Abstract
Thymoquinone (TQ) is a phytochemical compound found in the plant Nigella sativa. It has antioxidant and anti-cancer effects. This study investigated the effects of TQ on obesity and testicular structure of high-fat-diet (HFD) fed rats. Obese control (OC) and obese thymoquinone (OT) groups were fed a special diet containing 40% of total calories from fat. Non-obese control (NC) and non-thymoquinone (NT) groups were fed a standard diet for nine weeks. Then, intraperitoneal TQ injections were carried out to the OT and NT groups for six weeks and testes were removed. Catalase and myeloperoxidase activity were determined in rat testis tissue. Stereological, histopathological, and immunohistochemical changes were evaluated in the testes of the rats. In stereological studies, mean volumes of testis and seminiferous tubules, the number of spermatogenic cells and also Leydig cells in the OC group were reduced, but these values significantly increased in the OT group. Apoptotic cells were observed in the OC group in comparison to the OT group. The number of healthy sperms were reduced in the OC group, whereas the majority showed anomalies in the head, neck, and tail. The number of healthy sperm was increased and the anomalies significantly reduced by using TQ in both the NT, and especially the OT group. TQ like antioxidants may improve fertility by means of increasing the healthy sperm number and preventing sperm anomalies.
Introduction
In recent years, there has been an increase in the number of obesity associated conditions as a result of the increasing uptake of high-energy foods, decreasing physical activity, and a sedentary lifestyle. Oxidative stress caused by obesity is closely related to various diseases such as cardiovascular, hypertension, diabetes, cancers, and infertility [WHO Citation1997]. Obesity is characterized by increased fat accumulation in the body and it is related to many factors, and is a serious chronic disease. Obesity or excessive lipid accumulation are generally accompanied with increased glucose tolerance and rising serum triacylglycerol concentrations. Also, type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, infertility, and fast cancer progression are frequently seen in obese individuals [Galinier et al. Citation2005; Must and Anderson Citation2003; Wright et al. Citation1994]. The most important risk factors for obesity are excessive calorie intake, gender, age, education, marriage, and birth number. In addition, obesity can be caused by hereditary factors. More than 30% of the population in Turkey (men 7.9%, women 23.4%) are obese. Obesity is closely associated with many chronic diseases [Hatemi et al. Citation2002; Onat et al. Citation2002].
Known causes of male infertility are testicular torsion, trauma, undescended testis, hypogonadotropic hypogonadism, seminal tract infections, varicocele, and reproductive tract obstruction or gonadal dysgenesis. Lately, in some studies, body mass index (BMI) has been reported to negatively affect male fertility. Several studies revealed a direct correlation between high BMI and decreased free and total testosterone levels in the blood. In addition, obesity may cause oxidative stress as indicated by elevated oxidative stress markers paralleling decreased antioxidant defence enzymes. Oxidative stress causes damages on the major compounds in cells like lipids, proteins, DNA, and carbohydrates. All these changes may affect the semen parameters in obese cases [WHO Citation1997].
Nigella sativa is a flowering plant that belongs to the Ranunculaceae family. It has been used in many Middle Eastern countries as a natural remedy/treatment [Swamy and Tan Citation2000]. It contains volatile oil, fixed oil, quinines, proteins, alkaloids (nigellimine, nigellidine, nigellicine, and nigellamine), saponins (α-hederin), cumarins, vitamins, and minerals. The seeds or the oil of the seed have analgesic, anti-pyretic, anti-neoplastic, anti-fungal, anti-convulsant, anti-ulcer, anti-bacterial, anti-tumour, anti-diabetic, immune-modulator, anti-inflammatory, anti-viral, anti-hyperlipidemic, hepato-protective, anti-oxidant, anti-histaminic, anti-helminthic, and anti-hypertensive activities [Ali and Blunden Citation2003; Bruits and Bucar Citation2000]. Thymoquinone (TQ) is the most important phytochemical compound of the seeds and is responsible for many of these biological effects. But, the influence of TQ on obesity, obesity-induced testicular injury, and sperm production essentially is unknown. To address this issue the possible beneficial effect of TQ on the obesity related changes in adult rats’ testis and their functions were examined.
Results
Body and testis weight and mean seminiferous tubule and testis volume and cellular characteristics
During the experimental period, weight measurements were done weekly in the animals within the obese and non-obese groups. At the end of the 9th week, measured weight and length parameters were calculated using BMI, and those which were more than 5 kg/m2 were considered obese [Altunkaynak et al. Citation2008]. At the end of the study, the mean body weight and white adipose tissue around the testes in the obese control (OC) group was significantly more than that of the non-obese control (NC), and obese thymoquinone (OT) groups (p < 0.01). Following the TQ treatment, the body weight was significantly reduced in the non-obese thymoquinone (NT) (p < 0.05) and OT (p < 0.01) groups in comparison to those of the NC and OC groups. The testis weight and volume were reduced in the OC group in comparison with the NC group (p < 0.01), but the testis weight and volume increased after the TQ treatment of rats in the OT group, in comparison to the OC group (p < 0.001 and p < 0.05, respectively). The data show that the mean volume of the seminiferous tubule in animals of the OC group decreased in comparison to the NC group (p < 0.05). It was also obvious that the mean volume of the seminiferous tubules showed a significant increase in obese animals following the TQ treatment compared with those of the OC group (p < 0.05). There was no significant difference in these parameters between the NC and NT groups ().
Table 1. The mean body, testis, and peri-testicular adipose tissue (PTAT) weight (g), testis volume (cm3), and mean volume of the seminiferous tubules (mm3) in all groups.
Sperm morphological evaluation results in the samples of sperm smears in all groups are shown in . In the evaluation of the sperm morphology, the sperms generally had normal morphology in the NC and the NT groups and a few head, neck, and tail anomalies were observed (). But, the data revealed that the percent of sperms with normal morphology was significantly decreased (p < 0.01) and the percent of sperms with head, neck, or tail anomalies were significantly increased in the OC group compared to those of the NC group (p < 0.01). It was notable that the sperm tails were circled around the head in the OC group (). Many Dag defected sperms were formed, and tail-less sperms were observed (). Sperm anomalies were significantly reduced in the OT group in comparison to the OC group (). The TQ treatment in both the NT (p < 0.05) and OT (p < 0.01) groups caused an increase in the number of sperms with normal morphology and caused a decrease in the percentage of sperms with the head, neck, or tail anomalies.
Figure 1. The sperm smears belonging to non-obese control (A), obese control (B), non-obese thymoquinone (C), and obese thymoquinone (D) groups are shown. Arrowheads: normal sperm morphology; double arrowheads: Dag effect; white filled arrow: tail-less sperm; black arrow: sperms with head and tail anomaly. The general morphology of sperms is seen in the all groups. Tail-less sperms, sperms with head and tail anomalies and also Dag effect are seen in the sperm smears of the OC group. But following TQ application, sperms having normal morphology are seen in the sperm smears of the OT group. Dye: HE; Scale Bars: 25 µm.
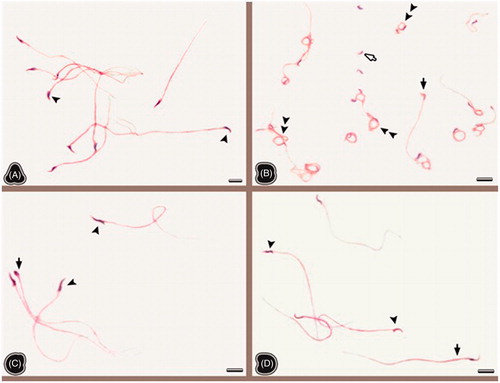
Table 2. The percentage of sperm with normal morphology or head, neck, and tail anomaly in all groups.
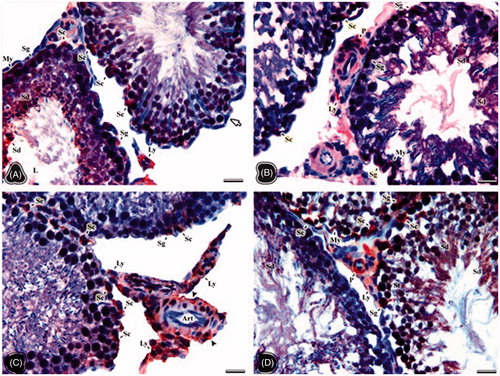
The red stained areas on testis samples of all groups were evaluated as positive in terms of luteinizing hormone (LH) receptor immunreactivity. The immunoreactivity was only observed in the cytoplasm of positive cells. So, no immunostaining was seen in the nuclei. The intensity of staining in the cell cytoplasm varied from cell to cell in the NC group. Strong positive immunoreactivity was observed in some Sertoli cell cytoplasms (). Slightly positive areas in the cell cytoplasm of spermatogonia, Sertoli, Leydig, and peritubular cells were seen in the OC group (). The strong immunoreactivity appeared in the cell cytoplasm of the endothelial cells of the vessels, connective tissue, and Sertoli and Leydig cells especially in the NT group (). However, the pericytes, smooth muscle cells on vessel walls showed strong immunoreactivity. The cell cytoplasm of Leydig cells, spermatocyte, and spermatids in the OT group exhibited more positivity than those in the OC group ().
Figure 2. The light microscopical sections of testis belonging to the non-obese control (A), obese control (B), non-obese thymoquinone (C), and obese thymoquinone (D) groups are shown. Sg: spermatogonia, Sc: sertoli cell, Sd: Spermatid, St: spermatocyte, Ly: Leydig cell, My: myoid cell, P: pericytes, L: tubule lumen. (A) Arrow: negative staining of Sertoli cell. (C) Art: arteriole, Arrowhead: vessel wall. Histological structure of testis is seen in the all groups. Luteinizing hormone (LH) receptor positivity is shown by immunohistochemistry in the nuclei of the positive stained cells (signed). LH receptor activity was observed in the cells occupying the connective tissue and vascular walls rather than tubular and Leydig cells of the obese control group. But following thymoquinone application, more positive cells were seen in the light microscopical sections of the obese thymoquinone group. Dye: anti-LH/choriogonadotrophic hormone (CG) receptor immune stain; Scale Bars: 25 µm.
Biochemical and histopathological analysis
The total number of Leydig cells significantly decreased in the OC group in comparison to the NC group (p < 0.01). After the TQ treatment, the total number of Leydig cells significantly increased in the OT group in comparison to that of the OC group. The total number of spermatogonia (p < 0.05), primary spermatocyte (p < 0.01), and round spermatids (p < 0.01) in animals in the OC group significantly decreased in comparison to the NC group. The TQ treatment in the OT group significantly increased the total number of spermatogonia (p < 0.05), primary spermatocyte (p < 0.01), and round spermatids (p < 0.01) compared to the OC group. Also, the total number of round spermatids in the NT group significantly increased in comparison to the NC group (p < 0.05; ).
Table 3. The total number (×106) of the different cell types of the testis seen in the all groups.
Catalase (CAT) and myeloperoxidase (MPO) enzyme activities in all groups are shown in . The CAT activity significantly increased in the OC group in comparison to the NC group (p < 0.05). But, the TQ treatment significantly lowered CAT activity in both the NT and OT groups compared to those of the NC and OC groups (p < 0.01). MPO activity significantly increased in the OC group in comparison to the NC group (p < 0.0001). Following the TQ treatment, the MPO activity was reduced the OT group in comparison to the OC group (p < 0.01).
Table 4. The values of catalase (CAT) and myeloperoxidase (MPO) enzyme activity in all groups.
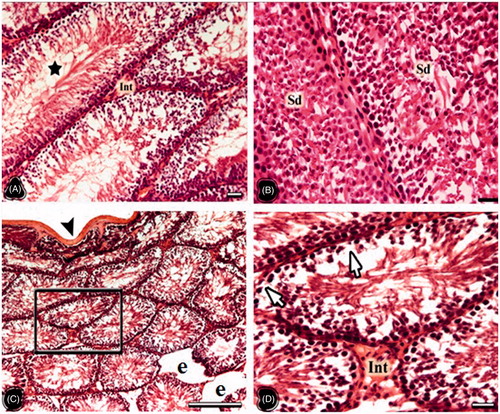
In the testes of the NC group, tunica albuginea, seminiferous tubuli, interstitial connective tissue and Leydig cells were observed in normal histological appearance. The cells belonging to the spermatogenic series were orderly arranged in the tubule wall (). Testes in the OC group presented as compromised and many seminiferous tubuli were atrophied. The seminiferous tubule wall in the OC group became thinner than that of the NC group, and the wall structure was degenerated. The separation from the interstitial area and the increase in the connective tissue were notable (). The testis cross sections were in normal histological structure in the NT group. The seminiferous tubules, spermatogenic cell series, and interstitial connective tissue also presented normal morphology (). The distances between germ cells in the OT group were extended. In some, the spermatogenic cell lines were damaged and the spermatogenesis was delayed. In these areas, the density of spermatids and spermatozoa in the tubule lumen were reduced compared to the NT group, but they were more than those of the OC group ().
Figure 3. The light microscopical sections of testis belonging to non-obese control (NC) and obese control (OC) groups are shown. (A and B) The light microscopical sections of testis belonging to the NC group. ST: seminiferous tubules, Black arrow: Tunica albuginea, Int: interstitial area, Fb: fibroblast, Ly: Leydig cell, M: macrophage, Framed area: spermatogenic cell series, Sg: spermatogonia, St: spermatocytes, Sd: spermatid, Sz: spermatozoon. (C and D) The light microscopical sections of testis belonging to the OC group. The stars show atrophic seminiferous tubules, asterisks show degenerated spermatogenic cell series, Arrowheads: the thin seminiferous tubule wall, e: enlarged peritubular tissue, Arrows: no spermatogenic cells within tubule epithelium, Art: arteriole. Histological structure of testis is seen in the NC and OC groups. In the OC group degenerations in the seminiferous tubules, enlarged peritubular tissue, and tubular atrophy are important findings. Dye: HE; Scale Bars: (A–C) 250 µm; (B–D) 25 µm.
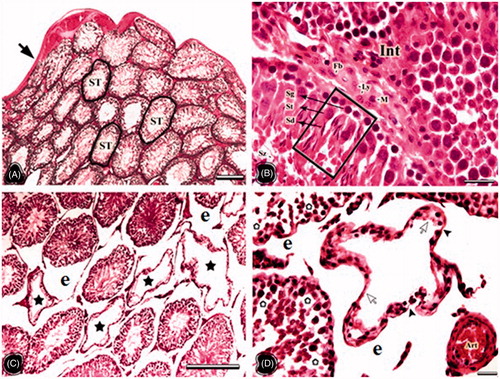
Figure 4. The light microscopical sections of testis belonging to the non-obese thymoquinone (NT) and obese thymoquinone (OT) groups are shown. (A and B) The light microscopical sections of testis belonging to the NT group. Star: intensive sperm in the tubule lumen, Sd: intensive spermatid in the tubule lumen, Int: interstitial area. (C and D) The light microscopical sections of testis belonging to the OT group. Arrowhead: tunica albuginea, e: enlarged peritubular tissue, arrows: germ cell loss. Framed area of C was indicated in D at higher magnification. Histological structure of testis is seen in the NT and OT groups. In the NT group more spermatogenic cells were seen on the seminiferous tubule walls compared to those of the NC group. Degenerated cells were decreased and no atrophic tubules were seen in OT group. Dye: HE; Scale Bars: (B–D) 25 µm; (A–C) 150 µm.
The apoptotic index of the all groups is shown in ; apoptotic activity was observed in few spermatogonia of the NC and NT groups (). There was no significant difference in terms of the incidence of the apoptotic spermatogenic cells among the NC, NT, and OT groups (p > 0.05; ). In comparison, OC apoptotic activity was observed in the Leydig, Sertoli, and peritubular myoid cells to a greater extent than those of the other groups (p < 0.01; ). However, apoptotic activity was reduced in the germ cells, especially in the spermatogonia of the OT group compared to the OC group (p < 0.01; ).
Figure 5. The light microscopical sections of testis belonging to the non-obese control (A), obese control (B), non-obese thymoquinone (C), and obese thymoquinone (D) groups are shown. Sg: spermatogonia, Sc: sertoli cell, Sd: spermatid, St: spermatocyte, Ly: Leydig cell, My: Myoid cell. Histological structure of testis is seen in all groups. Apoptotic activity is shown by TUNEL method in the nuclei of the positive stained cells (signed). The most apoptotic cells are seen in the OC group. But following TQ application, less apoptotic cells are seen in the light microscopical sections of the OT group. Dye: TUNEL stain; Scale Bars: 25 µm.
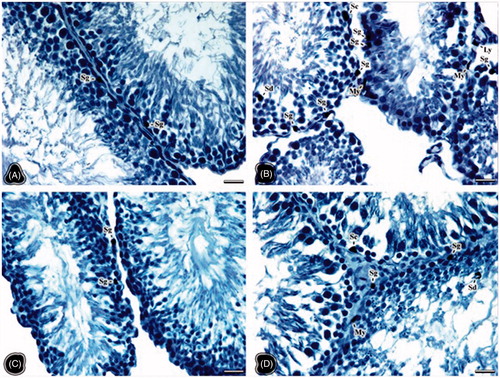
Table 5. Comparison of examined incidence of apoptotic cells with TUNEL staining.
Discussion
Obesity, containing social, genetic, metabolic, and physiological behavioral components is a complex, chronic disease [Heysmfıeld et al. Citation1989]. It is known that obesity is related to many chronic diseases [Abdel-Halim Citation2005; Buschemeyer and Freedland Citation2007; Gupta et al. Citation2010; Kutlutürk et al. Citation2011; Logue et al. Citation2011; Zimmerman et al. Citation2011]. The relationship between obesity and infertility has begun to be studied intensely [Abdel-Halim Citation2005; Hammoud et al. Citation2012]. In the studies of the last 50 years, it was detected that the sperm number was significantly reduced in obese individuals [Swan et al. Citation2000]. Increased oxidative stress was considered to be one of the major causes in the emergence of obesity complications [Bakos et al. Citation2011; Tunc et al. Citation2011]. Many studies reported that insufficiency occurred in antioxidant defence mechanisms of obese individuals, and this situation may cause different complications related to obesity [Colette et al. Citation2003; Puchau et al. Citation2010; Stefanović et al. Citation2008; Tunc et al. Citation2011]. Therefore, antioxidant treatment is a potential method for many diseases and it has become increasingly important in recent years [Puchau et al. Citation2010].
This study was carrried out to examine the effects of the oxidative stress caused by obesity, and the antioxidant feature of thymoquinone on male fertility was examined in high-fat-diet (HFD) fed obese rat testes. Gu et al. [Citation2012] observed that the body weight, visceral fat/body weight proportion, visceral fat, and insulin resistance index of a group of rats feeding on HFD during 22 weeks increased compared to another group feeding on a standard diet. Cattaneo et al. [Citation1997] observed that although obesity developed in a group of rats at the end of the nutrition period, on the contrary, no development occurred in another group of female rats feeding on HFD for 7 months. In our study, we observed that the mean weight of testes and the mean weight of the adipose tissue around the testis were rather higher in the OC group than in the NC group. It was observed that the weight increase followed increased fat around the testis in the OC group feeding on a HFD. The mean testis wet weight in the OC group was reduced more than the other experimental groups. We considered that the testes wet weight could have decreased due to obesity caused by HFD. The decrease in weight in the OT group as compared to that of the OC group showed that the TQ application may affect weight loss in obese individuals.
In the study of Jensen et al. [Citation2004], 1,558 cases were examined and in the individuals with BMI >25 kg/m2, the number and concentration of sperm was determined to be 22% lower than healthy controls. Fernandez et al. [Citation2011] showed increased estradiol level, reduced sperm activity, and lower sperm quality in rats feeding on HFD. They observed that HFD caused reproductive disorders and reduced the fertility potential of rats. In our work, germ cell number was reduced significantly in the OC group as compared to the NC group. The increase of the spermatid number in the OT group was supported by histological findings. TQ application increased the sperm number, and caused an increase in the seminiferous tubule volume, but this increase was little, therefore there was no important difference in terms of the testis volume.
Amirkhizi et al. [Citation2007] suggested that a long continuing period of obesity supports the increase of oxidative stress. In our study, there was a significant increase in the CAT activity of the OC group and after thymoquinone this level was decreased in both the NT and OT groups in comparison to the NC and OC groups. Ari et al. [Citation2008] observed that the MPO activity was significantly increased in the antioxidant condition criterion compared to the control group of rats fed a HFD. Accordingly, in our study, the MPO activity increased in the OC group compared to the NC group. These results are suggestive of an obesity caused inflammatory response in the testes, therefore the MPO activity increased significantly, and the oxidative damage was intensely based on obesity in this region. But, TQ application caused a significant decrease in the MPO activity in the OT group as compared to that of the OC group. Relating to our work, Badary et al. [Citation2000] determined that the TQ caused an important elevation of the antioxidant enzymes like SOD, CAT, and GPx.
Upon histopathologic evaluation, the histological images of the cells belonging to the seminiferous tubule, interstitial tissue, and spermatogenic series were normal in testes of the rats in the NC group. The spermatid and spermatozoon intensity was notable in the tubule lumen of the testes in the NT group. However, great damage was observed in the OC group. The degenerated tubules were seen which had no spermatogenic cells and thinner wall including only one layer of myoid cells. The tubules were in places which had thicker peritubular connective tissue than normal. However, these structures were not determined in the OT group, except for the loss of germ cells and separated tissues in some regions. This is similar to the work of Wang et al. [Citation2007] who showed spermatogenic cell decay and decreased mature sperm in an obese group of rats feeding on a HFD for 6 weeks. As a result, feeding on a HFD for a long time caused obesity on rats, and negatively affected the testis functions.
Immunohistochemical analysis evaluated the LH receptor using antibody localization and degree of staining in all groups. Immunoreactivity was reduced due to the heavy damage in the OC group. Biochemical studies indicated that the level of LH decreases in obese individuals [Alonso et al. Citation2012]. It appears that TQ caused an increase in the LH positivity and this may increase testosterone production. Hajshafiha et al. [Citation2013] have observed associations between BMI values and sexual hormone levels when comparing fertile and subfertile/infertile men.
There is only one study suggesting that obesity can increase apoptosis of spermatogenic cells in the rat testis [Chen et al. Citation2011]. In the current study, testis TUNEL staining indicated apoptotic activity was generally localized in the spermatogonia of the NC and NT groups. In the OT group, the spermatogonia and Sertoli cells in the damaged regions showed apoptotic activity, but the germ cell apoptosis was reduced significantly compared to the OC group. After the TQ treatment testicular damage was significantly reduced, and the apoptotic germ cell activity was also reduced.
The relationship between BMI and male infertility relating to the sperm number, activity, and morphology is being actively studied [Cabler et al. Citation2010; Mitchell et al. Citation2011; Mydlo Citation2004]. Hammoud et al. [Citation2008] suggested that the sperm number and motility were reduced, and sperm morphology was negatively affected, with the number of morphologically abnormal sperm increasing with obesity. In the study presented above the reduction in the number of sperm following coil-shaped, head, neck, and tail anomaly sperms were the most pronounced in the OC group. In comparison, these structures were reduced significantly in the OT group suggesting that sperm anomalies in obese rats were reduced with the application of thymoquinone. This supports the view that thymoquinone was effective over oxidative stress caused by obesity, increasing the sperm number. Perhaps the increase in the consumption of plants which contain antioxidants may be effective to avoid many diseases, and in addition, prevent and treat obesity and infertility problems.
Materials and Methods
Animals, diets, and dosage
This study was approved by the Experimental Research Ethical Committee of Ondokuz Mayıs University. In this experiment, 24 adult male Wistar albino rats aged 8–10 w with 200 g body weight were used. Rats were provided from the Experimental Animal Research and Application Center of Ondokuz Mayıs University (Samsun, Turkey). During the experiment, all rats were kept in 12:12-h day/night cycle at 22 ± 2°C with a humidity of 50 ± 5 percent. Rats were allowed to eat food and drink water ad libitum. The rats were randomly separated into 4 groups (n = 6). While the OC and OT groups, which would generate an obesity model, were fed on a special diet containing 40% calories from/of fat, the NC and NT groups were fed a standard commercial diet for 9 w. At the end of 9 weeks (63 d), obesity conditions were evaluated by calculating BMI.
Intraperitoneal injections were carried out simultaneously to the OT and NT groups. For the injections, TQ (Cataloque No: 274666, Sigma Aldrich, Interlab A.S. Istanbul, Turkey) was dissolved in phosphate buffered saline at a dose of 10 mg/kg/d. Injections were carried out daily during 42 d (6 w) by starting on the 64th day [Alenzi et al. Citation2010; Gokce et al. Citation2010].
After this treatment, all animals were anesthetized by intra muscular ketamine (Ketalar®-100 mg/kg; Pfizer, Istanbul, Turkey) and xylazine injection (Alfazyne®-5 mg/kg; Alfasan, Turkey). Then, the removed testes were prepared for histological, stereological, and biochemical studies, and morphological sperm analyses.
Histological studies
Sperm analysis
To evaluate the sperm morphology, the left testis was put under anaesthesia and ¼ section of it was dissected and semen samples were obtained. Then the semen samples were spread on the slides and stained with hematoxylin-eosin (HE). Afterwards, photographs were taken from the slides under the light microscope (Leica LMD 7000, Leica Inc; Wetzlar, Germany), and morphologically evaluated by the Kruger-Strict criteria. On each semen sample, 200 sperms were evaluated in terms of head, neck, and tail anomalies, and normal sperm parameters [Kruger et al. Citation1986].
Light microscopy
The right testis tissues were kept in 10% formalin for fixation. Remainders of the left testes (¾) were kept in the freezer at −80°C for biochemistry studies. Following the fixation, the testis samples were exposed to routine histological procedures and embedded in paraffin and serially cut by a rotary microtome (Leica RM2255; Nussloch, Germany) [Kumtepe et al. Citation2010]. The light microscopic sections at the thicknesses of 20 and 7 μm were used for stereological analysis and histopathological examination, respectively. All slides were stained with HE and examined under the light microscope (Leica LMD 7000, Leica Inc; Wetzlar, Germany).
TUNEL assay
To detect DNA breaks, in situ cell death detection kits for the TUNEL method were purchased from Roche Applied Science (Penzberg, Germany). The sections were mounted on positive charged slides, deparaffinized, and treated with proteinase K solution (20 μg/mL in PBS) for 15 min at room temperature. Subsequently, the sections were washed in distilled water and immersed in 3% hydrogen peroxide for 15 min. After several washings in PBS (50 mM sodium phosphate and 200 mM NaCl at pH 7.4), the sections were immersed in equilibration buffer at room temperature for 20 min. The sections were then incubated in terminal deoxynucleotidyl transferase (TdT) enzyme at 37°C for 1 h in a humidified chamber, and the reaction was stopped by immersion in a stop/wash buffer. After several washings, the sections were incubated in antidigoxigenin-peroxidase for 30 min at room temperature. The reaction was revealed with 0.06% 3.3-diaminobenzidine tetra hydrochloride (Sigma Chemical, Interlab A.S.; Istanbul, Turkey) in PBS for 3–6 min and the sections were counterstained with Mayer’s haematoxylin [Altunkaynak et al. Citation2009; Liu et al. Citation2002]. These sections were examined and photographed under a light microscope (Leica LMD 7000, Leica Inc; Wetzlar, Germany). Also, the number of apoptotic spermatogenic cells were calculated in five independent areas at high magnification (×400) followed by calculation, it is defined as the ratio of per 100 cells. Apoptotic index is calculated using the following formula [Selzner and Clavien Citation2000]:
Immunohistochemical staining of (LH/CG) receptor antibody
Immunohistochemical staining was applied by the streptavidin-peroxidase method. The LH/choriogonadotrophic hormone (CG) receptor antibody was used for the immunohistochemical study of the paraffin-embedded testis samples. Summarily, sections were incubated with 3% H2O2 for 10 min and treated and blocked in 5% BSA for 10 min at room temperature. Sections were then probed with the LH/CG receptor antibody (Catalogue Number: AB9549, Millipore; İstanbul, Turkey) at a concentration of 5 µg/mL for 1 h at room temperature. Then, sections were washed three times with PBS and incubated with biotinylated anti-rabbit IgG for a period of 30 min at 37°C. Immunological activity was detected with a streptavidin-biotin complex kit (ScyTek Laboratories, SitoGen Biomedikal Ltd; İstanbul, Turkey) and developed with diaminobenzidine tetrahydrochloride. The sections were counterstained with Mayer hematoxylin and images are obtained under a light microscope (Leica LMD 7000, Leica Inc) [Bekar et al. Citation2014].
Stereological studies
The number of Leydig cells, spermatogonia, spermatocytes, and spermatids were calculated by an optic dissector and unbiased counting frame. The mean volumes of seminiferous tubules and testis were calculated by the Cavalieri method.
Optical fractionator method
The stereological analyses were made by a special computerized analysis system (Sterioinvestigator® 9.0, Microbrightfield; Gantenbein LTD; Ankara, Turkey). Before beginning to work, the necessary strategy was determined by optical fractionator on the parts of the testis tissue [Altunkaynak et al. Citation2012]. According to that, the section thickness and section sampling fraction were determined as 20 µm and 1/250, respectively. After this sampling procedure, 16 sections were obtained from each testis. The frame area had 40 μm long edges and the area was 1600 μm2 (40 × 40), so as to count 6 interesting cells in each unbiased counting frame. Counting was made under 40× objective. The number of spermatogonia, spermatocytes, spermatids, and Leydig cells were counted on each testis sample by using 4 different markers (Supplementary Figure 1).
The total number of spermatogonia, spermatocytes, spermatids, and Leydig cells in the testis was estimated by the following formula:
Where, N is the total number of the interesting cell (spermatogonia, spermatocytes, spermatids, or Leydig cells), ΣQ is the counted number of the interesting cell, ASF is the area sampling fraction, and TSF is the thickness sampling fraction. The efficiency of the sampling procedure and convenience of sampled cells for the total number analysis of the interesting cell was checked by the estimation of the coefficient of error (CE) and coefficient of variation (CV), as previously described [Altunkaynak et al. Citation2011]. The CE of the sampling schedule of the testis was validated by a pilot study (CE must be ≤10%). It is also possible to estimate the CV within the testis in each group. These are valuable data to see whether the number of subjects in each group is adequate.
Cavalieri method
The volume of testes and seminiferous tubules was counted by using the Cavalieri principle [Altunkaynak and Altunkaynak Citation2007]. The area and volume were calculated on 7 μm cross sections with 1/250 sampling rate as to the applied systematic random sampling procedure. To estimate the testis volume, first, borders of the testicular area were determined under 5x objective, and the point interval was found as 150 μm by using a counting frame scale. Then, the counting frame scale was randomly put on the determined testis area and the hitting points over the testis were counted. Finally, the volume of each section was calculated by multiplying the area was calculated by multiplying the total number of these points and section thickness.
To calculate the mean volume of the seminiferous tubule, 10 tubules with drawn borders were randomly selected in each cross section, and the point interval was determined as 50 μm using a counting frame scale. This point counting scale was randomly put on the images of cross sections, and the points which hit over the selected tubules were counted. The mean volume of the seminiferous tubules was calculated by multiplying the total points over the selected tubules and section thicknesses. The value obtained from the calculation was divided to the number of selected tubules (10) to detect the mean volume of the seminiferous tubules (Supplementary Figure 1):
Where V is the volume of interest (seminiferous tubules, testis) in one section plane, t is the section thickness, a(p) is the interpoint area, and Σ is the number of points hitting the object of interest in that section. After this formula was applied to other sections, the total volume to be estimated was obtained from:
The volume fractions for each region were estimated by dividing the total point number that was superimposed on the related region by the sum of the points superimposed on the whole testis:
where P is the number of points hitting the region of interest or whole testis in that section.
Biochemistry
MPO activity
MPO activity was evaluated according to the modified method of Bradley et al. [Citation1982]. The homogenized samples were frozen and thawed 3 times, and then centrifuged at 1500 rpm for 10 min at 4°C. MPO activity was determined by adding 100 µL of the supernatant to 1.9 mL of 10 mmol/L phosphate buffers (pH 6.0) and 1 mL of 1.5 mmol/L o-dianisidine hydrochloride containing 0.0005% (wt/vol) hydrogen peroxide. The changes in the absorbance of each sample were recorded at 410 nm on a UV-vis spectrophotometer (Shimadzu UV-MINI 1240; Shimadzu; Istanbul, Turkey). MPO activity in all tissues was expressed as micromoles per min per milligram of tissue.
Catalase activity
Decomposition of H2O2 in the presence of CAT was performed at 240 nm. CAT activity was defined as the amount of enzyme required to decompose 1 nm of H2O2 min at 25°C and pH 7.8. Results were expressed as millimole per min per milligram of tissue (mmol/min/mg tissue) [Aebi Citation1984].
Statistical analyses
Statistical comparisons were made using SPSS 15.0 for Windows®. Significance among the incidence of TUNEL positive cells was determined by the aid of chi-square test and Fisher exact test. Stereological data were compared using a One-Way Anova in conjunction with the Bonferroni t test for all pair wise multiple comparisons. Differences were considered significant when p < 0.05. Data were presented as the Mean ± SEM.
| Abbreviations | ||
| BMI | = | body mass index |
| CAT | = | catalase |
| HE | = | hematoxylin-eosin |
| HFD | = | high-fat-diet |
| LH | = | luteinizing hormone |
| MPO | = | myeloperoxidase |
| NC | = | non-obese control |
| NT | = | non-obese thymoquinone |
| OC | = | obese control |
| OT | = | obese thymoquinone |
| TQ | = | thymoquinone |
Supplementary material available online
Supplementary Figure 1
Download Zip (7.1 MB)Declaration of interest
The authors report no conflicts of interest.
Author contributions
Conceived and designed the experiments: BZA, NHT; performed the experiments: NHT, MEA; Analyzed the data: BZA, MEA; Contributed reagents/materials/analysis tools: SK; Wrote the manuscript: BZA, NHT, SK. All authors approved revisions and the final paper.
Notes
Color versions of one or more of the figures in the article can be found online at the publisher's website.
References
- Abdel-Halim, R.E. (2005) Obesity. 1000 years ago. Lancet 366:16–22
- Aebi, H. (1984) Catalase in vitro. Medhods Enzymol 105:121--126
- Alenzi, F.Q., El-Bolkiny, Y.S. and Salem, M.L. (2010) Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. British Jof Biomed Sci 67:20–28
- Ali, B.H. and Blunden, G. (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17:299–305
- Alonso, M., Serrano, A., Vida, M., Crespillo, A., Hernandez-Folgado, L., Jagerovic, N., et al. (2012) Anti-obesity efficacy of LH-21, a cannabinoid CB(1) receptor antagonist with poor brain penetration, in diet-induced obese rats. Br J Pharmacol 165:2274–2291
- Altunkaynak, B.Z., Unal, D., Altunkaynak, M.E., Halici, Z., Kalkan, Y., Keles, O.N., et al. (2012) Effects of diabetes and ovariectomy on rat hippocampus (a biochemical and stereological study). Gynecol Endocrinol 28:228–233
- Altunkaynak, B.Z., Ozbek, E., Aydin, N., Aydin, M.D., Altunkaynak, M.E., Vuraler, O., et al. (2011) Effects of haloperidol on striatal neurons: Relation to neuronal loss (a stereological study). Folia Neuropathol 49:21–27
- Altunkaynak, B.Z. and Altunkaynak, M.E. (2007) Relationship of body weight and volume of liver. A morphometrical and stereological study. Saudi Med J 28:891–895
- Altunkaynak, M.E., Özbek, E., Altunkaynak, B.Z., Can, İ., Ünal, D. and Ünal, B. (2008) The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. J Anat 212:845–852
- Altunkaynak, M.E., Altunkaynak, B.Z., Unal, D., Vuraler, O. and Unal, B. (2009) morphological alterations of sciatic nerve during development from rat fetuses to adults: A histological, stereological and embryological study. Kafkas Univ Vet Fak Derg 15:687–696
- Amirkhizi, F., Siassi, F., Minaie, S., Djalali, M., Rahimi, A. and Chamari, M. (2007) Is obesity associated with increased plasma lipid peroxidación and oxidative stress in women. ARYA Atheroscler J 2:189–192
- Arı, Z., Ulman, C., Taneli, F., İşbilen, B., Uyanık, B.S. and Aldırmaz, H., et al. (2008) Yüksek yağ içerikli diyet ile beslenen sıçanların arka bacak kasında dehidroepiandrosteron sülfatın oksidan durum belirteçleri ile bakır ve çinko düzeylerine etkisi. Turk J Biochem 33:1–8
- Badary, O.A., Abdel-Naim, A.B., Abdel-Wahab, M.H. and Hamada, F.M. (2000) The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology 143:219–226
- Bakos, H.W., Mitchell, M., Setchell, B.P. and Lane, M. (2011) The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl 34:402–410
- Bekar, E., Altunkaynak, B.Z., Balcı, K., Aslan, G., Ayyıldız, M. and Kaplan, S. (2014) Effects of high fat diet induced obesity on peripheral nerve regeneration and levels of GAP 43 and TGF-β in rats. Biotech Histochem 89:446–456
- Bradley, P.P., Priebat, D.A., Christensen, R.D. and Rothstein, G. (1982) Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206
- Bruits, M. and Bucar, F. (2000) Antioxidant activity of Nigella sativa essential oil. Phytother Res 14:323–328
- Buschemeyer, W.C. and Freedland, S.J. (2007) Obesity and prostate cancer: Epidemiology and clinical implications. Eur Urol 52:331–343
- Cabler, S., Agarwal, A., Flint, M. and du Plessis, S.S. (2010) Obesity: Modern man’s fertility nemesis. Asian J Androl 12:480–489
- Cattaneo, L., De Gennaro Colonna, V., Zoli, M., Muller, E.E. and Cocchi, D. (1997) Hypothalamo-pituitary-IGF-1 axis in female rats made obese by overfeeding. Life Sci 61:881–889
- Chen, M.M., Lan, X.X., Li, C.Y., Tian, Z.M. and Chen, K.F. (2011) Diet-induced obesity increases the apoptosis of testicular spermatogenic cells in pubertal male rats. Zhonghua Nan Ke Xu 17:342–347
- Colette, C., Percheron, C., Pares-Herbute, N., Michel, F., Pham, T.C., Birillant, L., et al. (2003) Exchanging carbohydrates for monounsaturated fats in energy-restricted diets: Effects on metabolik profile and other cardiovascular risk faktors. Int J Obes Relat Metab Disord 27:648–656
- Fernandez, C.D., Bellentani, F.F., Fernandes G.S., Perobelli, J.E., Favareto, A.P., Nascimento A.F., et al. (2011) Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol 9:32
- Galinier, M., Pathak, A., Roncalli, J. and Massabuau, P. (2005) Obesity and Cardiac Failure. Arch Mal Coeur Vaiss 98:39–45
- Gökçe, A., Oktar, S., Koc, A., Gonenci, R., Yalcinkaya, F., Yonden, Z., et al. (2010) Protective effect of thymoquinone in experimental testicular torsion. Urol Int 85:461–465
- Gu, J.J., Gao, F.Y. and Zhao, T.Y. (2012) A preliminary investigation of the mechanisms underlying the effect of berberine in preventing high-fat diet-induced insulin resistance in rats. J Physiol Pharmacol 63:505–513
- Gupta, R.K., Chandra, A., Verm, A.K. and Kumar, S. (2010) Obstructive sleep apnoea: A clinical review. J Assoc Physicians India 58:438–441
- Hajshafiha, M., Ghareaghaji, R., Salemi, S., Sadegh-Asadi, N. and Sadeghi-Bazargani, H. (2013) Association of body mass index with some fertility markers among male partners of infertile couples. Int J Gen Med 6:447–451
- Hammoud, A.O., Wilde, N., Gibson, M., Parks, A., Carrell, D.T. and Meikle, A.W. (2008) Male obesity and alteration in sperm parameters. Fertil Steril 90:2222–2225
- Hammoud, A.O., Meikle, A.W., Reis, L.O., Gibson, M., Peterson, C.M. and Carrell, D.T. (2012) Obesity and male infertility: A practical approach. Semin Reprod Med 30:486–495
- Hatemi, H., Turan, N., Arık, N. and Yumuk, V. (2002) Türkiye obezite ve hipertansiyon taraması sonuçları. The results of obesity and hypertension screening in Turkey. Endokrinolojide yönelişler 11:1–15
- Heysmfıeld, S.B., Wang, J., Lichtman, S., Karnen, Y., Kehayias, J. and Pierson R.N. Jr (1989) Body composition in elderly subjects. A critical appraisal of clinical methodology. Am J Clin Nutr 50:1167–1175
- Jensen, T.K., Andersson, A.M., Jørgensen, N., Andersen, A.G., Carlsen, E., Petersen, J.H., et al. (2004) Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 82:863–870
- Kumtepe, Y., Odabasoglu, F., Karaca, M., Polat, B., Halici, M.B., Keles, O.N., et al. (2010) Protective effects of telmisartan on ischemia/reperfusion injury of rat ovary: Biochemical and histopathologic evaluation. Fertil Steril 93:1299–1307
- Kutlutürk, F., Öztürk B., Yıldırım, B., Özuğurlu, F., Çetin, İ., Etikan, İ., et al. (2011) Obezite prevalansı ve metabolik risk faktörleri ile ilişkisi: Tokat ili prevalans çalışması. obesity prevalence and ıts association with metabolic risk factors: Tokat province prevalence study. Türkiye Klinikleri J Med Sci 31:156–163
- Kruger, T.F., Menkveld, R., Stander, F.S., Lombard, C.J., Van der Merwe, J.P., van Zyl, J.A., et al. (1986) Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril 46:1118–1123
- Liu, J., Tian, Z., Gao, B. and Kunos, G. (2002) Dose-dependent activation of antiapoptotic and proapoptotic pathways by ethanol treatment in human vascular endothelial cells: Differential involvement of adenosine. J Biol Chem 277:20927–20933
- Logue, J., Murray, H.M., Welsh, P., Shepherd, J., Packard, C., Macfarlane, P., et al. (2011) Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart 97:564–568
- Mitchell, M., Bakos, H.W. and Lane, M. (2011) Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril 95:1349–1353
- Must, A. and Anderson, S.E. (2003) Effects of obesity on morbidity in children and adolescents. Nutr Clin Care 6:4–12
- Mydlo, J.H. (2004) The impact of obesity in urology. Urol Clin North Am 31:275–287
- Onat, A., Ceyhan, K., Başar, O., Erer, B., Toprak, S. and Sansoy, V. (2002) Metabolic syndrome: Major impact on coronary risk in a population with low cholesterol levels -a prospective and cross-sectional evaluation. Atherosclerosis 165:285–292
- Puchau, B., Ochoa, M.C., Zulet, M.A., Marti A., Martínez, J.A. and Members, G. (2010) Dietary total antioxidant capacity and obesity in children and adolescents. Int J Food Sci Nutr 61:713–721
- Selzner, M. and Clavien, P.A. (2000) Failure of regeneration of the steatotic rat liver: Disruption at two different levels in the regeneration pathway. Hepatology 31:35–42
- Stefanović, A., Kotur-Stevuljević, J., Spasić, S., Bogavac-Stanojević, N. and Bujisić, N. (2008) The influence of obesity on the oxidative stress status and the concentration of leptin in type 2 diabetes mellitus patients. Diabetes Res Clin Pract 79:156–163
- Swamy, S.M. and Tan, B.K., (2000) Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. Seeds. J Ethnopharmacol 70:10–17
- Swan, S.H., Elkin, E.P. and Fenster, L. (2000) The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environ Health Perspect 108:961–966
- Tunc, O., Bakos, H.W. and Tremellen, K. (2011) Impact of body mass index on seminal oxidative stress. Andrologia 43:121–128
- Wang, Y., Liu, X.P., Qin, D.N., Chen, S. and Li, Y.S. (2007) Diet-induced obesity affects testis development in pubertal rats. Zhonghua Nan Ke Xue 13:514–519
- WHO (1997) Prevention and management of the global epidemic of obesity. Report of the WHO Consultation on Obesity. World Health Organization, Geneva, Switzerland
- Wright, D.T., Cohn, L.A., Li, H., Fisher, B., Li, C. and Adler, K.B. (1994) Interactions of Oxygen radicals with airway epithelium. Environ Health Perspect 102:85–90
- Zimmerman, M., Hrabosky, J.I., Francione, C., Young, D., Chelminski, I., Dalrymple, K., et al. (2011) Impact of obesity on the psychometric properties of the diagnostic and statistical manual of mental disorders, fourth edition criteria for major depressive disorder. Compr Psychiatry 52:146–150

