Abstract
Cryopreservation of spermatozoa plays a significant role in reproductive medicine and fertility preservation. Chicken egg yolk is used as an extender in cryopreservation of human spermatozoa using glycerol egg yolk citrate (GEYC) buffered medium. Even though 50% survival of spermatozoa is generally achieved with this method, the risk of high levels of endotoxins and transmission pathogens from chicken egg yolk is a matter of concern. In the present study we attempted to establish a chemically defined cryopreservation medium which can replace the chicken egg yolk without affecting sperm survival. Ejaculates from 28 men were cryopreserved with GEYC based freezing medium or liposome encapsulated soy lecithin-cholesterol based freezing medium (LFM). The semen samples were subjected to rapid thawing after 14 days of storage in liquid nitrogen. Post-thaw analysis indicated significantly higher post-thaw motility and sperm survival in spermatozoa cryopreserved with LFM compared to conventional GEYC freezing medium. The soy lecithin and cholesterol at the ratio of 80:20 with sucrose showed the highest percentage of post-thaw motility and survival compared to the other compositions. In conclusion, chemically defined cryopreservation medium with liposome encapsulated soy lecithin and cholesterol can effectively replace the chicken egg yolk from human semen cryopreservation medium without compromising post-thaw outcome.
Introduction
In recent years, semen cryopreservation has become an integral aspect of reproductive medicine and fertility preservation. Semen cryopreservation and thawing is known to induce dramatic changes in the structure of spermatozoa. Despite its significance, even today we do not have an ideal cryopreservation medium which can prevent the loss of sperm function during the freeze-thaw process. The fertilization rate and pregnancy rate with frozen-thawed spermatozoa are demonstrated to be significantly lower than the spermatozoa from fresh human ejaculates [Yildiz et al. Citation2007] which is attributed to the loss of motility, viability, mitochondrial function, premature acrosome reaction, ultrastructural changes, and increased DNA damage due to the freeze-thaw process [Check et al. Citation1991; Critser et al. Citation1988; Cross and Hanks Citation1991; Kalthur et al. Citation2008].
The common extender present in conventional semen cryopreservation medium is hen’s egg yolk. It acts as an excellent reservoir of phospholipids and cholesterol and helps in replenishing these lipids which are lost from the sperm membrane during the freeze-thaw process. However, using egg yolk in human semen cryopreservation medium has certain drawbacks. The composition of egg yolk varies with the nutritional and health status of the hen. Apart from this, it can potentially harbor various infectious microorganisms and endotoxins [Jeyendran et al. Citation2008]. Due to these reasons, it is extremely difficult to have a good quality control on the outcome of semen cryopreservation when egg yolk based freezing mediums are used.
Substitution of egg yolk with lipoproteins and phospholipids have been tried in semen cryopreservation of human [Jeyendran et al. Citation2008] and lower animals [Aires et al. Citation2003; Thun et al. Citation2002]. Lipoproteins of animal origin may still carry the risk of endotoxins. To overcome this, we attempted to replace hen’s egg yolk with liposome encapsulated soy lecithin and cholesterol within the human semen cryopreservation medium.
Results
Formulation of liposomes
Lipid hydration successfully produced liposomes containing different lipid compositions. The composition of liposomes and results of size, poly dispersity index, and zeta potential are given in . The average vesicular size (before lyophilization) of all the batches of liposomes ranged between 246.66 and 283.00 nm. Lyophilization did not affect the size of liposomes and the liposomes after lyophilization showed a negligible increase (70–90 nm) in average size. Liposomal preparations of all the batches showed a low poly dispersity index of <0.330 before and after lyophilization. The zeta potential (before lyophilization) ranged between −36.67 to −47.33 mV indicating excellent physical stability of liposomes. There was no decrease in zeta potential (−29.33 to −40.67 mV) even after lyophilization, indicating the good physical stability of lyophilized liposomes. One batch of liposomes containing soy phosphatidyl choline (SPC) and cholesterol at 80:20 was prepared by using sucrose 10% w/w in aqueous dispersion medium. Inclusion of sucrose as a cryoprotectant decreased the size build up after lyophilization (<50 nm size increase after lyophilization). Also, there was no noticeable change in zeta potential with sucrose containing liposomes before and after lyophilization in comparison with the liposomes prepared without sucrose.
Table 1. Composition and size of liposome prepared using soy lecithin and cholesterol in various ratios.
Post-thaw motility
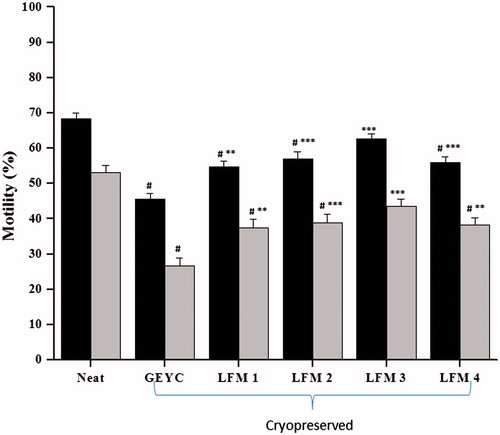
The sperm motility was graded under the microscope according to WHO [Citation2010] criteria as described in our earlier report [Kalthur et al. Citation2012]. The total and progressive motility in the neat sample (fresh ejaculate) was ∼70% and 52%, respectively (). Immediately after thawing, the motility was assessed in semen samples cryopreserved with glycerol egg yolk citrate (GEYC) and liposome based freezing medium (LFM). A significant decline (p < 0.001) in motility was observed in semen samples cryopreserved using conventional GEYC freezing medium. Cryopreservation using LFM with different concentrations of cholesterol and SPC exhibited similar responses except in LFM 3 (SPC 80: cholesterol 20). In this group, a significantly higher percentage of progressive motility was observed compared to the GEYC group (p < 0.001) and other groups (p < 0.05 vs. LFM 4; p < 0.01 vs. LFM 1).
Figure 1. Post-thaw motility in normozoospermic ejaculates cryopreserved with glycerol egg yolk citrate (GEYC) buffered and liposome based freezing medium (LFM). The spermatozoa were analyzed for the motility after thawing. The data from 28 subjects represented as the mean motility and the error bars represent the standard error of mean (SEM) value. Liposome based medium was prepared with liposomes encapsulated with soy lecithin and cholesterol at various ratios and designated as LFM1 (soy lecithin: cholesterol: 95:5), LFM2 (soy lecithin: cholesterol: 90:10), LFM3 (soy lecithin: cholesterol: 80:20), and LFM4 (soy lecithin: cholesterol: 70:30). The freeze-thaw process resulted in a significant reduction in total and progressive motility in post-thaw samples of GEYC, LFM1, LFM2, and LFM4 groups compared to that of the neat sample (#p < 0.001). Similarly, the total motility and progressive motility was higher in samples cryopreserved with liposome based medium compared to those cryopreserved with GEYC medium (**p < 0.01, ***p < 0.001). ▪: total motility; ▪: progressive motility.
Sperm yield
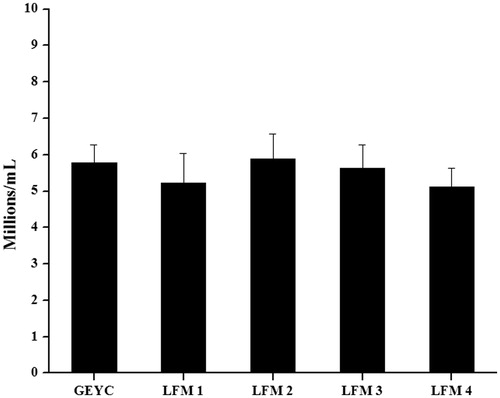
The post-thaw samples with equal sperm density from the GEYC and LFM groups were processed by swim up to extract the motile spermatozoa. There was no significant difference in the sperm yield among any of the groups. The GEYC group had a sperm yield of 5.78 ± 0.49, while it was 5.23 ± 0.81, 5.62 ± 0.66, 5.89 ± 0.69, and 5.11 ± 0.53 millions/ml in LFM1, LFM2, LFM3, and LFM4, respectively ().
Figure 2. Sperm yield by swim up technique in normozoospermic ejaculates cryopreserved with glycerol egg yolk citrate (GEYC) buffered and liposome based freezing medium (LFM). The liquefied semen samples from 28 normozoospermic subjects were divided into five equal parts after the analysis and cryopreserved with GEYC or LFM based cryopreservation medium. The LFM was prepared with liposomes encapsulated with soy lecithin and cholesterol at various ratios and designated as LFM1 (soy lecithin: cholesterol: 95:5), LFM2 (soy lecithin: cholesterol: 90:10), LFM3 (soy lecithin: cholesterol: 80:20), and LFM4 (soy lecithin: cholesterol: 70:30). The semen samples were thawed and the motile sperm were extracted by swim up technique. After 1 hour of incubation, the overlay was collected from which the sperm density was assessed. The data represents the mean sperm density in millions per ml and standard error of mean (SEM) value. Even though the sperm yield was higher in the LFM2 and LFM3 groups compared to the GEYC group, the difference was statistically non-significant (p > 0.05).
In vitro sperm survival
The motile spermatozoa from frozen-thawed semen samples cryopreserved with GEYC and LFM cryopreservation medium were collected by swim up. Their survival under in vitro conditions was assessed at 1, 4, and 12 hours after incubation (). At 1 hour, the GEYC group had ∼60% progressive motility which was higher in the LFM group. A significantly higher percentage of motile spermatozoa (p < 0.001) was observed in the LFM 3 group composed of SPC and cholesterol at the ratio of 80:20.
Table 2. Progressive motility in human spermatozoa subjected to freeze-thaw process using cryopreservation medium with liposomes of soy lecithin and cholesterol in various ratios.
A time-dependent drop in motility was observed in semen samples cryopreserved in GEYC medium. At 4 hours after incubation, the total and progressive motility declined by ∼11% and 15% compared to the respective motility type at 1 hour. At 12 hours after incubation, there was almost a 21% decline in total motility and a 31% decline in progressive motility compared to that at 1 hour. However, in the LFM 3 group, motility was retained even up to 12 hours after incubation and had a significantly higher percentage of total motile and progressive motile spermatozoa when compared to the GEYC group (p < 0.01 and p < 0.001, respectively). The in vitro survival was better in the LFM 3 group compared to the rest of the groups up to 12 hours.
Effect of sucrose in LFM
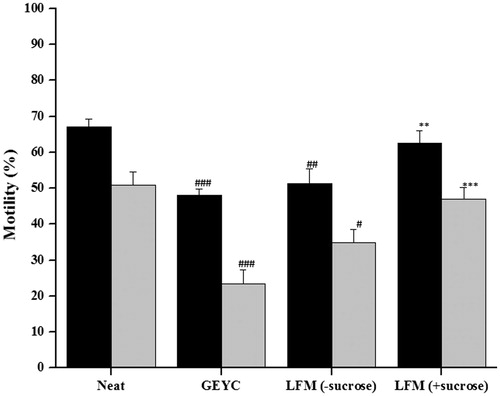
To check whether the presence of sucrose in the liposome preparation would affect the post-thaw sperm quality, we cryopreserved 10 ejaculates with GYEC medium and LFM medium (SPC: cholesterol 80:20) with and without sucrose. In both types, the LFM medium post-thaw motility was higher compared to the GEYC. However, a significantly higher total motility (p < 0.01) and progressive motility (p < 0.001) was found in ejaculates cryopreserved using LFM with sucrose compared to the GEYC group. The post-thaw motility was not significantly higher in LFM with sucrose. This indicates that the presence of sucrose in the liposome preparation does not interfere with but enhances sperm survival ().
Figure 3. Sperm motility in normozoospermic ejaculates frozen with liposome based cryopreservation medium (LFM) with or without sucrose. The semen samples from 10 normozoospermic subjects were divided into three parts. Semen samples were cryopreserved with glycerol egg yolk citrate (GEYC) medium, LFM3 medium (soy lecithin: cholesterol: 80:20) with sucrose (10% w/w) and LFM3 medium (soy lecithin: cholesterol: 80:20) without sucrose. The data represents the mean motility and standard error of mean (SEM). Both total and progressive motility was significantly lower in semen samples cryopreserved with conventional GEYC medium and LFM without sucrose (#p < 0.05, ##p < 0.01, ###p < 0.001). However, LFM with sucrose had a significantly higher percentage of total (**p < 0.01) and progressive motility (***p < 0.001) compared to samples cryopreserved in GEYC. ▪: total motility; ▪: progressive motility.
Discussion
During the freeze-thaw process human spermatozoa experiences oxidative, chemical, and physical stress [Alvarez and Storey Citation2003; Donnelly et al. Citation2001a,Citationb; Mazzilli et al. Citation1995]. Loss of motility [McLaughlin et al. Citation1992], loss of mitochondrial function [O’Connell et al. Citation2002], ultrastructural changes [Critser et al. Citation1988], membrane and DNA damage [Zribi et al. Citation2010] are the common consequences of this process which reduces the functional competence of the spermatozoa. Therefore, the pregnancy rate in assisted reproductive techniques using frozen-thawed human semen samples is generally lower than in the fresh ejaculate.
Glycerol egg yolk citrate buffered cryopreservation medium is routinely used for cryopreservation of human semen samples [Kalthur et al. Citation2008; Kotdawala et al. Citation2012]. Post-thaw survival of spermatozoa depends upon various factors such as quality of the ejaculate, freezing technique used, and composition of the freezing medium. In the present investigation we observed > 50% post-thaw survival of spermatozoa using GEYC medium. Even though this is an acceptable survival rate for human spermatozoa, the major concern of using GEYC medium is mainly due to the animal origin of egg yolk. The quality and composition of the egg yolk can change according to the nutritional status of the chicken egg. In addition, the chicken egg can harbor various microbial organisms and high levels of endotoxins. Due to these factors, achieving a consistent sperm survival following freeze-thaw process is technically challenging. To overcome these problems, in the present study we have attempted to replace the egg yolk with SPC and cholesterol encapsulated in the liposome form.
Soy phosphatidyl choline is considered to contain 10% of the phospholipids present in chicken egg yolk. Hong et al. [Citation1986] have demonstrated that the presence of phosphatidyl choline does not have any negative effect on human sperm motility. Therefore, one would expect that using SPC in semen cryopreservation medium may not have an adverse effect on spermatozoa. Earlier studies have shown that soy lecithin alone can replace the egg yolk from cryopreservation medium without compromising post-thaw sperm quality [Aires et al. Citation2003; Reed et al. Citation2009]. However, results of these studies indicate that the post-thaw sperm quality was either similar or marginally better than the egg yolk containing medium. In the present study we used liposome encapsulated SPC and cholesterol in the freezing medium which was found to have a significantly better post-thaw sperm function compared to the conventional egg yolk based freezing medium.
During the freeze-thaw process extensive changes take place in the sperm membrane. Along with transition of lipid molecules and peroxidation of polyunsaturated fatty acids, the loss of cholesterol is a predominant event [Drobnis et al. Citation1993]. Replenishment of cholesterol is very crucial to regain the membrane fluidity and function. In order to achieve this, in the present study we have used cholesterol along with SPC. In contrast to earlier studies we have encapsulated the SPC and cholesterol in liposomes. It is possible that since the liposomes behave like a cell membrane themselves, and have the property to adhere with the cells during freezing and thawing, exchange of cholesterol and phospholipids may occur more efficiently than when they are in the free form. This might explain the higher post-thaw survival and their longevity under in vitro conditions.
The data on post-thaw survival, sperm yield, and sperm survival in vitro clearly suggests that the optimum ratio of SPC and cholesterol in freezing medium for human ejaculate is 80:20. It is well established that poor quality ejaculates have a higher susceptibility to freeze-thaw-induced changes [Kalthur et al. Citation2008], which is attributed to the ultrastructural changes and/or their membrane composition. It will be interesting to determine whether similar results can be expected for a semen sample with abnormal semen parameters. However, in the present investigation, the efficacy of this chemically defined medium is assessed only in ejaculates with normal semen parameters.
A recent study by Del Valle et al. [Citation2012] indicates that SPC interferes with mitochondrial function of ram spermatozoa. In the present study even though we have not assessed mitochondrial function, the longevity in survival of spermatozoa cryopreserved in liposome based medium indicates that the substitution of egg yolk with SPC and cholesterol in liposome encapsulated form may not have any adverse effects on sperm mitochondrial function. However, this needs to be confirmed.
Sucrose is generally used in liposome preparation as a non-penetrating cryoprotectant that helps in stabilization of liposomes. It helps in decreasing the build-up in size of liposomal vesicles after lyophilzation [Capini et al. Citation2009]. Since it also acts as a non-penetrating cryoprotectant during semen cryopreservation and thawing [Critser et al. Citation1988], its presence in LFM was found to be beneficial for preventing loss of sperm function during the freeze-thaw process.
In conclusion, the results of the present study indicate that the liposome based cryopreservation medium containing soy lecithin and cholesterol at the ratio of 80:20 can replace egg yolk. The chemically defined cryopreservation medium can certainly help in quality control over semen cryopreservation protocol and avoid high risk of transmission of pathogenic microorganisms and endotoxins from animal origin. However, further studies are essential in this direction to ensure the superiority of chemically defined freezing medium over conventional glycerol egg yolk citrate buffered medium.
Materials and Methods
Chemicals
The following chemicals were used: soy phosphatidylcholine (soy lecithin; SPC; Sigma Aldrich, Cat No. P5638, USA); cholesterol (Sigma Aldrich, Cat No. C3292, USA); glucose (Sigma Chemicals, Cat No. G5146); fructose (Sigma Chemicals, Cat No. F3510); citric acid (Sigma Chemicals, Cat No. C7254); glycerol (Sigma Chemicals, Cat No. G9012); glycine (MERCK Chemicals, Cat No. 56-40-6, India); human serum albumin (Sigma Chemicals, Cat No. A1653).
Study subjects
The study was approved by the Institutional Ethics Committee of Kasturba Hospital, Kasturba Medical College, Manipal. Ejaculates from 28 normozoospermic men who attended the Andrology Laboratory of Manipal University, Manipal for routine semen evaluation were included in the study. Subjects with ejaculatory abstinence of 2–4 d were asked to provide the semen samples by masturbation. Upon liquefaction, the semen samples were used for microscopic analysis and cryopreservation. The subjects were explained about the study and a written consent was obtained from each subject.
Semen analysis
The liquefied semen samples were subjected to routine semen analysis. The sperm density in the semen samples were assessed using Makler’s counting chamber and expressed in millions per ml. The sperm motility, viability, and morphology were assessed in all the samples as described in the WHO [Citation2010] manual.
Preparation of liposomes
The liposomes of phosphatidylcholine and cholesterol in various ratios (95:05, 90:10, 80:20, and 70:30) were prepared. The composition of liposomes for 8 ml is given in . Briefly, accurately weighed quantities of soy phosphatidylcholine (soy lecithin; SPC) alone or SPC and cholesterol in different ratios were dissolved in 10 ml of chloroform in a 250 ml round bottomed (RB) flask. A thin lipid film was formed in the RB flask using a Rotary Flash evaporator (Rota-Vap, Buchi, Switzerland) at 40°C. To this, sucrose (5% w/v) was added and the flasks were continuously shaken for about 15–20 min at 40°C. The size and zeta potential of liposomes were determined using Nano ZS (Malvern Instruments, UK). After determining the size, the liposomal dispersions were lyophilized for 24–48 h.
Preparation of LFM
The secondary buffer was prepared by mixing one part of 303 mM glucose, one part of 303 mM fructose, and three parts of 100 mM citric acid in ratio 1:1:3. Medium was freshly prepared each time by mixing 6.8 ml of secondary buffer, 1.2 ml of glycerol, and 1% Glycine for 8 ml. This 8 ml of solution was taken and supplemented with about 450 mg of lyophilized liposomes of SPC and cholesterol in different ratios.
The conventional GEYC buffered medium was prepared by mixing 5.2 ml of the secondary buffer, 1.2 ml of glycerol, and 1.6 ml of egg yolk (from locally purchased hen egg) in ratio 13:3:4. The mixture was heat inactivated by incubating at 56°C for 30 min followed by the addition of 1% glycine.
Cryopreservation of human ejaculate
The ejaculates were cryopreserved in liquid nitrogen using GEYC as described previously [Kalthur et al. Citation2008], and served as control. Briefly, the liquefied semen samples were divided into aliquots of equal volume and mixed with cryopreservation medium in 1:1 ratio as: Control: semen sample + GEYC buffered cryopreservation medium; LFM 1: semen sample + liposome containing (SPC and cholesterol: 95:05) cryopreservation medium without egg yolk; LFM 2: semen sample + liposome containing (SPC and cholesterol: 90:10) cryopreservation medium without egg yolk; LFM 3: semen sample + liposome containing (SPC and cholesterol: 80:20) cryopreservation medium without egg yolk; LFM 4: semen sample + liposome containing (SPC and cholesterol: 70:30) cryopreservation medium without egg yolk. The samples were then transferred to cryovials and kept at 4°C for 10 min, −4°C for 10 min, in liquid nitrogen vapor (−160°C) for three min and finally plunged into liquid nitrogen (−196°C).
Thawing
After 14 days of storage in liquid nitrogen, the cryovials were removed and subjected to rapid thawing by placing the cryovials in running tap water for 5 min. The frozen samples were mixed with equal volume of Earle’s Balanced Salt Solution (EBSS) medium supplemented with 0.1% human serum albumin (HSA) and centrifuged at 1500 rpm for 8 min. This step was repeated twice to remove the cryoprotectants completely. The pellet was then re-suspended in 0.2 ml of EBSS medium and used for further analysis.
Assessing post-thaw motility and survival in spermatozoa
The motility of the spermatozoa was assessed from each group by placing approximately 10 µl of the sample on a clean microscopic slide and observing under the microscope at 400× magnification. The percentage of spermatozoa with progressive and non-progressive motility was counted separately and expressed in percentage.
Extraction of motile sperm by swim up technique
To assess the post-wash survival, the samples were centrifuged twice at 1200 rpm for 8 min, the supernatant was discarded, and 200 µl of EBSS with HSA medium was carefully layered on the pellet. The tubes were incubated at 37°C and 5% CO2 in air for 1 h. The layered medium containing motile spermatozoa were carefully collected without disturbing the pellet and transferred to a new tube. The motility was assessed from this sample at 1, 4, and 12 h of incubation.
Statistical analysis
Data are presented as Mean ± SE. The validity of the data was analyzed by one way analysis of variance (ANOVA) using Bonferroni post-test using GraphPadInstat 3.0 software (GraphPad Software, Inc., San Deigo, CA, USA). Statistical significance was set at 0.05.
Declaration of interest
The authors have no declarations of interest to report.
Author contributions
Study concept, study design, and manuscript preparation: GK; Study design, liposome preparation: SM; Performed all the experiments: SRS; Preparation and characterization of liposomes: KA, HJ, JM, ARH; Statistical analysis and critical reading of manuscript: PK; Helped in designing the study and manuscript writing: SKA.
| Abbreviations | ||
| GEYC | = | glycerol egg yolk citrate |
| SPC | = | soy phosphatidyl choline |
| LFM | = | liposome based freezing medium |
| EBSS | = | Earle’s balanced salt solution |
| HSA | = | human serum albumin |
| RB | = | round bottomed flask |
| ANOVA | = | analysis of number of variance |
References
- Aires, V.A., Hinsch, K.D., Mueller-Schloesser, F., Bogner, K., Mueller-Schloesser, S., and Hinsch, E. (2003) In vitro and in vivo comparison of egg yolk-based and soybean lecithin-based extenders for cryopreservation of bovine semen. Theriogenology 60:269–79
- Alvarez, A.J., and Storey, B.T. (2003) Evidence that membrane stress contributes more than lipid peroxidation to sublethal cryodamage in cryopreserved human sperm: glycerols and other polyols as sole cryoprotectant. J Androl 14:199–208
- Capini, C., Jaturanpinyo, M., Chang, H.I., Mutalik, S., McNally, A., Street, S., et al. (2009) Antigen-specific suppression of inflammatory arthritis using liposomes. J Immunol 182:3556–65
- Check, M.L., Check, J.H., and Long, R. (1991) Detrimental effects of cryopreservation on the structural and functional integrity of the sperm membrane. Arch Androl 27:155–60
- Critser, J.K., Huse-Benda, A.R., Aakes, D.V., Arneson, B.W., and Ball, G.D. (1988) Cryopresevation of human spermatozoa III. The effect of cryoprotectants on motility. Fertil Steril 50:314–20
- Cross, N.L., and Hanks, S.E. (1991) Effects of cryopreservation on human sperm acrosomes. Hum Reprod 6:1279–83
- Del Valle, I., Gomez-Duran, A., Holt, W.V., Muino-Blanco, T., and Cebrian-Perez, J.A. (2012) Soy lecithin interferes with mitochondrial function in frozen-thawed ram spermatozoa. J Androl 33:717–25
- Donnelly, E.T., Steele, E.K., McClure, N., and Lewis, S.E. (2001a) Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod 16:1191–9
- Donnelly, E.T., McClure, N., and Lewis, S.E. (2001b) Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 76:892–900
- Drobnis, E.Z., Crowe, L.M., Berger, T., Anchordoquy, T.J., Overstreet, J.W., and Crowe, J.H. (1993) Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J Exp Zool 265:432–7
- Hong, C.Y., Sheih, C.C., Wu, P., Huanq, J.J., and Chiang, B.N. (1986) Effect of phosphatidylcholine, lysophosphatidylcholine, arachidonic acid and docosahexaenoic acid on the motility of human sperm. Int J Androl 9:118–22
- Jeyendran, R.S., Acosta, V.C., Land, S., and Coulam, C.B. (2008) Cryopreservation of human sperm in a lecithin-supplemented freezing medium. Fertil Steril 90:1263–5
- Kalthur, G., Adiga, S.K., Upadhya, D., Rao, S., and Kumar, P. (2008) Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril 89:1723–7
- Kalthur, G., Salian, S.R., Keyvanifard, F., Sreedharan, S., Thomas, J.S., and Kumar, P. (2012) Supplementation of biotin to sperm preparation medium increases the motility and longevity in cryopreserved human spermatozoa. J Assist Reprod Genet 29:631–5
- Kotdawala, A.P., Kumar, S., Salian, S.R., Thankachan, P., Govindraj, K., Kumar, P., et al. (2012) Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J Assist Reprod Genet 29:1447–53
- Mazzilli, F., Rossi, T., Sabatini, L., Pulcinelle, F.M., Rapone, S., Dondero, F., et al. (1995) Human sperm cryopreservation and reactive oxygen species (ROS) production. Acta Europaea Fertilitatis 26:145–8
- McLaughlin, E.A., Ford, W.C., and Hull, M.G. (1992) The contribution of the toxicity of a glycerol-egg yolk-citrate cryopreservative to the decline in human sperm motility during cryopreservation. J Reprod Fertil 95:749–54
- O’Connell, M., McClure, N., and Lewis, S.E. (2002) The effect of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 17:704–9
- Reed, M.L., Ezeh, P.C., Hamic, A., Thompson, D.J., and Caperton, C.L. (2009) Soy lecithin replaces egg yolk for cryopreservation of human sperm without adversely affecting postthaw motility, morphology, sperm DNA integrity, or sperm binding to hyaluronate. Fertil Steril 92:1787–90
- Thun, R., Hurtado, M., and Janett, F. (2002) Comparison of Biociphos-Plus and TRIS-egg yolk extender for cryopreservation of bull semen. Theriogenology 57:1087–94
- WHO. (2010) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. World Health Organization. 5th ed. Cambridge, UK: Cambridge University Press UK
- Yildiz, C., Ottaviani, P., Law, N., Ayearst, R., Liu, L., and McKerlie, C. (2007) Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction 133:585–95
- Zribi, N., Feki Chakroun, N., El Euch, H., Garqouri, J., Bahloul, A., and Ammar Keskes, L. (2010) Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 93:159–66

