Abstract
It is necessary to regularly monitor thyroid function status during pregnancy. The repeated tests on serum thyroid hormones are invasive and can be uncomfortable. Sampling urine may provide an effective alternative. The primary aim of this study was to investigate if there is a correlation between the serum and urine levels of thyroid hormones during pregnancy. The secondary aim was to investigate their variation during pregnancy. This study collected the serum specimens of 30 healthy pregnant women at 9–12, 14–17, 23–26, and 37–40 weeks of gestation, respectively, simultaneously along with random urine specimens. This study compared the median levels of free triiodothyronine (FT3), free thyroxine (FT4), and thyrotropin (TSH) in serum and urine among four gestational stages. The differences were statistically significant (p < 0.05). There were positive correlations between serum FT3 (sFT3) and uFT3/uRBP (the ratio of urine FT3(uFT3) and urine retinol binding protein (uRBP)), r = 0.38 (I2 = 0%, 95% CI: 0.21 ∼ 0.54), serum FT4 (sFT4) and uFT4/uRBP (the ratio of urine FT4 (uFT4) and uRBP), r = 0.29 (I2 = 68.9%, 95% CI: 0.07 ∼ 0.51), and no correlation between serum TSH (sTSH) and uTSH/uRBP (the ratio of urine TSH (uTSH) and uRBP), r = 0.11 (I2 = 86.7%, 95% CI: −0.24 ∼ 0.45). In conclusion, the levels of sFT3, sFT4, uFT3/uRBP, and uFT4/uRBP continued to decrease until the 27th week of gestation, when it was almost invariant. The levels of uFT3/uRBP and uFT4/uRBP correlated well with the sFT3 and sFT4 during pregnancy, which may provide a more convenient and secure way to monitor the maternal thyroid function status during pregnancy.
Introduction
Thyroid hormones play essential roles in differentiation, growth, and metabolism. Maternal thyroid hormones are very important not only to the mother but also to the fetus [Patel et al. Citation2011; Sahay and Nagesh Citation2012]. Their transfer keeps contributing to fetal serum thyroid hormones and protecting the neurodevelopment until delivery [Obregon et al. Citation2007]. Thyroid dysfunction during pregnancy can cause abortion, premature stillbirth, fetal intrauterine growth retardation, congenital malformation, neurodevelopment disorders, and newborn thyroid dysfunction [Abalovich et al. Citation2002; Inoue et al. Citation2009]. If thyroid dysfunction is detected early and immediately, the treatment would be effective with very little detriment to the mother and the fetus [Sahay and Nagesh Citation2012]. Hence, it is very important to monitor the maternal thyroid function status periodically during pregnancy.
Urine is the terminal metabolite of the body, and urinary analysis is a feasible, noninvasive, and efficient strategy for researching both urinary [Lei et al. Citation2013] and endocrine disease [Chu et al. Citation2013]. Thyrotropin (TSH) is a glycoprotein [Pierce et al. Citation1971] having a molecular mass of approximately 28,000 daltons [Moura and Moura Citation2004]. Biologically active TSH is composed of glycosylated α and β subunits [Vamvakopoulos and Kourides Citation1979]. The latter carries the TSH-specific immunological and biological information that was detected in the serum and urine specimens in this study. It has been reported that kidney is the major metabolic site for TSH. The renal handling of TSH involves both glomerular filtration and tubular re-absorption, such that urinary TSH excretion increases when serum TSH is increased [Yoshida et al. Citation1988].
The thyroid hormones are synthesized by the thyroid gland and secreted directly into the circulation. The primary biological active form is free triiodothyronine (FT3), which is mainly derived from deiodination of free thyroxine (FT4) in peripheral tissues. It exerts its intracellular effects by binding to the receptors (e.g., TRα-1) [Yen Citation2001]. Almost all (more than 99%) of the circulating T4 and T3 are bound to thyroid hormone binding proteins and delivered to tissues. However, only the unbound thyroid hormone fractions are partly filtered by the glomerulus and excreted into urine after exerting their biological function [Shakespear and Burke Citation1976]. Urinary FT3 appeared to be directly derived from serum FT3 with partial renal tubular excretion, while urinary excretion of FT4 involved both glomerular filtration and tubular re-absorption [Burke and Shakespear Citation1976]. Renal clearance of un-conjugated thyroid hormones correlated well with serum free hormone levels in normal subjects [Burke et al. Citation1972].
Circulating blood volume and renal function is altered during pregnancy [Sturgiss et al. Citation1996], which makes it more difficult to detect the urinary thyroid hormones during pregnancy. However, this study eliminated the interference in the assay from maternal altered renal function by simultaneously analyzing and referring the data to the level of urine retinol binding protein (uRBP) which is a marker of tubular function. Using this as a platform we measured the urinary excretion of thyroid hormone parameters as a function of pregnancy. The results are discussed below.
Results
Variations in serum and urine thyroid hormones during pregnancy
showed the median, 25th percentile, and 75th percentile for the serum FT3 (sFT3), the ratio of urine FT3 and uRBP (uFT3/uRBP), serum FT4 (sFT4), the ratio of urine FT4 and uRBP (uFT4/uRBP), serum TSH (sTSH), and the ratio of urine TSH and uRBP (uTSH/uRBP). The t1, t2, t3, and t4 represented the 9–12, 14–17, 23–26, and 37–40 weeks of gestation, respectively. The normal distribution and equal variance assumptions were all denied (p < 0.05, p < 0.10). The Friedman Repeated Measures analysis of variance (ANOVA) on Ranks was selected to further process the data. The Student-Newman-Keuls Test indicated that the differences in the sFT3, uFT3/uRBP, sFT4, uFT4/uRBP, sTSH, and uTSH/uRBP among four stages were all significant (p < 0.05).
Table 1. Levels of thyroid hormones in serum and urine at four different gestational ages.
Accordingly, the concentration of sFT3 kept dropping from t1 4.554 pmol/L to t3 3.448 pmol/L (p < 0.05), and was almost invariant at t3 3.448 pmol/L vs. t4 3.510 pmol/L (p > 0.05) (). The level of uFT3/uRBP was almost invariant at t1 0.338 mg/g vs. t2 0.323 mg/g (p > 0.05), then kept descending from t2 0.323 mg/g to t4 0.183 mg/g (p < 0.05) ().
Figure 1. The levels of maternal thyriod hormones in serum and urine during pregnancy. The y-axis showed the levels of maternal sFT3 (A), sFT4 (B), sTSH (C), uFT3/uRBP (D), uFT4/uRBP (E), and uTSH/uRBP (F), respectively. The t1, t2, t3, and t4 of x-axis represented the 9–12, 14–17, 23–26, and 37–40 weeks of gestation, respectively. Each point corresponds to the median level of individual specimen obtained at each gestational age range. The P75 and P25 represented the 75th and 25th percentile of them, respectively. sFT3: serum free triiodothyronine; sFT4: serum FT4; sTSH: serum TSH; uFT3/uRBP: the ratio of urine FT3 and retinol binding protein; uFT4/uRBP: the ratio of uFT4 and uRBP; uTSH/uRBP: the ratio of uTSH and uRBP.
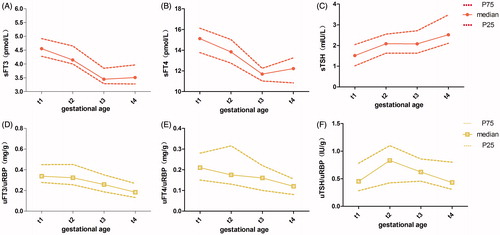
The concentration of sFT4 kept dropping from t1 15.125 pmol/L to t3 11.694 pmol/L (p < 0.05), and increased slightly at t3 11.694 pmol/L vs. t4 12.222 pmol/L (p < 0.05) (). The level of uFT4/uRBP decreased at t1 0.211 mg/g vs. t4 0.117 mg/g, t2 0.175 mg/g vs. t4 0.117 mg/g, and t3 0.160 mg/g vs. t4 0.117 mg/g (p < 0.05), the differences of other adjacent points were not statistically significant (p > 0.05) ().
The concentration of sTSH increased at t1 1.515 mIU/L vs. t2 2.085 mIU/L (p < 0.05), and was almost invariant at t2 2.085 mIU/L vs. t3 2.080 mIU/L (p > 0.05), then it kept rising at t3 2.080 µIU/ml vs. t4 2.520 mIU/L (p < 0.05) (). The level of uTSH/uRBP increased at t1 0.454 IU/g vs. t2 0.830 IU/g (p < 0.05), then it kept dropping from t2 0.830 IU/g to t4 0.432 IU/g (p < 0.05) ().
Correlation of thyroid hormones between serum and urine during pregnancy
The individual correlation coefficients (r) of thyroid hormones between serum and urine were calculated. The 95% CI for r was calculated after standardizing by Fisher’s Zr transformation and reverse transformation. The heterogeneity of the analysis (I2) was less than 50% and the fixed-effects model was used for meta-analysis. The combined correlation coefficient between sFT3 and uFT3/uRBP for 30 cases of pregnant women was 0.38 (I2 = 0%, 95% CI: 0.21 ∼ 0.54) ().
Figure 2. Correlation of thyroid hormones in serum and urine during pregnancy. A) Correlation of sFT3 and uFT3/uRBP, B) Correlation of sFT4 and uFT4/uRBP, and C) Correlation of sTSH and uTSH/uRBP. Study ID presents the serial number of subjects; r presents the Pearson correlation coefficient; Overall (the last row of the results) showed the pooled correlation coefficients and corresponding 95% confidence intervals. sFT3: serum free triiodothyronine; uFT3/uRBP: the ratio of urine FT3 and retinol binding protein; sFT4: serum FT4; uFT4/uRBP: the ratio of uFT4 and uRBP; sTSH: serum TSH; uTSH/uRBP: the ratio of uTSH and uRBP.

The heterogeneity of the analysis (I2) was greater than 50% and the random-effects model was used for meta-analysis. The combined correlation coefficient between sFT4 and uFT4/uRBP was 0.29 (I2 = 68.9%, 95% CI: 0.07 ∼ 0.51) (). The combined correlation coefficient between sTSH and uTSH/uRBP was 0.11 (I2 = 86.7%, 95% CI: −0.24 ∼ 0.45), which indicated extreme heterogeneity (I2 > 75%) among these individuals ().
Discussion
The results of this study indicate that the levels of uFT3/uRBP and uFT4/uRBP are well correlated with the sFT3 and sFT4 during pregnancy. The levels of thyroid hormones are different depending on the gestational age [Wang et al. Citation2011; Yu et al. Citation2010], so it is necessary to monitor the thyroid function status periodically during pregnancy. Although the fetal thyroid gland begins to develop at 5–6 weeks of gestation, the function of trapping iodine and synthesizing thyroid hormones does not begin until 12–14 weeks, while significant hormonal secretion is not seen till 20 weeks. The fetal hypothalamic–pituitary– thyroid axis begins functioning early in the third trimester, but fetal thyroid hormones do not reach adult levels until birth [Obregon et al. Citation2007; Patel et al. Citation2011]. Therefore, maternal thyroid hormones must play an essential role in ensuring normal fetal development, in organ systems like the central nervous system [Vermiglio et al. Citation2004]. Clinical studies have suggested that maternal thyroid dysfunction can cause abortion, premature stillbirth, fetal intrauterine growth retardation, congenital malformation, and newborn thyroid dysfunction and neurodevelopment disorders [Allan et al. Citation2000; Casey et al. Citation2005; Rashid and Rashid Citation2007].
This study analyzed the serial changes of sFT3, sFT4, and sTSH at four different gestational ages. Overall, there was an inconsecutive rise in sTSH during pregnancy, followed by a progressive decrease in the sFT3 and sFT4 until the 27th week of gestation (the third trimester), when it was almost invariant. Previous studies showed that sTSH in early pregnancy was suppressed by human chorionic gonadotropin (HCG). This shares 85% sequence identity with the β-subunit of TSH and has a mild thyrotropic activity [EI Baba and Azar Citation2012]. Before 12 weeks of gestation, a decrease in sTSH was associated with high HCG values. After that, an increase in serum TSH was associated with the slow decrease in HCG [Glinoer Citation1997] in agreement with previous reports of others [Bocos-Terraz et al. Citation2009; Ardawi et al. Citation2002].The likely reasons were that the consumption of maternal thyroid hormones was increased with the increment in basal metabolic rate [Piers et al. Citation1995], and the consumption of iodine was increased with the increments in urinary iodine extraction [Bodzek et al. Citation2006] and fetal growth during pregnancy [Alvarez-Pedrerol et al. Citation2009].
Maternal renal function undergoes many alterations during a normal pregnancy. Compared with non-pregnant values, effective renal plasma flow (ERPF) increases by 80 percent during early pregnancy but falls significantly during the third trimester. The glomerular filtration rate (GFR), however, remained at a level 50 percent above the non-pregnant value throughout pregnancy. The filtration fraction (GFR/ERPF) was significantly reduced during early pregnancy but rose to a value equivalent to the non-pregnant value during the third trimester [Dunlop Citation1981; Larsson et al. Citation2010; Sturgiss et al. Citation1996]. This makes it more difficult to detect the urinary thyroid hormones during pregnancy, while the 21 KD RBP that is easily filtered by the glomerulus and reabsorbed in proximal tubules could be indicative of alterations of maternal renal function [Beetham et al. Citation1988; Cheung et al.Citation1989]. Hence, the urinary thyroid hormone parameters were analyzed after correction with uRBP in this study.
This study showed that the levels of serum and urinary thyroid hormones during pregnancy seemed to have a regularity of fluctuation accompanying gestational age. We also analyzed their correlation between the serum and urine specimens during pregnancy. The results from the meta-analysis showed that, there was a positive correlation between sFT3 and uFT3/uRBP, r = 0.38 (I2 = 0%, 95% CI: 0.21 ∼ 0.54) and a positive correlation between sFT4 and uFT4/uRBP, r = 0.29 (I2 = 68.9%, 95% CI: 0.07 ∼ 0.51). On the whole, the correlation (r) and homogeneity (I2) of the former were better than that of the latter. Previous studies also showed that the affinity of T4 to urinary proteins was stronger than that of T3, and an increase of protein excretion caused the marked increase of T4 excretion [Burke Citation1976]. The excreted T3 may reflect a loss of the daily production of the hormone more exactly.
There was an extreme heterogeneity among the 30 cases of normal pregnant women when we analyzed the correlation between sTSH and uTSH/uRBP for these individuals. The homogeneity analysis indicated that the individual differences were significant. There may be no obvious correlation between sTSH and uTSH/uRBP. As shown in , between the 9th and 17th weeks of gestation, the levels of uTSH/uRBP increased accompanying sTSH, and then the course of change in sTSH and uTSH/uRBP levels was a mirror image of each other.
In summary, the levels of sFT3, sFT4, uFT3/uRBP, and uFT4/uRBP kept dropping until the 27th week of gestation. The levels of uFT3/uRBP and uFT4/uRBP correlated well with the sFT3 and sFT4 during pregnancy. However, uFT3 is more accurate and useful than uFT4 for clinical examination but this awaits further independent study.
Materials and Methods
Subjects and design of study
This study was designed and undertaken in the Beijing Shijitan Hospital. The inclusion criteria were as follows: (i) pregnant women, 20–35 years old, (ii) between 9 and 12 weeks of gestation, (iii) singleton pregnancies, and (iv) 0.27mIU/L < sTSH < 2.5mIU/L, and other biochemical parameters of thyroid function were all normal. The exclusion criteria were as follows: (i) no kidney or liver dysfunction, (ii) no family genetic disease, (iii) no other history of autoimmune diseases, (iv) no life history in the areas of endemic goiter, (v) no history of excessive iodine intake, (vi) no medical history affecting thyroid function, (vii) no other metabolic disorders, and (viii) no smoking.
From October 2011 to March 2012, 37 cases of pregnant women were recruited with informed consent, which was in accordance with the provisions of the Helsinki Declaration and approved by the Ethics Committee of Beijing Shijitan Hospital. They all received a questionnaire on their medical history and were willing to accept physical examination. Thirty cases of pregnant women completed their routine antenatal examination and gave birth to children in Beijing Shijitan Hospital (4 cases of pregnant women did not complete their routine antenatal examination in Shijitan Hospital and 3 cases of them developed gestational diabetes mellitus in the second trimester). The 30 cases of involved women were aged 28.27 ± 0.42 y and during their first prenatal visit the body mass index (BMI) was 20.63 ± 0.36 kg/m2, the gestational age was 11.00 ± 0.23 weeks, and the sTSH concentration was 1.51 ± 0.10 mIU/L.
Specimen collection and processing
The serum specimens (8–12 a.m.) of the 30 cases of pregnant women were collected consecutively at 9–12, 14–17, 23–26, and 37–40 weeks of gestation (represented by t1, t2, t3, and t4, respectively), with the spot urine specimens simultaneously. The serum was prepared by centrifugation of the blood (1,780 x g for 5 min) within 2 h after acquisition. The cells and other non-soluble materials were cleared from the spot urine specimens by brief centrifugation (360 x g for 5 min) as soon as collection. Aliquots of serum and centrifuged urine specimens were stored at −80°C immediately.
Electrochemical luminescence immunoassay (ECLIA) for FT3, FT4, and TSH
The serum and random urine specimens were thawed (4°C) and centrifuged (1,780 g for 10 min), and then the supernatant was collected. The concentrations of FT3 (pmol/L), FT4 (pmol/L), and TSH (mIU/L) were detected using ECLIA on the Roche cobs e601 automatic electrochemical luminescence immunity analyzer (Roche Diagnostics GmbH, Switzerland). FT3 and FT4 were detected by a competitive method measured within range from 0.400 pmol/L to 50.000 pmol/L and 0.300 pmol/L to 100.000 pmol/L, respectively, while TSH by double-antibody sandwich method was measured within the range from 0.005mIU/L to 100.000mIU/L.
Competitive enzyme immunoassay (EIA) for uRBP
The supernatants of random urine specimens were obtained by the above methods. The concentration of uRBP (ng/ml) was determined using a commercially available EIA kit according to the manufacturer's instructions (EIA-RBP-1, Human/Mouse/Rat RBP4 Enzyme Immunoassay Kit, Raybiotech, Inc., Atlanta, America). The absorbance was read using Bio-Rad 680 microplate reader (Bio-Rad, Calfornia, America).
Statistical analysis
The concentrations of sFT3 (pmol/L), sFT4 (pmol/L), sTSH (mIU/L), uFT3 (pmol/L), uFT4 (pmol/L), uTSH (mIU/L), and uRBP (ng/ml) were measured. The levels of uFT3/uRBP (=uFT3*0.651/uRBP (mg/g)), uFT4/uRBP (=uFT4*0.77688/uRBP (mg/g)), and uTSH/uRBP (=uTSH*1000/uRBP (IU/g)) were calculated. The results are expressed as median values with 25–75th percentile.
The variation of thyroid hormone parameters in maternal serum and urine were analyzed during pregnancy. Repeated measures analysis of variance (ANOVA) was conducted by SigmaStat version 3.5. If assumptions of normality and equal variance were violated, then the alternative parametric test, i.e., LSD or nonparametric test, i.e., Student-Newman-Keuls was used for multiple comparisons of the repeatedly measured data among different measurement times. p Values of <0.05 from 2-sided tests were considered to indicate statistical significance.
To increase the statistical power of our analyses, the Pearson correlation coefficients (r) of thyroid hormone parameters between serum and urine during pregnancy were pooled [Nalls et al. Citation2013]. The STATA version 11.0 software was applied in this study. Firstly, we calculated the Pearson correlation coefficients (r) of each parameter between the serum and urine of each subject. Secondly, all of the r were standardized (Zr) using Fisher’s Zr transformation and reverse transformation. Then 95%CI for r was calculated, first using Zr and then reverse transformed from Zr to r. Thirdly, we estimated a pooled r with 95% CI based on fixed- and random-effects models depending on the heterogeneity of the analysis. I-squared (I2, ranges between 0 and 100%, I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity) indicates the proportion of variability in effect sizes due to heterogeneity. When I2 > 50% indicated heterogeneity across studies, the random-effects model was used for meta-analysis, otherwise the fixed-effects model was calculated.
Declaration of interest
This research was supported by National Key Technology R&D program (No.2013BAI12B01-4) and Beijing Physical Science Fund (No.7122086). The authors report no declarations of interest.
Author contributions
Conceived and designed the experiments: MZ; Performed the experiment: JC, QM; Analyzed the data: MZ, JC, XZ, TL; Contributed reagents/material/analysis tools: MZ, XZ, TL, QM; Wrote the Manuscript: MZ, JC, HZ.
| Abbreviations | ||
| FT3 | = | free triiodothyronine |
| FT4 | = | free thyroxine |
| TSH | = | thyrotropin |
| RBP | = | retinol binding protein |
| sFT3 | = | serum FT3 |
| sFT4 | = | serum FT4 |
| sTSH | = | serum TSH |
| uFT3 | = | urine FT3 |
| uFT4 | = | urine FT4 |
| uTSH | = | urine TSH |
| uRBP | = | urine RBP |
| uFT3/uRBP | = | the ratio of uFT3 and uRBP |
| uFT4/uRBP | = | the ratio of uFT4 and uRBP |
| uTSH/uRBP | = | the ratio of uTSH and uRBP |
| BMI | = | body mass index |
| ECLIA | = | electrochemical luminescence immunoassay |
| EIA | = | competitive enzyme immunoassay |
| ANOVA | = | analysis of variance |
| rs | = | correlation coefficients |
Acknowledgments
We gratefully acknowledge all volunteers for the generous donation of urine and serum specimens as well as the staff in the Clinical Laboratory of Beijing Shijitan Hospital for enthusiastic assistance. We would like to thank Professor Dr. Hongyuan Wang (Peking University Health Center) for suggesting opinions about statistical analysis.
References
- Abalovich, M., Gutierrez, S., Alcaraz, G., Maccallini, G., Garcia, A. and Levalle, O. (2002) Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 12:63–8
- Allan, W.C., Haddow, J.E., Palomaki, G.E., Williams, J.R., Mitchell, M.L., Hermos, R.J., et al. (2000) Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 7:127–30
- Alvarez-Pedrerol, M., Guxens, M., Mendez, M., Canet, Y., Martorell, R., Espada, M., et al. (2009) Iodine levels and thyroid hormones in healthy pregnant women and birth weight of their offspring. Eur J Endocrinol 160:423–9
- Ardawi, M.S., Nasrat, H.A. and Mustafa, B.E. (2002) Urinary iodine excretion and maternal thyroid function. During pregnancy and postpartum. Saudi Med J 23:413–22
- Beetham, R., Dawnay, A., Menabawy, M. and Silver, A. (1988) Urinary excretion of albumin and retinol-binding protein during normal pregnancy. J Clin Pathol 41:1089–92
- Bocos-Terraz, J.P., Izquierdo-Alvarez, S., Bancalero-Flores, J.L., Alvarez-Lahuerta, R., Aznar-Sauca, A., Real-López, E., et al. (2009) Thyroid hormones according to gestational age in pregnant Spanish women. BMC Res Notes 2:237
- Bodzek, P., Olejek, A. and Zamłyński, J. (2006) Iodine excretion with urine and thyrotrophic hormone concentration in normal and complicated pregnancies in the industrial region of iodine deficiency. Wiad Lek 59:612–17
- Burke, C.W. and Shakespear, R.A. (1976) Triiodothyronine and thyroxine in urine. II. Renal handling, and effect of urinary protein. J Clin Endocrinol Metab 42:504–13
- Burke, C.W., Shakespear, R.A. and Fraser, T.R. (1972) Measurement of thyroxine and triiodothyronine in human urine. Lancet 2:1177–9
- Casey, B. M., Dashe, J. S., Wells, C. E., McIntire, D.D., Byrd, W., Leveno, K.J., et al. (2005) Subclinical Hypothyroidism and Pregnancy Outcomes. Obstet Gynecol 105:239–45
- Cheung, C.K., Lao, T. and Swaminathan, R. (1989) Urinary excretion of some proteins and enzymes during normal pregnancy. Clin Chem 35:1978–80
- Chu, L., Fu, G., Meng, Q., Zhou, H. and Zhang, M. (2013) Identification of urinary biomarkers for type 2 diabetes using bead-based proteomic approach. Diabetes Res Clin Pract 101:187–93
- Dunlop, W. (1981) Serial changes in renal haemodynamics during normal human pregnancy. Br J Obstet Gynaecol 88:1–9
- EI Baba, K.A. and Azar, S.T. (2012) Thyroid dysfunction in pregnancy. Int J Gen Med 5:227–30
- Glinoer, D. (1997) The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 18:404–33
- Inoue, M., Arata, N., Koren, G. and Ito, S. (2009) Hyperthyroidism during pregnancy. Can Fam Physician 55:701–3
- Larsson, A., Palm, M., Hansson, L.O. and Axelsson, O. (2010) Cystatin C and modification of diet in renal disease (MDRD) estimated glomerular filtration rate differ during normal pregnancy. Acta Obstet Gynecol Scand 89:939–44
- Lei, T., Zhao, X., Jin, S., Meng, Q., Zhou, H. and Zhang, M. (2013) Discovery of potential bladder cancer biomarkers by comparative proteomics and analysis. Clin Genitourin Cancer 11:62–5
- Moura, E.G., Moura, C.C. (2004) Regulation of thyrotropin synthesis and secretion. Arq Bras Endocrinol Metabol 48:40–52
- Nalls M.A., Duran R., Lopez G., Kurzawa-Akanbi M., McKeith I.G., Chinnery P.F., et al. (2013) A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 70:727–35
- Obregon, M.J., Calvo, R.M., Del Rey, F.E. and de Escobar, G.M. (2007) Ontogenesis of thyroid function and interactions with maternal function. Endocr Dev 10:86–98
- Patel, J., Landers, K., Li, H., Mortimer, R.H. and Richard, K. (2011) Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab 22:164–70
- Pierce, J.G., Liao, T., Howard, S.M., Shome, B. and Cornell, J.S. (1971) Studies on the structure of thyrotropin: its relationship to luteinizing hormone. Recent Prog Horm Res 27:165–212
- Piers, L.S., Diggavi, S.N., Thangam, S., van Raaij, J.M., Shetty, P.S. and Hautvast, J.G. (1995) Changes in energy expenditure, anthropometry, and energy intake during the course of pregnancy and lactation in well-nourished Indian women. Am J Clin Nutr 61:501–13
- Rashid, M. and Rashid, M.H. (2007) Obstetric management of thyroid disease. Obstet Gynecol Surv 62:680–8
- Sahay, R.K. and Nagesh, V.S. (2012) Hypothyroidism in pregnancy. Indian J Endocrinol Metab 16:364–70
- Shakespear, R.A. and Burke, C.W. (1976) Triiodothyronine and thyroxine in urine. I. Measurement and application. J Clin Endocrinol Metab 42:494–503
- Sturgiss, S.N., Wilkinson, R. and Davison, J.M. (1996) Renal reserve during human pregnancy. Am J Physiol 271:F16–F20
- Vamvakopoulos, N.C. and Kourides, I.A. (1979) Identification of separate mRNAs coding for the alpha and beta subunits of thyrotropin. Proc Natl Acad Sci USA 76:3809–13
- Vermiglio, F., Lo Presti, V.P., Moleti, M., Sidoti, M., Tortorella, G., Scaffidi, G., et al. (2004) Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab 89:6054–60
- Wang, Q.W., Yu, B., Huang, R.P., Cao, F., Zhu, Z.Q., Sun, D.C., et al (2011) Assessment of thyroid function during pregnancy: the advantage of self-sequential longitudinal reference intervals. Arch Med Sci 7:679–84
- Yen, P.M. (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–142
- Yoshida, K., Sakurada, T., Kaise, K., Kaise, N., Nomura, T., Itagaki, Y., et al. (1988) Measurement of thyroid stimulating hormone (TSH) in human urine. Endocrinol Jpn 35:733–9
- Yu, B., Wang, Q.W., Huang, R.P., Cao, F., Zhu, Z.Q., Sun, D.C., et al. (2010) Establishment of self-sequential longitudinal reference intervals of maternal thyroid function during pregnancy. Exp Biol Med (Maywood) 235:1212–15

