-Tonsy, M.H., Sharma, R.K., Meyer, A., (2003) Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 79(3):1597–1606.
- Simon, L., Brunborg, G., Stevenson, M., Lutton, D., McManus, J. and Lewis, S.E.M. (2010) Clinical significance of sperm DNA damage in assisted reproductive outcome. Hum Reprod 25(7):1594–1608.
- Simon, L., Lutton, D., McManus, J. and Lewis, S.E.M. (2011) Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and IVF success. Fertil Steril 95(2):652–657.
- Sun, J.G., Jurisicova, A. and Casper, R.F. (1997) Detection of deoxyribonucleic acid fragmentation in human sperm: Correlation with fertilization in vitro. Biol Reprod 56:602–607.
- Tesarik, J., Mendoza-Tesarik, R. and Mendoza, C. (2006) Sperm nuclear DNA damage: update on the mechanism, diagnosis and treatment. Reprod BioMed Online 12(6):715–721.
- Tomlinson, M.J., Kessopoulou, E. and Barratt, C.L. (1999) The diagnostic and prognostic value of traditional semen parameters. J Androl 20:588–593.
- Tomlinson, M.J., Moffatt, O., Manicardi, G.C., Bizzaro, D., Afnan, M. and Sakkas, D. (2001) Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod 16(10):2160–2165.
- Turner, R.M. (2006) Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev 18:25–38.
- Urata, K., Narahara, H., Tanaka, Y., Egashira, T., Takayama, F. and Miyakawa, I. (2001) Effect of endotoxin-induced reactive oxygen species on sperm motility. Fertil Steril 76(1):163–166.
- van Uem, J.F., Acosta, A.A., Swanson, R.J., Mayer, J., Ackerman, S.B., Burkman, L.J., (1985) Male factor evaluation in vitro fertilization: Norfolk experience. Fertil Steril 44:375–383.
- van Weert, J.M., Repping, S., Van Voorhis, B.J., van der Veen, F., Bossuyt, P.M. and Mol, B.W. (2004) Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: a meta-analysis. Fertil Steril 82:612–620.
- Varghese, A.C., Bragais, F.M., Mukhopadhyay, D., Kundu, S., Pal, M., Bhattacharyya, A.K. and Agarwal, A. (2009) Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia 41:207–215.
- Virro, M.R., Larson-Cook, K.L. and Evenson, D.P. (2004) Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 81(5):1289–1295.
- Wald, M. (2008) Enhancement of human sperm motility. J Urol 180(2):442–443.
- Wassarman, P.M. (1999) Fertilization in animals. Dev Genet 25:83–86.
- World Health Organization. (1992) WHO Laboratory Manual for the Examination of Human Sperm and Sperm-Cervical Mucus Interaction. Cambridge University Press, Cambridge, UK.
- Yan, J.G., Agresti, M., Bruce, T., Yan, Y.H., Granlund, A. and Matloub, H.S. (2007) Effects of cellular phone emissions on sperm motility in rats. Fertil Steril 88:957–964.