Abstract
This study was aimed to investigate the long-term effect of vasectomy using the bonnet monkey (Macaca radiata) as a primate animal model. Animals weighing around 6 to 8 kg were randomly chosen for bilateral, unilateral vasectomy and sham-control. The postoperative periods of six months and two years were considered as short and long-term, respectively. Sperm were collected and subjected to analysis before euthanasia. The testes and epididymides were excised from euthanized animals then embedded in paraffin. Normal histological changes were observed in sham-operated animals and short-term contralateral testes. In contrast, marked alterations were observed in the testes and epididymides of both short and long-term groups. Seminiferous epithelium was thinned out showing marked depletion of germ cells in long-term; only a thin layer of Sertoli cells, spermatogonia, and fewer spermatocytes were seen. Exfoliation of germ cells and the occurrence of multinucleated giant cells were common features in these tubules. The epididymal tubular lumens were greatly dilated with accumulated spermatozoa in short and long-term animals; significant defects were observed in the epithelium of the long-term animals. Microscopic spermatic granulomas were noticed in epididymides and the vas deferens. Large granulomas were seen in long-term vasectomized monkeys, frequently compressing the surrounding structures. These granulomas could be visualized in ultrasound, however, only at the late stage of its occurrence. Sperm collected from the unilateral vasectomized animals showed a poor motility score in the capillary mucus penetration test (CMPT). Results indicate that the changes observed after vasectomy might be due to pressure initially, whereas in the long-term the damage was supplemented by autoimmune attack. With immunoglobulin (IgG) deposition in contra-lateral unoperated testis of unilateral vasectomized animals it also showed degenerative changes and a concomitant drop in sperm quality. Although, granulomatous reactions were observed in the epididymis and vas deferens but testes were spared from such reactions even in the long-term.
Introduction
At present, vasectomy seems to be the major permanent method of male contraception. Many experimental studies were employed to understand the consequences of vasectomy especially to make this method more suitable as a reversible method of male contraception. From the literature it is quite clear that understanding the mechanism and arriving at a consensus on sequelae of vasectomy seems to be very difficult. Several experiments have been done to study the effect of vasectomy in various animals like mice, shrew, guinea pig, rat, rabbit, hamster, bull, monkey [Aitken et al. Citation1999; Alexander Citation1972; Bedford Citation1976; Flickinger Citation1975; Igboeli and Rakha Citation1970; McGlynn and Erpino Citation1974; Singh and Chakravarty Citation2000; Singh and Dominic Citation1981], and human [Jarow et al. Citation1985; McDonald Citation1996] yet there are conflicting reports on vasectomy sequelae. Evidence suggests that alteration in testis and epididymis is primarily due to intraluminal pressure as a result of sperm accumulation [Flickinger et al. Citation1987; Muir et al. Citation1976]. The duct system of human and monkey appear to react to vasectomy in a similar way. With the exception of monkey, the other common laboratory species do not seem to be the ideal models to study the overall response of the reproductive tract to vasectomy [Bedford Citation1976]. The reaction of the male duct system to vasectomy varies among species. Consideration of such variations is essential to avoid misinterpretation of results while carrying them from animal studies to human.
In countries like India, vasectomy is considered to be a very popular and safe method of sterilization, though the once high acceptance level has decreased [Labrecque et al. Citation2005]. This likely reflects fear of surgery, cardiovascular problems, and risk of prostatic and testicular cancers, and mistakenly linking vasectomy with reduction in sexual potency [Puri et al. Citation2000], contraindications like scrotal pathology, haematoma, allergy to local anaesthesia, genito-urinary or groin infections, and sperm granulomas [Lipshultz and Banson Citation1980; Silber Citation1998]. Apart from the above, non-scientific reasons, so called myths and taboos among the people of India were also suggested for the drop in acceptance of vasectomy [Saoji et al. Citation2013; Sharma Citation2006]. However, vasectomy is still a widely accepted procedure and men seeking reversal in India are few. Hence, the problem of reversal failure has not surfaced unlike in the western world.
In a report, WHO has claimed vasectomy to be a safe procedure and further supported its usage [Waites Citation1998]. The quantum of vasectomy in India since 1950 is enormous and in comparison there are few studies reported from India (both animal and human studies) on sequelae of vasectomy [Choudhuri Citation1975; Indian Council for Medical Research Citation1983a, Citation1983b; Joshi Citation1981; Lohiya and Tiwari Citation1983, Citation1984; Lohiya et al. Citation1987; Platz et al. Citation1997; Singh and Dominic Citation1981; Soonawalla Citation1999; Singh and Chakravarty Citation2000; Thakore and Patel Citation1972; Tripathy et al. Citation1994]. It is mandatory to continue monitoring the safety and possibility of reversal of the procedure. The aim of this study was to understand the effects of vasectomy in testes and epididymides in a long-term postoperative period, using bonnet monkey as an animal model (a native of tropical regions of India). By using a non-human primate model for this study the data can be phylogenetically valid in terms of vasectomy follow-up research [Bedford Citation1976].
Results
Semen collection
Electro-ejaculation by the penile method yielded a good result, semen was in the form of a coagulum, which took around 30 minutes to completely liquefy. The semen collected from bilaterally vasectomized monkeys showed the absence of sperm and unilaterally vasectomized monkeys showed reduced sperm count when compared to the sham-operated control. The progressive movement in capillary mucus penetration test (CMPT) was analysed. The following semen parameters were analyzed: volume of semen, sperm number, percentage of motile sperm, percentage of abnormal sperm, motility score, CMPT scores, and fructose level (data are presented in ).
Table 1. Various parameters observed through semen analysis.
Ultrasound imaging
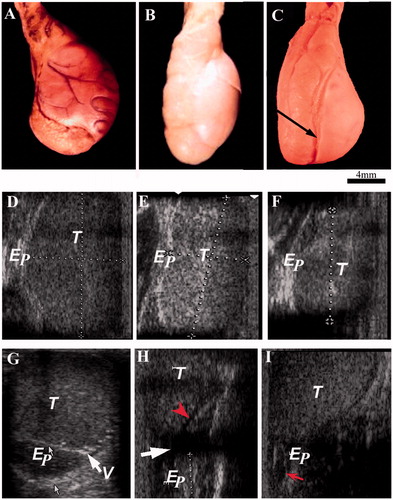
The testis was highly echogenic, epididymis was medium echogenic, and vas deferens was hypoechoic. In the long-term vasectomized animals, the spermatic granulomas were seen close to the upper pole of the testis, which was seen as a mixed echoic structure. In the short-term animals, spermatic granuloma could not be seen. In all the vasectomized animals, enlargement of the mediastinum and epididymis was obvious ().
Figure 1. Presenting gross morphology pictures of testis and epididymis and their corresponding ultrasound images taken from various experimental groups. A–C) shows the morphology of testis and epididymis in sham-control, short-term and long-term animals, respectively. Arrow indicates the impression of epididymis on the testis. D) shows the ultrasound images of scrotal content. D–F and G–I) are images along longitudinal and transverse plains, respectively. Arrow and arrow head (red) in H indicates dilated mediastinum and epididymis, respectively. Arrow indicates dilated epididymis in I. T: testis; Ep: epididymis; V: vas deferens.
Hormone analysis
The hormone analyses of total testosterone and estradiol levels were compared between the control, unilateral, and bilateral vasectomy groups. No significant change in hormone levels of vasectomized animals was observed when compared to the control ().
Table 2. Gross measurement of testis and epididymis.
Morphological observations
The gross measurements of organs in sham operated, short-term, and long-term animals are presented in . Morphologically, the testes and epididymides from control animals showed normal features. In vasectomized animals, the degree of distension of the epididymis and vas deferens and the size of the spermatic granulomas were grossly inversely proportional. In short-term animals, the distension was obvious and spermatic granuloma was small. In long-term animals, the distension was comparatively less while the size of the spermatic granuloma was large ().
Table 3. Estimation of testosterone and estradiol.
Histopathology
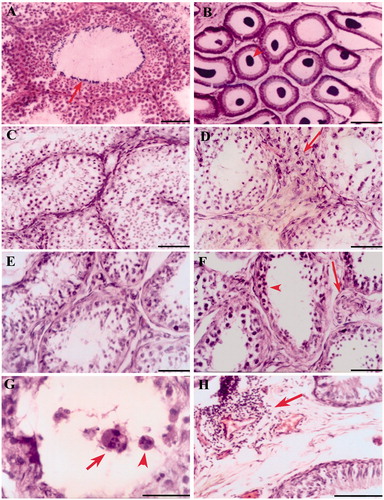
As presented in , histologically, the testis and epididymis revealed several pathological changes in vasectomized animals when compared to control animals. The changes seen in the vasectomized animals were: hypertrophy of myoid layer; distorted seminiferous tubules with multinucleated giant cells; increase in the connective tissue proportion of testis; distended tubules filled with sperm cells; microscopic granulomatous reactions in epididymides and infiltrations at many places in the proximal segment of the vas deferens; and hyaline degeneration of cells of the duct system. Relatively, the degree of histopathological changes was less in short-term animals than in long-term animals. The sham–operated control testes and epididymides had no inflammatory reaction and demonstrated normal histo-architecture as described elsewhere [Prakash et al. Citation2009].
Figure 2. Photomicrograph of testis and epididymis stained with hematoxylin and eosin taken from various experimental groups. (A and B) shows the photomicrographs of testis (complete spermatogenesis - arrow) and epididymis (sperm accumulation - arrowhead) from sham-operated control. (C and D) are testis from short-term, and (E and F) are from long-term of unoperated and operated sides testes, respectively. The arrows in D and F indicate sites of germ cell leakage and hypertrophic changes in the interstitium. The arrowhead indicates depleted epithelium (F). (G) shows a seminiferous tubule taken from long-term animal with multinucleated giant cells, a large cell with multiple nucleuses with vacuole (arrow), and another cell with interconnected four distinct nucleuses (arrowhead). (H) a site of infiltration or inflammatory reaction around a capillary (vacuities like change) in epididymal interstitium from long-term animal was shown (arrow). Scale bar = 80 µm.
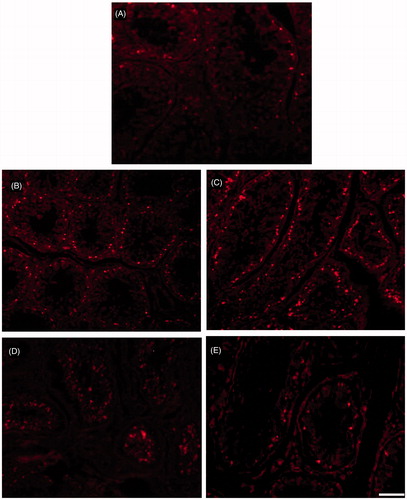
Immunohistological staining of testis sections showed deposition of immunoglobulin (IgG) only in long-term bilateral and unilateral testes. However, deposition was restricted to the basal compartment of the seminiferous tubules and not elsewhere. In unilateral long-term animals, the immunoglobulin depositions were seen in both operated and unoperated testes. However, the quantum of deposition in the unoperated side was not as severe as in the operated side (). The number of apoptotic cells identified by TUNEL was greater in seminiferous epithelium of both short and long-term bilateral and unilateral vasectomized animals when compared to the control ().
Figure 3. Fluorescent microscopic image of immunohistological staining of testis sections demonstrating deposition of anti-Monkey immunoglobulin (IgG)-FITC after vasectomy. (A) shamcontrol; (B) unoperated testes; and (C) operated testes. Arrow heads indicate the IgG deposition in the basal compartment of the seminiferous epithelium. In all testes IgG deposition was restricted to the basal compartment of the seminiferous tubules and not elsewhere. In unilateral vasectomized animals, the immunoglobulin depositions were seen in both operated and unoperated testes. However, the quantum of deposition in the unoperated side was not as severe as in the operated side. Scale bar = 80 µm.
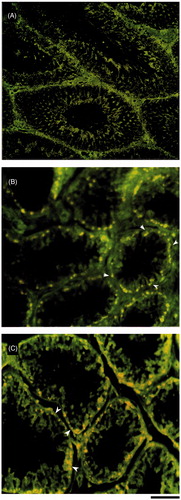
Figure 4. Photomicrograph of fluorescent microscopic images of TUNEL apoptotic positive cells (TMR red) in testis from various experimental groups. TUNEL positive cells (bright fluorescent spots) in seminiferous epithelium of the testis. (A–E) from sham-control, unoperated and operated side short-term testes, and unoperated and operated side long-term testes, respectively. The number of apoptotic cells identified by TUNEL was greater in seminiferous epithelium of both short and long-term bilateral and unilateral vasectomized animals when compared to the control. When compare to long-term testis short-term testis shows more apoptotic positive cells. Scale bar = 80 µm.
Discussion
We have investigated the post vasectomy response in unilateral and bilateral models in bonnet monkey and analyzed the changes on testis and epididymis in short and long-term periods. The bonnet monkey is a tropical inhabitant of the Indian subcontinent sharing reasonable similarity with human in terms of testis and its duct system. The sham-operated control showed normal histological testes and epididymides architecture and almost similar to normal monkey data reported earlier [Prakash et al. Citation2009] and ruled out any iatrogenic effect. After vasectomy, the testes and epididymides underwent gross alterations in both short-term and long-term animals. Morphologically, increase in weight and tremendous dimensional change in testes and epididymides were observed after vasectomy in both short-term and long-term vasectomized monkeys. The accumulation of sperm and the tight fibrous capsule could have produced the observed dimensional change in testis and epididymis. This may influence the micro-architecture of these organs. The distension of the testes and epididymides were obvious in six months. In two years after vasectomy the distension appears to be reduced but showed distortion or dimensional change, with distended epididymis that had created its mark on the testis.
The changes seen in monkey testes and epididymides in response to the vasectomy can be due to the nature of the duct system. In the bonnet monkey, epididymal leakage is minimal and epididymal granulomas are few and microscopic, indicating more resistance to leakage, with moderate distensibility. Testis showed extensive damage primarily induced by increased intra-luminal pressure with corresponding variation in weight and dimension, apart from severely affected spermatogenesis. The pathological changes in testes after vasectomy are closely related to the variation in the distensibility of the duct system, spermatogenic rate, and sperm transit time in the epididymis [McDonald Citation1996, Citation2000] and supported by the vasectomy reversal (vaso-vasostomy) that stops further progression of vasectomy induced pathological changes [Flickinger et al. Citation1987]. The epididymal dysfunction can be the primary cause for the failure of vasectomy reversal [McDonald Citation1996] and has been reported to inflict changes in mRNA expression in epididymal tubules [Doiron et al. Citation2003]. Understanding their physiological functions and the mechanisms regulating differential gene expression along the epididymal tubules can possibly shed more light towards success of the vasectomy reversal. Genetic studies may unravel the mechanism of systemic post vasectomy sequelae or disease [Wheeler et al. Citation2011].
Generally, the exposure of sperm to host immunity results in spermatic granuloma or an inflammatory reaction in the testes [Agnarsdottir et al. Citation2006; McDonald Citation2000]. There are studies describing the lack of an inflammatory reaction in normal testis after various types of trauma such as needle prick and injection of testicular substances [Itoh et al. Citation1999]. Surprisingly, even studies involving the rhesus monkey did not note testicular granuloma [Alexander Citation1972; Chapman et al. Citation1978]. A similar environment may be present in humans as many studies in vasectomized men reported alterations in testis, without reference to infiltrations in the testis [Bigazzi Citation1981; Choi and Reiner Citation1983; Jarow et al. Citation1985; Jarow et al. Citation1994; Jenkins et al. Citation1979; Mehrotra et al. Citation1985]. However, there is no confirmation of this condition long-term after vasectomy.
Several mechanisms were proposed as the root cause for the resistance of testis connective tissue to the inflammatory reaction. Perhaps the blood capillaries in the testis are relatively resistant to infiltration as compared to those in the epididymis and vas deferens [Itoh et al. Citation1995; Itoh et al. Citation1999]. This could be achieved by faster lymphatic drainage in testis removing the antigens before they evoke an immune response [Itoh et al. Citation1999] and immunosuppressive activity of Leydig cells [Born and Wekerle Citation1982]. The antigenic property of spermatozoa changes rapidly during passage through the testis to epididymis [Bohring and Krause Citation2001]. These studies indicate that granulomatous reaction may be stimulated against epididymal sperm but not against sperm or germ cells in the testis. In the present study testis showed a lack of spermatic granuloma/inflammation even up to two years after vasectomy but showed regions of extensive degeneration and scarring. Experimental studies on smaller animals like ram and guinea pig have shown that passage of immunoglobulin into the seminiferous tubules is limited by a peritubular barrier formed by vascular elements, interstitial cells, basement membranes, and Sertoli cells [Johnson and Setchell Citation1968]. Despite such mechanisms, reports about the presence of immunoglobulins in testis are not uncommon [Itoh et al. Citation1999; Itoh et al. Citation2005].
Potency of anti-sperm antibodies to induce testicular damage or any cardiovascular problem in general has been debated over a long period of time without any conclusion. While there have been reports about immunoglobulin induced damage to testis [Tung and Alexander Citation1977; Bigazzi Citation1981], it is also believed that presence of anti-sperm antibodies in testis or duct system is not an indication of antibody attack on testis rather an indication of previous antigen escape [Johnson Citation1970; McDonald Citation2000]. There seems to be marked species variations in the occurrence and distribution of immune complex deposition in testis, as Flickinger et al. [Citation1990] has reported that the appearance of immune reaction vary among strains of Lewis rat and Wistar rat and also among individuals of the same strain. Factors like phylogenetic position, genetic composition, differences between individuals in the rate of spermatozoal breakdown, etc. may play an important role in the anti-sperm antibody production and its subsequent attack on the testis after vasectomy.
In the present study, the deposition of immunoglobulin was restricted to the basal layer and does not pass further. This may be due to the barrier formed by Sertoli cells as described by Dym [Citation1973] in Macaca mulatta and Macaca nemestrina. In unilaterally vasectomized animals, the deposition of IgG is found in the unoperated side, but without marked degenerative changes by light microscopic observation. This indicates that sperm antigens escaped from the operated side induce antibody production and find their way into unoperated side testis, systemically. Similar to this previous report, IgG deposition has not induced observable degenerative changes in contralateral testis. However, increased immune complex deposition very close to Sertoli cell culmination may yield a patho-physiological effect by altering ipsilateral testis and spermatogenesis leaving behind stem cells and immature cells in the long-term. The ability of the Sertoli-Sertoli junction to withstand this immune complex in further course of post vasectomy period could be an interesting study and also the fate of circulating immune complex (CIC).
The CMPT analysis implicates the role of immunological factors on sperm [Fjallbrant Citation1968]. The sperm analysis by CMPT has shown poor forward movement after vasectomy (unilateral), which is an indication of alteration in sperm fertilizing ability [Ola et al. Citation2003]. This may contribute to the observed failure in vasectomy reversal including the retrieved sperm from testis or epididymis [Abdelmassih et al. Citation2002; McVicar et al. Citation2005].
Another major factor is the accumulation of reactive oxygen species (ROS) [Shapiro Citation1998]. Accumulation of sperm in the epididymis following vasectomy may lead to deregulation of ROS generation, causing oxidative stress and epididymal dysfunction [McVicar et al. Citation2005], and lead to functional and structural damage in the sperm [Suresh et al. Citation2010]. Similarly, the observed increase in apoptotic cells in testis of vasectomized animals could be ROS mediated [Wang et al. Citation2012] and thus reduce sperm yields during biopsy after vasectomy with increasing years [O’Neill et al. Citation2007]. Consequently, TUNEL positive cells were more abundant in short-term as compared to long-term post vasectomy indicating reduced spermatogenesis in the latter. Several pathway apoptotic mediators after vasectomy include p53-Bax [Shiraishi et al. Citation2001], Fas-FasL mediated [O’Neill et al. Citation2007] and survivin downregulation and Bax over expression [Al-Maghrebi et al. Citation2011]. These will be interesting aspects to explore in vasectomized subjects.
In conclusion, an increase or decrease in weight or dimension of testes and epididymides after vasectomy certainly forms an indication for pathological changes. Consequently, ultrasound can be more useful in diagnosing the testicular and paratesticular lesion. Observations show that the scrotal sonography can be considered to be sensitive in detecting intra-scrotal abnormalities after vasectomy in bonnet monkeys. The utility of ultrasound in diagnosing spermatic granuloma was discussed elsewhere [Prakash et al. Citation2002]. The seminal vesicles and prostate were normal. There is no major alteration in testosterone and estrodiol levels after vasectomy even in the long-term vasectomized groups. On the whole results from the study (especially in long-term) indicate that achieving fertility is not an assured possibility in vasectomized men who want reversal of the procedure. Nevertheless, in India, this procedure is still considered to be a very good choice without alarming side effects, where men opting for reversal are negligible when compared to other parts of the world.
Materials and Methods
The bonnet monkey was used as a non-human primate model for this study. The animals required for the research were procured from the Wild Life Park at Guindy in Chennai after being permitted by the Conservator of Forests, Tamil Nadu. Healthy adult male monkeys of body weight ranging from 6 to 8 kg were selected for the study. Fertility of the animals was confirmed by semen analysis during the quarantine period. Quarantine procedures and animal maintenance was done according to the recommendation of the Canadian Council Guide to the Care and use of Experimental Animals [1993] and the Indian National Science Academy, New Delhi guidelines [1992]. The ethical committee of Dr. A.L.M. PGIBMS, University of Madras approved the protocol of the work. Details of animal maintenance were described elsewhere [Prakash et al. Citation2002]. Animals were grouped as sham-operated control (i.e., vas deferens was exposed, not severed), unilateral and bilateral vasectomized animals short-term (6 months) and long-term (2 years), respectively, for each group (n = 5). For the CMPT cervical mucus was collected from female monkeys in the colony.
Surgical procedure and postoperative care
Animals were anaesthetized using thiopentone sodium (Pentothal© Abbott Laboratories, India) at a dosage of 30 mg/Kg i.p. Intra-operative monitoring of vital signs was done using Biomonitor© (Kody’s Medical Electronics, India). Under aseptic conditions, a vertical incision for 1–2 cm was made over the scrotum just lateral to the root of the penis. The spermatic cord was exposed and its layers were incised exposing the vas deferens. Care was taken to avoid any sort of vascular injury. Vas deferens was gently squeezed to move the sperm from the site of incision. After making sure that the vas deferens was devoid of sperm at the surgery site, two ligatures were made one at either end using 5-0 silk suture threads, excluding any blood vessels along with the ligature. The blood vessels were separated from the vas deferens by using an operative microscope under 22× magnifications. The intermittent segment of about 1 cm was incised and removed. The wound was closed in layers. No antibiotic was administrated postoperatively. Analgesics were administered for a postoperative period of 2 d, after which no medication was given. The postoperative period was uneventful.
Semen collection
Semen collection in the monkey was done once in every 6 months, by penile method. Collection was performed by electro-ejaculation using monophasic alternate current stimulator with intermittent charges of 20 volts delivered at a frequency of 10–20 impulses/sec for a period of 25–50 sec. During the procedure the limbs of the animals were restrained with minimal stress, and the animal was moved to a convenient position for electrode placing. If ejaculation did not occur within one min, then voltage was increased periodically at a rate of 0.5 volts until the ejaculation occurred. At any given time, the voltage used did not exceed 30 volts [Mastroianni and Mason Citation1969; Ramesh et al. Citation1998]. Various semen parameters were assessed including (progressive movement of sperm) CMPT [Fjallbrant Citation1968].
Ultrasound imaging
One day before euthanasia, scrotum and its contents were examined ultrasonographically using Colour Doppler (HDI 1500, Philips, ATL India Ltd.). High frequency probe (5–12 MHz) was used. Animals were anaesthetized during this procedure. The animal was placed in supine position with hind limb stretched apart with slightly extended thigh and flexed leg. The ultrasound transmission gel was applied after clipping the scrotal hairs. Transverse and sagittal scanning of the scrotal content was made.
Hormone analysis
The blood was collected from femoral or saphenous vein, at the time of vasectomy and also periodically at a postoperative interval of 3 months. The serum was separated, and testosterone and estradiol were measured by using radio-immuno assay (RIA) [Anletta Citation1974].
Euthanasia and tissue processing
Animals were sacrificed at the end of intended experiments by overdose of anaesthesia (thiopentone sodium). Immediately after the respiration ceased, the animals were fixed by trans-cardiac perfusion with formal saline after flushing the blood with normal saline. Post mortem and gross measurements were recorded. Histopathology examinations were carried out using paraffin-processed sections stained with Harris haematoxylin and counter stained with eosin. Immuno-staining was done by using anti-monkey IgG (whole molecule) FITC labelled (Sigma-Aldrich, St. Louis, USA) after antigen retrieval. Localization of apoptotic cells in testis was done using in-situ cell death detection kit-TMR red for formalin-fixed paraffin sections as per manufacturer protocol (Roche Diagnostics GmbH, Mannheim, Germany). The stained sections were observed under bright-field and epi-fluorescent microscopes (Nikon Corporation, Tokyo, Japan) accordingly.
Declaration of interest
Part of this work was from Dr. S. Prakash’s PhD thesis submitted to the University of Madras. The authors report no conflicts of interest.
Author contributions
Conceived and designed the experiments: PS, KK; Performed the experiments: PS; Analyzed the data: PS, KK; Contributed reagents/materials/analysis tools: PS, KK; Wrote the manuscript: PS.
| Abbreviations | ||
| CMPT | = | capillary mucus penetration test |
| IgG | = | immunoglobulin |
| vaso-vasostomy | = | vasectomy reversal |
| CIC | = | immune complex |
| ROS | = | reactive oxygen species |
References
- Abdelmassih, V., Balmaceda, J., Tesarik, J., Abdelmassih, R., and Nagy, N. (2002) Relationship between time period after vasectomy, and the reproductive capacity of sperm obtained by epididymal aspiration. Hum Reprod 17:736–40
- Agnarsdottir, M., Carlen, B., and Willen, R. (2006) Malacoplakia and spermatic granuloma complicating vasectomy. Ups J Med Sci 111:227–30
- Aitken, H., Kumarakuru, S., Reid, O., Milne, E.W., Bennett, N.K., and McDonald, S.W. (1999) Degenerated tubules in the guinea pig testis after long-term vasectomy or sham-operation. Clini Anat 13:6–10
- Alexander, N.J., and Tung, K.S. (1977) Immunological and morphological effects of vasectomy in the rabbit. Anat Rec 188:339–50
- Alexander, N.J. (1972) Vasectomy: Long-term effects in the rhesus monkey. J Reprod Fertil 31:399–406
- Al-Maghrebi, M., Kehinde, E.O., and Anim, J.T. (2011) Survivin downregulation is associated with vasectomy-induced spermatogenic damage and apoptosis. Med Princ Pract 20:449–54
- Anletta, F.J., Caldwell, B.V., and Hamilton, G.L. (1974) Androgens: Testosterone and Dihydrotestosterone. In: Methods of Hormone Radio Immunoassay. Jaffe, B.M., and Behram, N.R., eds. New York: Academic Press, p. 520
- Bedford, J.M. (1976) Adaptations of the male reproductive tract and the fate of spermatozoa following vasectomy in the rabbit, rhesus monkey, hamster and rat. Biol Reprod 14:118–42
- Bigazzi, P. (1981) Immunologic effects of vasectomy in men and experimental animals. Prog Clini Bio Res 70:461–76
- Bohring, C., and Krause, W. (2001) Differences in the antigen pattern recognized by antisperm antibodies in patients with infertility and vasectomy. J Urol 166:1178–80
- Born, W., and Wekerle, H. (1982) Leydig cells non specifically suppress lymphoproliferation in vitro: implications for the testis as an immunologically privileged site. Am J Reprod Immunol 2:291–5
- Chapman, G.E., Hartman, P.G., Cary, P.D., Bradbury, E.M., and Lee, D.R. (1978) A nuclear-magnetic-resonance study of the globular structure of the H1 histone. Eur J Biochem 86:35–44
- Choi, Y.J., and Reiner, L. (1983) Autoimmune response following vasectomy. N Y State J Med 83:819–22
- Choudhuri, A. (1975) Demographic and socio-economic study of mass vasectomy. J Indian Med Assoc 64:106–8
- Doiron, K., Légaré, C., Saez, F., and Sullivan, R. (2003) Effect of vasectomy on gene expression in the epididymis of cynomolgus monkey. Biol of Reprod 68:781–8
- Dym, M. (1973) The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec 175:639–56
- Fjallbrant, B. (1968) Sperm antibodies and sterility in men. Acta Obstet Gynec Scand 47:5--38
- Flickinger, C.J., Herr, J.C., Caloras, D., Sisak, J.R., and Howards, S.S. (1990) Inflammatory changes in the epididymis after vasectomy in the Lewis rat. Biol Reprod 43:34–45
- Flickinger, C.J., Herr, J.C., Howards, S.S., Caloras, D., Yarbro, E.S., and Spell, D.R. (1987) The influence of vasovasostomy on testicular alterations after vasectomy in Lewis rats. Anat Rec 217:137–45
- Flickinger, C.J. (1975) Fine Structure of the Rabbit Testis after Vasectomy. Biol Reprod 13:61–7
- Igboeli, G., and Rakha, A.M. (1970) Bull testicular and epididymal functions after long-term vasectomy. J Anim Sci 3:72–5
- Indian National Science Academy, New Delhi (1992) Guidelines for care and use of animals in scientific research. 1st Edition, Bengal Offset Works, New Delhi, India
- Itoh, M., Chen, X.H., Takeuchi, Y., and Miki, T. (1995) Morphological demonstration of the immune privilege in the testis using adjuvants: tissue responses of male reproductive organs in mice injected with Bordetella pertussigens. Arch Histol Cytol 58:575–9
- Itoh, M., Terayama, H., Naito, M., Ogawa, Y., and Tainosho, S. (2005) Tissue microcircumstances for leukocytic infiltration into the testis and epididymis in mice. J Reprod Immunol 67:57–67
- Itoh, M., Xie, Q., Miyamoto, K., and Takeuchi, Y. (1999) Major differences between the testis and epididymis in the induction of granulomas in response to extravasated germ cells. I. A light microscopical study in mice. Int J Androl 22:316–23
- Jarow, J.P., Budin, R.E., Dym, M., Zirkin, B.R., Noren, S., and Marshall, F.F. (1985) Quantitative pathologic changes in the human testis after vasectomy. A controlled study. N Engl J Med 14:1252–6
- Jarow, J.P., Goluboff, E.T., Chang, T.S., and Marshall, F.F. (1994) Relationship between antisperm antibodies and testicular histologic changes in humans after vasectomy. Urology 43:521–4
- Jenkins, I.L., Muir, V.Y., Blacklock, N.J., Turk, J.L., and Hanley, H.G. (1979) Consequences of vasectomy: an immunological and histological study related to subsequent fertility. Br J Urol 51:406–10
- Johnson, M.H. (1970) Changes in the blood-testis barrier of the guinea-pig in relation to histological damage following iso-immunization with testis. J Reprod Fertil 22:119–27
- Johnson, M.H., and Setchell, B.P. (1968) Protein and immunoglobulin content of rete testis fluid of rams. J Reprod Fertil 17:403–6
- Joshi, U.M. (1981) Endocrine and accessory sex organ function after vasectomy and vasovasostomy. Arch Androl 7:187–91
- Labrecque, M., Pile, J., Sokal, D., Kaza, R.C.M., Rahman, M., Bodh, S.S., et al. (2005) Vasectomy surgical techniques in South and South East Asia. BMC Urology 5:10
- Lipshultz, L.I., and Banson, G.S. (1980) Vasectomy: an anatomical, physiological and surgical review. In: Regulation of male fertility. Cunningham, G.R., Schill G.R., Hafez E.S.E., eds. Hague: Martinus Nijhoff, pp. 159–86
- Lohiya, N.K., and Tiwari, S.N. (1983) Vasectomy in langur monkeys (Presbytis entellus entellus dufresne). Arch Androl 11:85–7
- Lohiya, N.K., and Tiwari, S.N. (1984) Effect of vasectomy on biochemical constituents of the blood in langur monkey–a 2 1/2 years follow up. Indian J Physiol Pharmacol 28:306–10
- Lohiya, N.K., Tiwari, S.N., Ansari, A.S., and Watts, N. (1987) Long-term vasectomy effects on testis and accessory sex organ function in langur monkey. Acta Eur Fertil 18:207–11
- Indian Council for Medical Research. (1983a) Long-term effects of vasectomy 1983. Part I: Biochemical parameters. An ICMR (Indian Council of Medical Research) Task Force Study on Regulation of Male Fertility (surgical approaches). Contraception 28:423–8
- Indian Council for Medical Research. (1983b) Long-term effects of vasectomy 1983. Part II: Immunological parameters. An ICMR Task Force study on regulation of male fertility (surgical approaches). Contraception 28:527–41
- Mastroianni, L., and Mason, W.A. (1963) Collection of monkey semen by electro-ejaculation. Proc Soc Exp Biol Med. 112:1025--7
- McDonald, S.W. (1996) Vasectomy review: sequelae in the human epididymis and ductus deferens. Clin Anat 9:337–42
- McDonald, S.W. (2000) Cellular responses to vasectomy. Int Rev Cytol 199:295–339
- McGlynn, J.M., and Erpino, M.J. (1974) Effects of vasectomy on the reproductive system and sexual behaviour of rats. J Reprod Fertil 40:241–7
- McVicar, C., O’Neill, D., McClure, N., Clements, B., McCullough, S., and Lewis, S. (2005) Effects of vasectomy on spermatogenesis and fertility outcome after testicular sperm extraction combined with ICSI. Hum Reprod 20:2795–800
- Mehrotra, R., Nath, P., Singh, K.M., Tandon, P., Kumar, H., Pandey, R.K., et al. (1985) Changes in seminiferous tubules after vasectomy. Indian J Pathol Microbiol 28:371–8
- Muir, V.Y., Turk, J.L., and Hanley, H.G. (1976) Comparison of allergic aspermatogenesis with that induced by vasectomy. I. In vivo studies in the guinea-pig. Clin Exp Immunol 24:72–80
- Olfert, E.D., Cross, B.M., and McWilliam, A.A. (1993) Guide to the care and use of experimental animals. Canadian Council for Animal Care. Vol. 1, 2nd edition. Ottawa, Canada
- O’Neill, D., McVicar, C., McClure, N., Maxwell, P., Cooke, I., Pogue, K., et al. (2007) Reduced sperm yield from testicular biopsies of vasectomized men is due to increased apoptosis. Fertil Steril 87:834–41
- Ola, B., Afnan, M., Papaioannou, S., Sharif, K., Björndahl, L., and Coomarasamy, A. (2003) Accuracy of sperm-cervical mucus penetration tests in evaluating sperm motility in semen: a systematic quantitative review. Hum Reprod 18:1037–46
- Platz, E.A., Yeole, B.B., Cho, E., Jussawalla, D.J., Giovannucci, E., and Ascherio, A. (1997) Vasectomy and prostate cancer: a case control study in India. Int J Epidemiol 26:933–7
- Prakash, S., Kamakshi, K., and Muthusamy, R. (2002) Spermatic granuloma in bonnet monkeys -An ultrasonographic study. Folia Veterinaria 44:175–8
- Prakash, S., Suresh, S., and Prithiviraj, E. (2009) Anatomical aspects of the male reproductive system in the bonnet monkey (Macaca radiata). Anat Sci In 84:53–60
- Puri, C.P., Gopalkrishnan, K., and Iyer, K.S. (2000) Constraints in the development of contraceptives for men. Asian J Androl 3:179–90
- Ramesh, V., Ramachandra, S.G., Krishnamurthy, H.N., and Rao, A.J. (1998) Electroejaculation and seminal parameters in bonnet monkeys (Macaca radiata). Androl 30:97–100
- Suresh, S., Prithiviraj, E., and Prakash, S. (2010) Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl 33:22–32
- Saoji, A., Gumashta, R., Hajare, S., and Nayse, J. (2013) Denial mode for vasectomy among married men in central India: causes and suggested strategies. J Psychol Psychother 3:1--4 , doi: 10.4172/2161-0487.1000120
- Shapiro, R.H., Muller, C.H., Chen, G., and Berger, R.E. (1998) Vasectomy reversal associated with increased reactive oxygen species production by seminal fluid leukocytes and sperm. J Urol 166:1565–71
- Sharma, R.P. (2006) No scalpel vasectomy advocacy and community mobilisation–a personal experience. J Indian Med Assoc 104:134–7
- Shiraishi, K., Naito, K., and Yoshida, K. (2001) Vasectomy impairs spermatogenesis through germ cell apoptosis mediated by the p53-bax pathway in rats. J Urol 166:1565–71
- Silber, S.J. (1998) Vasectomy. In: Encyclopedia of reproduction. Knobil, E., Neill, J.D., eds. California: Academic Press, pp. 977–85
- Singh, S.K., and Chakravarty, S. (2000) Histologic changes in the mouse testis after bilateral vasectomy. Asian J Androl 2:115–20
- Singh, S.K., and Dominic, C.J. (1981) Effect of vasectomy on the testis and accessory sex glands of the musk shrew, Suncus murinus L. Endokrinologie 77:137–46
- Soonawalla, F.P. (1999) Vasectomy-safety and reversibility. In: Male contraception: present and future. Rajalakshmi M., Griffin P.D., eds. New Delhi: New Age Int (P) Ltd, pp. 251–63
- Thakore, V., and Patel, V. (1972) A world record in sterilization. Indian J of Public Health 16:183–6
- Tripathy, S.P., Ramachandran, C.R. and Ramachandaran, P. (1994) Health consequences of vasectomy in India. Bull World Health Organ 72:779–81
- Tung, K.S., and Alexander, N.J. (1977) Immunopathologic studies on vasectomized guinea pig. Biol Reprod 17:241–54
- Waites, G.M.H. (2003) Development of methods of male contraception: Impact of the World Health Organization Task Force. Fertil Steril 80:1--15
- Wang, X., Zhang, Y., Chen, Z., and Huang, X. (2012) Pathological influences of twelve months vasectomy on the reproductive tissues in rabbits. Adv Sex Med 2:3–9
- Wheeler, K., Tardif, S., Rival, C., Luu, B., Bui, E., Del Rioc, R., et al. (2011) Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc Natl Acad Sci USA 108:7511–16

