Abstract
The incidence of diabetes mellitus is rapidly increasing in the world. One of the complications of diabetes includes disturbance of the reproductive tract, such as infertility, erectile dysfunction, and endocrine disruption. Nitric oxide (NO) is a free radical produced by most cells including the human male and female reproductive tracts. NO has a dual role where low concentrations are essential for homeostatic cellular biology and physiology, but high levels have detrimental effects relating to cellular damage from this reactive oxygen species (ROS). 8-hydroxydeoxyguanosine (8-OHdG) is an oxidized nucleoside of DNA that is currently used as a biomarker of cellular oxidative stress, where urinary levels can correlate with diabetic nephropathy and retinopathy. Our aim was to investigate the relationship between nitrate/nitrite levels and 8-OHdG levels in the semen of diabetic and non-diabetic men. Concentrations of nitrate/nitrite and 8-OHdG were examined in seminal plasma of 32 diabetic and 35 non-diabetic men. The level of nitrate/nitrite was assayed by colorimetric reaction and 8-OHdG was measured by ELISA. Our results showed that the seminal plasma nitrate/nitrite levels were significantly higher in the diabetic group (p < 0. 01). There were also significantly higher 8-OHdG levels in diabetic men compared to non-diabetic men (p < 0.05). Regression analysis indicated that in diabetic men, nitrate/nitrite levels correlated well with 8-OHdG levels (r = 0.64, p < 0.001). A significant trend between nitrate/nitrite and sperm parameters was not observed. Our data suggests that high levels of nitrate/nitrite in the semen of diabetic men is suggestive of reactive oxygen species induced DNA damage that is correlated with 8-OHdG levels but not sperm parameters. These results support the further investigation of NO and 8-OHdG as biomarkers for assessing male infertility.
Introduction
Diabetes mellitus is a significant global contributor to morbidity and mortality in developed as well as developing countries. According to a report from the World Health Organization (WHO), 220 million people worldwide were diagnosed and affected with diabetes in 2009 [WHO Citation2009]. The diabetic sequel is accompanied by both macro vascular and micro vascular complications [Yonekura et al. Citation2005]. An important complication of diabetes is disturbances in the male reproductive system that can affect the majority of patients [Amaral et al. Citation2006]. Some studies on the relationship between diabetes, semen parameters, and male reproductive tracts have been reported, but results are conflicting [Dinulovic and Radonjic Citation1990; Handelsman et al. Citation1985; Niven et al. Citation1995; Ricci et al. Citation2009]. Other than the detection of severe abnormalities in semen parameters (such as azoospermia), routine semen analysis is currently neither sensitive nor specific for evaluating fertility dysfunction in males as a consequence of diabetes [Jequier Citation2005].
Reports in animal models have shown that diabetes has harmful effects on the function of male reproduction and can impair fertility [Cameron et al. Citation1990; Scarano et al. Citation2006]. In human, these studies are rare and the value of conventional semen analysis is unclear. Agbage et al. [2007] reported a high level of sperm nuclear DNA fragmentation in diabetic men compared with non-diabetic men. Furthermore, pregnancy rates of samples with high levels of sperm DNA fragmentation are significantly lower than DNA intact sperm [Evenson and Wixon Citation2006]. Oxidative stress plays a key role in male infertility where, in semen of infertile men, high levels of reactive oxygen species (ROS) have been detected [Cocuzza et al. Citation2007]. High levels of ROS and oxidative stress are associated with DNA damage and can be assessed by oxidative damage to DNA via the nucleoside metabolite, 8- hydroxydeoxyguanosine (8-OHdG) [Kasai et al. Citation2005]. Nitric oxide (NO) plays a key role in many physiological phenomena and is also a ROS that has high reactivity, which is potentially toxic to cells and macromolecules [Burney et al. Citation1999a]. We aimed in this study to establish the effect of NO and 8-OHdG in the semen of diabetic and non-diabetic men.
Results
The mean age of diabetic and non-diabetic subjects was 35.84 ± 8.89 and 32.58 ± 5.68 years, respectively. Characteristics of semen quality are summarized in . Serum volume, concentration, morphology, and motility showed no significant difference between the two groups. Fasting blood glucose and HbA1c were significantly higher (p < 0.001, ) in diabetic compared non-diabetic men. No significant difference was found in the lipid profiles of diabetic and non-diabetic men ().
Table 1. Semen characteristics and biochemical data of diabetic and non-diabetic men.
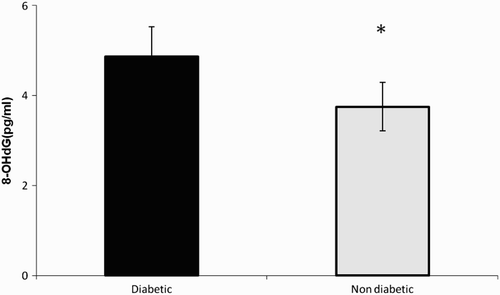
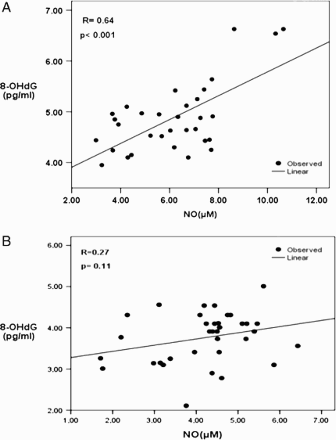
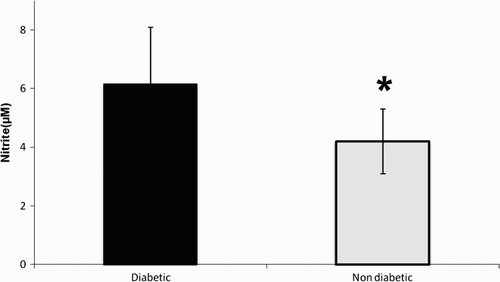
The NO metabolites values were 6.16 ± 1.93 and 4.2 ± 1.4 µM in diabetic and non-diabetic subjects, respectively (). Nitrate/nitrite levels were significantly elevated in the seminal plasma of diabetic men in comparison to non-diabetic subjects (p < 0.01). The levels of 8-OHdG were 4.86 ± 0.67 and 3.75 ± 0.54 (pg/mL) in diabetic and non-diabetic groups, respectively (). 8-OHdG levels were elevated significantly in seminal plasma of diabetic men in comparison to non-diabetic men (p < 0.05). A significant positive correlation between nitrate/nitrite and 8-OHdG levels was observed in the seminal plasma (r = 0.64, p < 0.001, ) of diabetic men. No significant correlation was observed between seminal plasma nitrate/nitrite and the concentration (r = 0.18, p = 0.31), motility (r = 0.00, p = 0.94), and morphology (r = 0.1, p = 0.15) of spermatozoa in diabetic men.
Figure 1. Seminal plasma levels of nitrite in diabetic and non-diabetic men. Nitrate/nitrite was quantified by colorimetric assay. The optical density was measured at 540 nm and the concentration was determined by standard curve. The * indicates significance at p < 0.01.
Discussion
In this study, we assessed semen characteristics from diabetic and non-diabetic men. We did not find a significant difference in sperm characteristics between these two groups however a significant increase in markers of ROS, including NO metabolites, and DNA damage (8-OHdG) was observed in the semen of diabetic men. These findings are in line with other studies indicating difficulty in determining fertility status for diabetic patients by routine semen analysis [Agbaje et al. Citation2007]. NO is produced during NO synthesis (NOS) in various tissues of the human male reproductive system including testes, epididymis, vas deferens, blood vessels, and spermatozoa [Revelli et al. Citation2001]. Our study showed the existence of nitrate/nitrite in seminal plasma that confirms previous studies [Revelli et al. Citation2001; Rosselli et al. Citation1995]. As observed in the present study, nitrate/nitrite in seminal plasma of diabetic men with normal standard sperm parameters was significantly higher than those of the non-diabetic group with normal standard sperm characteristics. This finding can have important diagnostic and prognostic value in the management of male infertility in diabetic patients. Elevated cell damage may lead to low fertility in diabetic men who have normal semen analysis.
Cosentino et al. [1997] reported that prolonged exposure to high glucose concentrations increases eNOS gene expression, protein expression, NO2− release, and production of O2− in endothelial cells. NO can react with O2− and oxygen to form peroxynitrite (ONOO-) that is a powerful radical and can induce DNA damage [Burney et al. Citation1999b]. To determine oxidative DNA damage, we measured the levels of 8-OHdG (as DNA oxidative marker) in the seminal plasma of two groups. Our findings showed a significant increase of 8-OHdG in seminal plasma of diabetic men compared with non-diabetic men. As observed in this study, nitrate/nitrite levels are positively associated with 8-OHdG levels in seminal plasma of diabetic men indicating the occurrence of high oxidative stress in the semen of diabetic men due to the production of NO and its metabolites. Inoue and Kawanishi [1995] reported increased levels of 8-OHdG induced by NO in calf thymus DNA. NO and its derivatives can be due to DNA damage directly or indirectly. Direct damage includes DNA base deamination, peroxynitrite-induced adduct formation, and single strand breaks in the DNA [Felley-Bosco Citation1998]. Indirect damage is due to the interaction of NO reactive species with other macromolecules.
Our findings do not show a significant correlation between seminal plasma nitrate/nitrite concentration and semen parameters such as motility, morphology, and concentration of spermatozoa in the diabetic and non-diabetic group. It must be noted however, all our data were within WHO reference intervals [WHO 1999]. In another study, Revelli et al. [2001] demonstrated no correlation between nitrite concentration and semen parameters, but others showed detrimental effects of higher NO on sperm parameters [Nobunaga et al. Citation1996; Rosselli et al. Citation1995; Wu et al. Citation2004]. In conclusion, this study demonstrates that high levels of NO metabolites are correlated with 8-OHdG concentrations in the seminal plasma of diabetic men. In a recent study, we showed a high level of advanced glycation end-products (AGEs) in seminal plasma of diabetic men [Karimi et al. Citation2011]. The molecular mechanism of increased nitrate/nitrite formation in diabetic conditions needs further investigation.
Materials and Methods
Subjects
Diabetic males (Type 1, n = 17; Type 2, n = 15) aged 22–46 y attending the Diabetes Research Center (Hamadan University of Medical Sciences, Hamadan, Iran), and who were diagnosed with diabetes 5 y prior to the study, were recruited to participate in this study. Subjects with Type 2 diabetes were married and had children and Type 1 diabetic males were single. The participants were on standard medication regimens based on the requirements of their diabetic condition. Non-diabetic subjects (n = 35) were recruited who were age matched, healthy, normospermic, had normal blood sugar and were married and had children. Exclusion criteria included leukocyte concentration >106/mL in ejaculate, history of smoking, varicocoele, occupational exposure to chemicals or excessive heat, and specimens with hyper-viscosity. Written informed consent for participation was obtained and the project was approved by the Research Ethics Committee of Hamadan University of Medical Sciences.
Semen samples were collected by masturbation after 3 d of abstinence according to WHO [1999] recommendations and conventional semen analysis was performed. Seminal plasma was separated by centrifugation (Hermle, Z 230A, Wehingen, Germany) of the samples at 600 x g for 10 min and stored at −80oC. The pellet containing sperm was stored in aliquots of 20 × 106 sperm at -80oC.
Peripheral blood samples were taken after 12–14 h of overnight fasting. Serum glucose was performed using a glucose assay kit (Pars Azmun, Tehran) and lipid profiles were determined using Ziest Chem (Tehran, Iran) kits according to the manufacturer's instructions. HbA1c was quantified using BioSystems kit (736CA, Barcelona Spain) based on the manufacturer's protocol.
Measurement of total NO products in seminal plasma
Nitrate/nitrite was quantified using a colorimetric assay kit (Cayman Chemical, Cat# 780001, Ann Arbor, MI, USA) as indicated in the manufacturer's instructions. Briefly, 80 µL of samples and controls were added in to each well of a 96-well plate. 10 µL of the Enzyme Cofactor mixture and 10 µL of Nitrate Reductase were added to each well followed by incubation at room temperature for two h. After incubation, 50 µL of Griess Reagent R1 and immediately 50 µL of Griess Reagent R2 were added to each well and incubated for 10 min. The optical density was measured using a Tecan Sunrise microplate reader (Tecan, Grödig, Austria) at 540 nm and the concentration was determined using the appropriate standard curve.
8-OHdG assay
8-hydroxy-2-deoxyguanosine (8-OHdG) levels were measured in seminal plasma using a competitive ELISA kit (Cayman chemical, Cat# 589320, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Statistical Analysis
Statistical analysis was performed using SPSS 11 software and an independent sample t-test was used to compare the mean difference between test and control samples. Association between variables was tested using linear regression analysis and Pearson's correlation coefficient. Data were represented as mean ± standard deviation (SD) and a p < 0.05 was considered statistically significant.
Abbreviations
| NO: | = | nitric oxide |
| NOS: | = | nitric oxide synthesis |
| 8-OHdG: | = | 8 – hydroxydeoxyguanosine |
| ROS: | = | reactive oxygen species |
| AGE: | = | advanced glycation endproduct. |
Acknowledgments
We would like to thank the staffs of the Diabetes Research Center and the laboratory of Fatemiyeh Hospital for their assistance in collecting patient data.
Declaration of interest: This study was supported by Hamadan University of Medical Science. The authors report there are no conflicts of interest.
Reference
- Agbaje, I.M., Rogers, D.A., McVicar, C.M., McClure, N., Atkinson, A.B., Mallidis, C., (2007) Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod 22:1871–1877.
- Amaral, S., Moreno, A.J., Santos, M.S., Seica, R., and Ramalho-Santos, J. (2006) Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology 66:2056–2067.
- Burney, S., Caulfield, J. L. , Niles, J. C., Wishnok, J. S., and Tannenbaum, S.R. (1999b) The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res 424:37–49.
- Burney, S., Niles, J.C., Dedon, P.C., and Tannenbaum, S.R. (1999a) DNA damage in deoxynucleosides and oligonucleotides treated with peroxynitrite. Chem Res Toxicol 12:513–520.
- Cameron, D.F., Rountree, J., Schultz, R.E., Repetta, D., and Murray, F.T. (1990) Sustained hyperglycemia results in testicular dysfunction and reduced fertility potential in BBWOR diabetic rats. Am J Physiol 259:E881–889.
- Cocuzza, M., Sikka, S.C., Athayde, K.S., and Agarwal, A. (2007) Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol 33:603–621.
- Cosentino, F., Hishikawa, K., Katusic, Z.S., and Luscher, T.F. (1997) High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96:25–28.
- Dinulovic, D., and Radonjic, G. (1990) Diabetes mellitus/male infertility. Arch Androl 25:277–293.
- Evenson, D.P., and Wixon, R. (2006) Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology 65:979–991.
- Felley-Bosco, E. (1998) Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Metastasis Rev 17:25–37.
- Handelsman, D.J., Conway, A., Boylan, L.M., Yue, D.K., and Turtle, J.R. (1985) Testicular function and glycemic control in diabetic men. A controlled study. Andrologia 17:488–496.
- Inoue, S., and Kawanishi, S. (1995) Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett 371:86–88.
- Jequier, A.M. (2005) Is quality assurance in semen analysis still really necessary? A clinician's viewpoint. Hum Reprod 20:2039–2042
- Karimi, J., Goodarzi, M.T., Tavilani, H., Khodadadi, I., and Amiri, I. (2011) Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res Clin Pract 91:61–66.
- Kasai, H., Svoboda, P., Yamasaki, S., and Kawai, K. (2005) Simultaneous determination of 8-hydroxydeoyguanosine, a marker of oxidative stress, and creatinine, a standardization compound, in urine. Ind Health 43:333–336.
- Niven, M. J. , Hitman, G.A., and Badenoch, D.F. (1995) A study of spermatozoal motility in type 1 diabetes mellitus. Diabet Med 12:921–924.
- Nobunaga, T., Tokugawa, Y., Hashimoto, K., Kubota, Y., Sawai, K., Kimura, T., (1996) Elevated nitric oxide concentration in the seminal plasma of infertile males: nitric oxide inhibits sperm motility. Am J Reprod Immunol 36:193–197.
- Revelli, A., Bergandi, L., Massobrio, M., Lindblom, B., Bosia, A., and Ghigo, D. (2001) The concentration of nitrite in seminal plasma does not correlate with sperm concentration, sperm motility, leukocytospermia, or sperm culture. Fertil Steril 76:496–500.
- Ricci, G., Catizone, A., Esposito, R., Pisanti, F.A., Vietri, M.T., and Galdieri, M. (2009) Diabetic rat testes: morphological and functional alterations. Andrologia 41:361–368.
- Rosselli, M., Dubey, R.K., Imthurn, B., Macas, E., and Keller, P J. (1995) Effects of nitric oxide on human spermatozoa: evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum Reprod 10:1786–1790.
- Scarano, W.R., Messias, A.G., Oliva, S.U., Klinefelter, GR., and Kempinas, W.G. (2006) Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl 29:482–488.
- WHO. (2009) World Health Organization Diabetes: Fact sheet No. 321. http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
- WHO. (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th edition, Cambridge University Press, NY, USA.
- Wu, T.P., Huang, B.M., Tsai, H.C., Lui, M.C., and Liu, M.Y. (2004) Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryonic development. Arch Androl 50:173–179.
- Yonekura, H., Yamamoto, Y., Sakurai, S., Watanabe, T., and Yamamoto, H. (2005) Roles of the receptor for advanced glycation endproducts in diabetes-induced vascular injury. J Pharmacol Sci 97:305–311.