Abstract
In order to investigate the effects of molybdenum (Mo) on sperm parameters and testicular oxidative stress, the ICR strain of adult mice were exposed to different doses of molybdenum for a sub-acute toxicity test. Compared to the control, our results showed that the sperm parameters, including the epididymis index, sperm motility, sperm count, and morphology, increased by a moderate dose of Mo (25 mg/L), but were negatively affected at high doses (≥ 100 mg/L). In addition, the changes of sperm parameters were accompanied with changes of the superoxide dismutase (SOD) activities, the glutathione peroxidase (GPx) activities, and the malondialdehyde (MDA) levels in testes. In conclusion, Mo affects the sperm quality through regulating the testicular oxidative stress in a complex manner.
Introduction
A variety of endocrine disrupting chemicals have been released into the environment in the rapid industrial progress, which may exert adverse health effects in human and animals [Carlsen et al. Citation1992; Khurana et al. Citation2000; Friedmann Citation2002]. Molybdenum (Mo) is an essential trace element in animals and humans. It has been identified as part of the active sites of over 50 enzymes, and may promote normal cell function possibly by catalyzing a variety of hydroxylation, oxygen atom transfer and other oxidation-reduction reactions [Hille et al. Citation1998]. Molybdenum is also an endocrine disruptor and has been widely present and detected in our food and water [Underwood Citation1981; Mills and Davis Citation1987; Kargar et al. Citation2011; Yu et al. Citation2011]. In addition, Mo is broadly used in industrial production, such as metallurgical processes, the manufacture of electronic products, glass, ceramics, lubricants, catalysts, pigments and nano materials [Pandey and Singh Citation2002; CDC 2005; Braydich-Stolle et al. Citation2005; Ema et al. Citation2010]. Furthermore, Mo is also an environmental pollutant discharged from uranium processing, combustion processing, contact lens solutions, and the color additives in cosmetics [ACGIH 1995]. This wide distribution greatly increases the risk of animals and humans exposed to the high level of Mo in the environment. For example, molybdenum concentrations have risen to 0.2 mg/L in areas near mining sites, however, the WHO recommends a maximum level of molybdenum in drinking water of 0.07 mg/L [WHO 1993].
Excessive amounts of Mo can induce reproductive toxicity, especially for male animals and humans [Thomas and Moss 1951; Sharma et al. Citation2004; Bersényi et al. Citation2008]. In rats, ingestion of a high dose of Mo caused decreased sperm motility, count, morphologic abnormalities, epididymis weight decline, and testis histopathologic changes [Pandey and Singh Citation2002, Lyubimov et al. Citation2004.], and fertility [Wirth and Mijal Citation2010]. A reduction in the germ cells and mature spermatocytes in rabbits has been observed [Bersényi et al. Citation2008] as well as a decline in sperm quality and morphology in humans [Meeker et al. Citation2008]. However, the mechanism of Mo affecting the reproductive function of male animals is still not very clear.
Oxidative stress is a powerful mechanism that can lead to sperm damage, deformity, and eventually male infertility [Makker et al. Citation2009]. Sharma et al. [2004] showed that Mo induced oxidative stress in crossbred calves. Spalj et al. [2012] suggested that titanium-molybdenum generated oxidative stress in mouse fibroblasts cultures. Antioxidants provide a defense against oxidative stress.
Seminal plasma contains three main enzymatic antioxidants: superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Spermatozoa possess primarily enzymatic antioxidants, with SOD being the most predominant [Makker et al. Citation2009]. Superoxide dismutase in conjugation with CAT and GPx scavenge both intracellular and extracellular superoxide radicals and inhibit lipid peroxidation [Agarwal and Prabhakaran Citation2005]. Malondialdehyde (MDA) is one of the byproducts of lipid peroxidation that indirectly reflects the level of peroxidation and the degree of cell injury [Sharma et al. Citation2004]. This byproduct has been used in various biochemical assays to monitor the degree of oxidative damage sustained by spermatozoa [Aitken et al. Citation1989; Aitken and Fisher Citation1994]. Oxidative stress can be evaluated by detecting the activities of SOD and GPx, as well as the MDA level in the tissue. Up to now, there are no data available for the oxidative stress of testicular tissue caused by Mo on mice.
This study has been undertaken to evaluate the effect of orally administered Mo on the sperm parameters of the epididymis index, sperm motility, count, and morphology changes. The oxidative stress of testis was considered as a function of the levels of MDA, SOD, and GPx in mice.
Results and Discusssion
The epididymis index, sperm motility, and count are summarized in . The change in morphology at a range of Mo concentrations is shown in . Molybdenum at 25 mg/L improved the sperm quality (P < 0.01), but Mo ≥100 mg/L negatively affected the sperm quality (P < 0.05); Mo at 12.5 and 50 mg/L did not affect sperm quality significantly.
Figure 1. Photomicrographs of the commonly observed abnormal sperms from the 100 mg/L molybdenum dose group. A) normal morphology; B) big head (arrow heading); C) pin head; D) no tail attached; E) without the main section; F) without characteristic curvature; G) two main sections; H) rolled into a spiral; and I) no head attached.
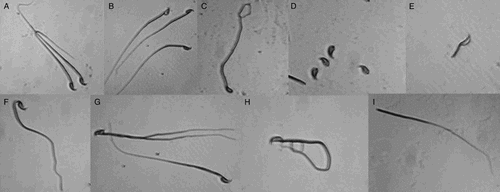
Table 1. Effects of molybdenum treatments on sperm parameters in mice.
As shown in , compared to control, Mo at ≥100 mg/L decreased the activities of SOD and GPx, yet the content of MDA significantly increased. At 25 mg/L Mo markedly improved the activities of SOD and GPx, but did not change MDA. At a concentration that ranged from 12.5 and 50 mg/L only GPx activity decreased significantly. The level of SOD and MDA did not markedly change.
Table 2. Effects of molybdenum treatments on the SOD, GPx, and MDA levels of testes in mice.
The results presented in this study show that the changes of sperm quality are accompanied by alterations in oxidative stress in mice exposed to the various oral doses of Mo. Sperm quality significantly improved and oxidative stress decreased within the 25 mg/L group. On the contrary, sperm quality significantly decreased and oxidative damage increased within the ≥100 mg/L group.
Oral administration of 25 mg/L Mo significantly improved the sperm quality as shown by a significant decrease in the average epididymis index, sperm motility, and count as well as a significant decrease in the sperm abnormality rate (). This is probably due to the enhancement of testicular function marked by a reduction in the degree of oxidative stress as shown by a significant decrease in the activity of both SOD and GPx, and a considerable increase in MDA in testicular tissue (). The effects of Mo on reproductive improvement have been described in several in vitro studies. Our previous study showed that at 5 µg/ml Mo is likely to improve the development of mouse embryos cultured in vitro [CitationBi et al. 2012]. In contrast, Braydich-Stolle et al. [2005] observed that in vitro 5 µg/ml and 10 µg/ml of Mo nano-particles seem to promote plasma membrane leakage of mouse spermatogonial stem cell lines. Molybdenum at ≥100 mg/L negatively impacted sperm quality and increased the oxidative damage in testicular tissue. There is little information in the literature on the in vivo effect of Mo on male mouse reproductive parameters. However, similar phenomena have been observed in other animals and humans. Pandey and Singh [2002] reported a dose-dependent degeneration of testicular morphology and function with declining sperm concentration, motility, normal morphology, and epididymides in rats after oral administration of sodium molybdate at a dose level of ≥ 30 mg/kg body weight. Similarly, Lyubimov et al. [2004] observed that a significant reduction of epididymal weight, sperm count, motility, morphologic abnormalities, and histopathologic changes in testis and epididymis occurred in the rats treated by tetrathiomolybdate at 12 mg/kg/day for 2 months. Bersényi et al. [2008] revealed a reduction in the number of germ cells and mature spermatocytes in the testes, and an appearance of a large number of syncytial giant cells and degenerated cells among the spermatogenic cells in the seminiferous tubules of rabbits fed carrots containing 39 mg Mo/kg dry matter as compared to animals given uncontaminated samples. Meeker et al. [2008] found dose-dependent trends between Mo and declined sperm quality and morphology in humans. In addition, mice exposed to ≥100 mg/L Mo in the present study, like the report in bull calves fed by high dietary intakes of Mo [Thomas and Moss 1951], exhibited a complete lack of libido, and sterility. This probably reflects the response to marked damage of the interstitial cells and germinal epithelium with impaired spermatogenesis in the testes. The results in our study are consistent with the above in the effect of Mo on the epididymides, sperm abnormality, motility, and count. This may be mediated through oxidative stress in testicular tissue. A significant decrease in the SOD and GPx level, and an increase in MDA level, suggests that Mo at a high dose induces oxidative damage of testicular tissue. The MDA content of testis increased sharply, indicating that a high dose of Mo induces lipid peroxidation that is directly reflected by increased oxidative stress. Accompanying this, is a significant increase of MDA and a significant decrease in activities of SOD and GPx. At this high dosage level a reduction in the capacity of testis to eliminate radicals and catalyze the conversation of superoxide to oxygen and hydrogen peroxide to protect the structures and functions of cell membranes from the interference and damage by peroxides is apparent [Ola-Mudathir et al. Citation2008; Portugal-Cohen et al. Citation2010]. Similar phenomena were observed in rabbits. Bersényi et al. [2008] observed that high dietary Mo (39 mg Mo/kg dry matter) can generate free radicals or reactive intermediates, resulting in altering MDA and GPx activity.
In conclusion, molybdenum affects sperm quality through regulating the testicular oxidative stress in a complex manner. Male reproductive parameters apparently improved at moderate doses (25 mg/L), but were significantly repressed at high doses (≥ 100 mg/L). The change in the levels of SOD, GPx, and MDA indicate that the dual functions of Mo on sperm quality are likely to be mediated through oxidative stress in testicular tissue.
Materials and Methods
Chemicals
Unless otherwise stated, all components used in the present study were procured from Sigma–Aldrich Corp. (St. Louis, MO, USA).
Animals
All of the following studies were approved by the Animal Care and Use Committee of Henan University of Science and Technology. The ICR strain adult (3 to 4 weeks of age) male mice weighing 30-35 g were used for the acute toxicity experiments. All mice used in this study were maintained under Good Laboratory Practice (GLP) conditions. The mice had free access to drinking distilled water and commercial standard pellet diet.
Exposure of mice to molybdenum
A sub-acute toxicity test was performed in this study. Sixty healthy ICR strain mice of proven fertility were divided randomly into six groups of 10 animals each. The mice in different groups were respectively allowed free access to the distilled water containing the Mo (as sodium molybdate dihydrate) at concentrations of 0, 12.5, 25, 50, 100, or 200 mg/L for 14 d. These doses were based on a previous study [Schroeder and Mitchener Citation1971] and similar to human exposures [Huang et al. Citation2011].
Collection of testes and epididymides
The testicular tissues of mice from each group were used for determination of SOD, GPx, and MDA, and epididymides for collecting the sperm. The mice were sacrificed by cervical dislocation on day 14 of the experiment. Testes and epididymides were quickly removed and weighed. The epididymides were placed into 37°C preheated saline, the cauda epididymis was lacerated for incubation of sperm. The testes were put in 4°C precooled saline in a refrigerator, then transferred into −20°C before homogenate preparation.
Evaluation of sperm parameters
Semen samples were collected after incubation for 30 min, and semen analysis was conducted following the World Health Organization protocol [WHO 1999]. Sperm concentration (million sperm per milliliter), percent motile sperm, and sperm morphology were investigated in this study. The concentration of immobilized sperm was determined on a hemacytometer. Sperm motility was evaluated within 1 hr after collection. Percent motile was the sum of the percentages with rapid linear progression (3 to ≥ 4) and slow linear progression (≥ 2). Sperm morphology (percent normal forms) was determined using air-dried smears stained with a modified Wright-Giemsa stain. At least 200 sperm in four different areas of the slide were evaluated according to Kruger's strict criteria [Kruger et al. Citation1988].
Detection of MDA, SOD, and GPx levels in testes
The testicular tissue stored at −20°C were homogenized at 4°C after adding pre-cooled 0.9% saline in the ratio of 1:9. When testicular tissues were disrupted, the homogenate was centrifuged at 3,000 × g for 10 min at 4°C. The supernatant was used for the assay of SOD, GPx, and MDA according to the instructions for these kits (Nanjing Jiancheng Bioengineering Institute, China).
Statistical analyses
Five replicates for each experiment were conducted. All of the data were expressed as the mean ± standard deviation (SD) of the mean and statistically analyzed by single variable of the general linear model using SPSS Statistics 17. Differences between experimental groups were considered significant at P < 0.05 and extreme at P < 0.01.
Acknowledgments
Dr. Chunjie Zhang, Dr. Yinju Li, and Dr. Yumei Liu are thanked for assistance in usage of instruments. Ms. Kerry Alicia Webber is thanked for assistance in preparing the manuscript.
Declaration of interest: This work was supported by the National Natural Science Foundation of China (Project No. 30901026) and the Higher Education Finance Scheme for Young Backbone Teachers in Henan Province (Project No. 2010GGJS-071), China. The authors report no declarations of interest.
Author contributions: Conceived and designed the experiments: F-JL, Z-JY, X-WZ, Y-LZ; Performed the experiments: X-WZ, QQ, YB; Analyzed the data: X-LC, L-JJ, X-GM; Contributed reagents/materials/analysis tools: RS; Wrote the manuscript: F-JL, X-WZ, Y-LZ, RS.
References
- ACGIH (American Conference of Governmental Industrial Hygienists) y(1995) 1995-1996 Threshold Limit Values (TLVs) for chemical substances and physical agents and biological exposure indices (BEIs). Cincinnati, OH: ACGIH.
- Agarwal, A. and Prabhakaran, S.A. (2005) Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Ind J Exp Biol 43:963–974.
- Aitken, J. and Fisher, H. (1994) Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 16:259–267.
- Aitken, R.J., Clarkson, J.S. and Fishel, S. (1989) Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod 41:183–197.
- Bersényi, A., Berta, E., Kádár, I., Glávits, R., Szilágyi, M. and Fekete, S.G. (2008) Effects of high dietary molybdenum in rabbits. Acta Vet Hung 56:41–55.
- Bi, C.M., Zhang, Y.L., Liu, F.J., Zhou, T.Z., Yang, Z.J., Gao, S.Y. (2013) The effect of molybdenum on the in vitro development of mouse preimplantation embryos. Syst Biol Reprod Med 59:69–73.
- Braydich-Stolle, L., Hussain, S., Schlager, J.J. and Hofmann, M.C. (2005) In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci 88:412–419.
- Carlsen, E., Giwereman, A., Keiding, N., Skakkebaek, N.E. (1992) Evidence for decreasing quality of semen during past 50 years. BMJ 305:609–613.
- CDC (Centers for Disease Control and Prevention) (2005) Third National Report on Human Exposure to Environmental Chemicals. Washington, DC, 52.
- Ema, M., Kobayashi, N., Naya, M., Hanai, S. and Nakanishi, J. (2010) Reproductive and developmental toxicity studies of manufactured nanomaterials. Reprod Toxicol 30:343–352.
- Friedmann, A.S. (2002) Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol 16:275–279.
- Hille, R., Rétey, J., Bartlewski-Hof, U. and Reichenbecher, W. (1998) Mechanistic aspects of molybdenum-containing enzymes. FEMS Microbiol Rev 22:489–501.
- Huang, J., Wu, J., Li, T., Song, X., Zhang, B., Zhang, P. (2011) Effect of exposure to trace elements in the soil on the prevalence of neural tube defects in a high-risk area of China. Biomed Environ Sci 24:94–101.
- Kargar, M., Khorasani, N., Karami, M., Rafiee, G.R. and Naseh, R. (2011) Study of aluminum, copper and molybdenum pollution in groundwater sources surrounding (Miduk) Shahr-E-Babak copper complex tailings dam. World Academy of Science, Engineering and Technology 76:412–416.
- Khurana, S., Ranmal, S. and Ben-Jonatllan, N. (2000) Exposure of new born male and female rats to environmental estrogens: delayed and sustained hyperolaetinemia and alterations in estrogen receptor expression. Endocrinology 141:4512–4517.
- Kruger, T.F., Acosta, A.A., Simmons, K.F., Swanson, R.J., Matta, J.F. and Oehninger, S. (1988) Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 49:112–117.
- Lyubimov, A.V., Smith, J.A., Rousselle, S.D., Mercieca, M.D., Tomaszewski, J.E., Smith, A.C., (2004) The effects of tetrathiomolybdate (TTM, NSC-714598) and copper supplementation on fertility and early embryonic development in rats. Reprod Toxicol 19:223–233.
- Makker, K., Agarwal, A. and Sharma, R. (2009) Oxidative stress & male infertility. Indian J Med Res 129:357–367
- Meeker, J.D., Rossano, M.G., Protas, B., Diamond, M.P., Puscheck, E., Daly, D. (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116:1473–1479.
- Mills, C.F. and Davis, G.K. (1987) Molybdenum, In: Trace Elements in Human and Animal Nutrition, 5th edn, ed. Mertz, W., Academic Press, San Diego, CA pp. 429–463
- Ola-Mudathir, K.F., Suru, S.M., Fafunso, M.A., Obioha, U.E. and Faremi, T.Y. (2008) Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxieol 46:3604–3611.
- Pandey, R. and Singh, S.P. (2002) Effects of molybdenum on fertility of male rats. Biometals 15:65–72.
- Portugal-Cohen, M., Numa, R., Yaka, R. and Kohen, R. (2010) Cocaine induces oxidative damage to skin via xanthine oxidase and nitric oxide synthase. J Dermatol Sci 58:105–112.
- Schroeder, H.A. and Mitchener, M. (1971) Toxic effects of trace elements on the reproduction of mice and rats. Arch Environ Health 23:102–106.
- Sharma, S., Kaur, R. and Sandhu, H.S. (2004) Effect of subacute oral toxicity of molybdenum on antioxidant status in cross bred cow calves. Ind J Anim Sci 74:734–736.
- Spalj, S., Mlacovic Zrinski, M., Tudor Spalj, V. and Ivankovic Buljan, Z. (2012) In-vitro assessment of oxidative stress generated by orthodontic archwires. Am J Orthod Dentofacial Orthop 141:583–589.
- Thomas, J.W. and Moos, S. (1951) The effect of orally administered molybdenum on growth spermatogenesis and testes histology of young dairy bulls. J Dairy Sci 34:929–934.
- Underwood, E.J. (1981) Trace metals in human and animal health. J Hum Nutr 35:37–48.
- WHO (World Health Organization) (1993) Guidelines for drinking water quality. Second edition. World Health Organisation, Geneva.
- WHO (World Health Organization) (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. New York: Cambridge University Press.
- Wirth, J.J. and Mijal, R.S. (2010) Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med 56:147–167.
- Yu, C., Xu, S., Gang, M., Chen, G. and Zhou, L. (2011) Molybdenum pollution and speciation in Nver River sediments impacted with Mo mining activities in western Liaoning, northeast China. Int J Environ Res 5:205–212.
