Abstract
Background and aims. The preliminary results of a DNA barcoding study of the doryctine fauna of parasitoid wasps from the Chamela–Cuixmala Biosphere Reserve in Mexico, a region dominated by tropical dry forest, are presented. So far, three field trips have been carried out to the reserve and 468 specimens have been collected, of which 407 cox1 sequences were obtained.
Materials and methods. The general mixed Yule-coalescent model was applied to a phylogram to investigate the number of evolutionary units that can be detected from the DNA sequence data examined.
Results. A total of 185 barcoding species assigned to 20 identified doryctine genera were discriminated using the above model, 115 of which belong to the speciose genus Heterospilus, pointing out the extraordinary species richness of this subfamily of insects in a Mexican tropical dry forest.
Conclusion. On the basis of the DNA barcodes generated, Ptesimogastroides Braet & van Achterberg is proposed to be a junior synonym of Ptesimogaster Marsh syn. nov. Neoheterospilus was also found deeply nested within a large Heterospilus clade, suggesting the paraphyly of the latter genus.
Introduction
DNA barcoding (Hebert et al. Citation2003) has proved to be a valuable tool for the rapid identification of species, especially in the case of megadiverse, poorly known fauna. In particular, this molecular technique has shown to be useful in many cases in assessing the actual diversity of small, morphologically conserved invertebrate taxa (e.g. Smith et al. Citation2008, Citation2009; Monaghan et al. Citation2009). In addition to considerably accelerating the process of species delimitation in those groups, DNA barcoding analyses also inherently help to solve some of their taxonomic problems. For instance, DNA barcoding studies (in animals, usually a fragment of the cox1 mitochondrial [mt] DNA gene) may reveal conspecificity among specimens of distinct sexes that were previously considered to be allospecific (e.g. Sheffield et al. Citation2009), allow association of different semaphoronts of a given species (e.g. Gossner and Hausmann Citation2009), detect cryptic species (e.g. Desneux et al. Citation2009; Li et al. Citation2010), or can be used to select candidate taxa for phylogenetic analyses with a vast taxon sampling (Hajibabaei et al. Citation2007). Moreover, although regarded with caution, DNA barcoding could also help to give insights into the taxonomic placement of problematic taxa based on the tree-building methods that are performed.
With over 1000 described species and more than 180 recognized genera, the braconid wasp subfamily Doryctinae represents a prime example of an understudied highly diverse group of insects. Doryctine wasps are known to attack mainly xylophagous or bark-boring coleopteran larvae; however, other host groups (e.g. species of Lepidoptera and Diptera) and biologies (e.g. gall association, termitophyly) have also been recorded for members of this subfamily (Belokobylskij Citation1992; Wharton and Hanson Citation2005). Higher level classification within the Doryctinae has been the subject of extensive confusion due to its extensive morphological homoplasy, and the erection of genera based only on the combination of characters or trends, which probably has arisen in a number of non-monophyletic supraspecific taxa (CitationBelokobylskij et al. 2004; Zaldívar-Riverón et al. Citation2008).
Here, we show the preliminary results of an ongoing DNA barcoding study of the doryctine species richness from the Chamela–Cuixmala biosphere reserve, a region dominated by tropical dry forest that is located near the Pacific coast of Jalisco, Mexico. We employ the general mixed Yule-coalescent (GMYC) model to estimate species boundaries using the standard animal DNA barcoding marker (∼650 bp of the cox1 mtDNA gene). Our results obtained after only three collecting trips carried out so far have not only unveiled an extraordinary species richness for the Doryctinae but also allowed for some taxonomic inferences within the group.
Materials and methods
Study site and specimen sampling
Specimens belonging to the braconid wasp subfamily Doryctinae were mostly collected in the Chamela biological station of the Instituto de Biología, Universidad Nacional Autónoma de México (UNAM). This station is located within the Chamela–Cuixmala biosphere reserve, near the Pacific coast in the state of Jalisco, Mexico. Insects were collected in two different vegetation types, tropical dry and tropical subdeciduous forests, the first being the predominant type of vegetation in the region. Three field trips of 6–8 days duration each were carried out during June, September, and November 2009. In all the trips, specimens were collected with two malaise traps, two to four light traps, 100–300 yellow pan traps, and a minimum of four sweep nets for at least 4 h per day.
A 615–658 bp fragment corresponding to the standard animal DNA barcoding marker (cytochrome c oxidase subunit I mtDNA gene) was amplified for the collected samples using both LepF1/LepRI (Hebert et al. Citation2004) and LCO1490/HCO2198 (Folmer et al. Citation1994) primers. All specimens were preserved in absolute ethanol and kept at − 20°C until they were processed. For most of the specimens, a single leg was removed, placed in a 96-well lysis plate, and posted to the University of Guelph for DNA extraction, amplification, and sequencing. DNA extractions and amplifications were carried out at the Instituto de Biología, UNAM, for about one-quarter of the specimens. PCR amplification products of these samples were placed in 96-well lysis plates containing 6.25 μl of 10% trehalose each well and also posted to the University of Guelph for DNA sequencing (see laboratory protocols in Smith et al. Citation2009). Sequences were edited with Sequencher 4.0.5 (Gene Codes Corp.) and aligned manually based on their translated amino acids.
All of the cox1 sequences generated are deposited in GenBank (accession numbers GU715182-288, HM420734-5, HM434309-544, HM882254, HQ200960-201008, 201239-54). These sequences and all the specimen information are available in the project file “Parasitoid Wasps (Braconidae: Doryctinae) of Chamela–Cuixmala Biosphere Reserve” (ASDOR project) in the projects section of the Barcode of Life Data Systems (www.barcodinglife.org).
Species boundaries analyses
All the collected specimens were identified to genus level using the relevant literature (Marsh Citation1997, Citation2002; Braet et al. Citation2003; Belokobylskij Citation2006; Gomes and Penteado-Dias Citation2007) and subsequently mounted after generating their DNA sequences. Species boundaries were assessed using the GMYC approach (Pons et al. Citation2006; Fontaneto et al. Citation2007), which requires a fully resolved topology with branch length estimates. A Bayesian analysis using BEAST 1.5.3 (Drummond and Rambaut Citation2007) was therefore carried out in order to reconstruct a fully resolved topology with branch length estimates, removing first all the duplicate haplotypes with Collapse 1.2 (Posada Citation2004). The analysis performed used a relaxed lognormal clock, and branch lengths were estimated using a coalescent prior and a GTR+I+Γ model of evolution (Lanave et al. Citation1984; Yang Citation1994) with unlinked codon positions. The latter model was selected using the program jModelTest (Posada Citation2008) and the Akaike Information Criterion (Akaike Citation1974). The coalescent prior gives more conservative results, as it is more likely to ignore a coalescent—speciation transition since the GMYC employs a coalescent as the null model to explain branching patterns (Pons et al. Citation2006; Monaghan et al. Citation2009). Moreover, the latter prior has been found to perform better for estimating branch lengths than the Yule prior for relaxed lognormal clock estimates, therefore, giving more accurate species boundaries’ estimates (Monaghan et al. Citation2009).
The Bayesian analysis was run for 20 million generations, sampling trees every 1000 generations. Stationarity was detected to occur after 700,000 generations using Tracer 1.4 (CitationRambaut and Drummond 2007), although we followed a conservative approach and deleted the trees sampled from the first 10 million generations. The remaining sampled trees were used to build a maximum clade credibility tree with TreeAnnotator v.1.5.3 (CitationRambaut and Drummond 2008).
The GMYC approach for species delimitation was carried out using the SPLITS package for the R statistical environment (from http://r-forge.r-project.org/projects/splits), running both the single and the multiple threshold optimizations (Monaghan et al. Citation2009) in order to compare their performance with the data set examined.
Results and discussion
Species diversity
A total of 468 specimens were collected during the three field trips performed, of which we were able to generate barcoding sequences (between 483 and 658 bp in length) for 407 of them. The number of haplotypes used for the analyses was 275. Application of the single and the multiple threshold GMYC models using the phylogram reconstructed with BEAST with a lognormal coalescent approach yielded 186 and 178 putative species (“barcoding” species), (CI = 182–190, 174–183; -lnL of null model = 23929.2, 3929.2; -lnL of GMYC model = 23995.7, 4016.7, respectively). Of these species, 54 and 79 were recovered as separate clusters (i.e. clades with two or more haplotypes) and 132 and 99 as singletons (i.e., represented by a single haplotype), respectively. Inspection of the results obtained from both analyses revealed that, even when it resulted in a lower number of barcode species, the multiple-threshold GMYC model frequently considered the existence of two or more species within various clusters of sequences that evidently belonged to a single lineage. We, therefore, only included the results derived from the single-threshold GMYC model.
The 185 barcode species delimited with the single-threshold GMYC model were assigned to 20 identified doryctine genera (), of which 115 species belonged to Heterospilus Haliday. Specimens belonging to two additional genera, Notiospathius Matthews and Marsh and cf. Barbalhoa Marsh, were also collected, although failure to amplify the barcoding region for their assigned specimens prevented their inclusion in the analyses. The barcoding species accumulation curve including the samples from the three collecting trips that were carried out clearly reveals that the number of evolutionary units discriminated in the site of study has not reached an asymptote (); and therefore, a considerable number of species are still expected to be discovered during subsequent trips. This species diversity, however, still needs to be confirmed using other sources of evidence, such as using an additional nuclear marker and/or confirming it with morphological examination of the barcoding species involved (Monaghan et al. Citation2009).
Table I. Number of specimens and barcode species recovered for each identified genus.
Figure 1. DNA barcode accumulation curve (obtained from the Barcode of Life Data Systems; www.barcodinglife.org) for the doryctine wasp species collected during three field trips carried out in June, September, and November 2009.
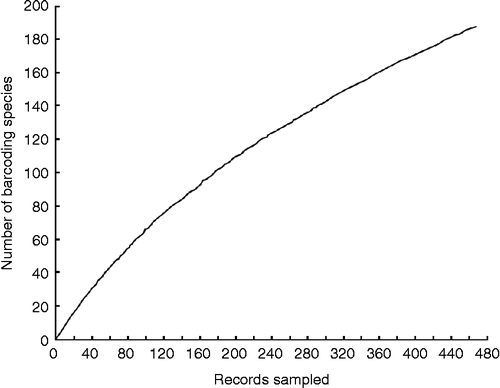
Our preliminary results support previous DNA barcoding studies in other geographic regions that have revealed the existence of an extraordinary but highly overlooked species diversity of small invertebrates not only in the tropics (e.g. Smith et al. Citation2008; Monaghan et al. Citation2009) but also in temperate zones (Sheffield et al. Citation2009; Smith et al. Citation2009). Regarding our group of study, the parasitoid wasp subfamily Doryctinae is known to be particularly diverse in the tropics (Belokobylskij Citation1992; Marsh Citation2002), although it has been virtually neglected in the Mexican territory since only 33 described species have been reported to occur in this country, five of which belong to Heterospilus (Yu et al. Citation2005). Our ongoing study will give insight into the actual species richness of this speciose group of insects in a Mexican tropical dry forest, which has traditionally been considered to have a lower biodiversity than other tropical ecosystems.
Taxonomic inferences
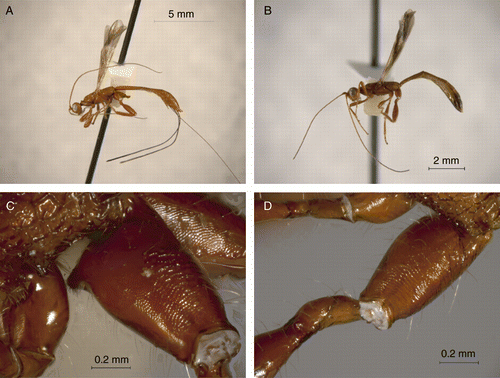
Our DNA barcoding sequences detected that the specimens of two of the genera that were identified, Ptesimogaster Marsh and Ptesimogastroides Braet and van Achterberg (,b), share a same haplotype and therefore they might be conspecific, pending additional molecular evidence. In any case, this finding reveals that, whether these two taxa are conspecific or not, they actually are very closely related, and we therefore propose Ptesimogastroides to be a junior synonym of Ptesimogaster syn. nov. (type species: P. parkeri Marsh 1965). The described species of Ptesimogastroides are only distinguished from the species of Ptesimogaster by the absence of a distinctive antero-ventral basal tubercle on the hind coxa (Braet et al. Citation2003) (). This result supports other studies (Zaldívar-Riverón et al. Citation2007, Citation2008) that have shown that the latter morphological feature, traditionally considered a major character to separate groups of genera within the Doryctinae, is considerably variable and thus cannot be employed alone to separate genera nor to propose new genus group names in the subfamily. Interestingly, the specimens collected from the latter two genera are a male and a female, so further collections will help to determine whether the presence or absence of a tubercle in the hind coxa actually represents sexual dimorphism or individual variation within a species.
Figure 2. Lateral view of the body of (a) Ptesimogaster sp. and (b) Ptesimogastroides sp. Hind coxa of (c) Ptesimogaster sp. and (d) Ptesimogastroides sp.
The phylogenetic analysis performed with the cox1 sequences recovered a large Heterospilus clade, but with the specimens assigned to Neoheterospilus Belokobylskij deeply nested within it. Despite being regarded with caution when inferring evolutionary relationships when it is analyzed separately due to its high substitution rate, it has been observed that the cox1 gene possesses reliable phylogenetic signal at different taxonomic levels; therefore, it is not rare that barcoding studies may accurately recover clusters of genera, tribes, and even families (e.g. Sheffield et al. Citation2009). The results obtained in this work point out the paraphyletic condition of the speciose genus Heterospilus at least with respect to Neoheterospilus, which is congruent with the morphological features displayed by the two genera involved. The species of Neoheterospilus are only distinguished from the ones of Heterospilus by having a highly modified, unusually shaped ovipositor (Belokobylskij Citation2006; CitationMartínez and Zaldívar-Riverón, in press). This conclusion, however, needs to be confirmed with additional molecular markers.
Acknowledgements
The authors thank Ma Cristina Mayorga-Martínez for mounting part of the specimens examined in this study and Andrew Polaszek, Estrella Hernández and Valeria B. Salinas-Ramos for their assistance in the field. The present research was supported by grants from the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO, México; grant number HB033) and the Consejo Nacional de Ciencia y Tecnología (CONACyT; apoyo para la red temática del código de barras de la vida) (to A.Z.R.).
Declarations of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akaike H. 1974. A new look at the statistical identification model. IEEE Trans Automatic Control. 19:716–723.
- Belokobylskij SA. 1992. On the classification and phylogeny of the Braconid wasps subfamilies Doryctinae and Exothecinae (Hymenoptera, Braconidae). Part I. On the classification, 1. Entomol Obozrenie 71:900–928 (in Russian). Entomol Rev 1993. 72 109: 137 (English translation).
- Belokobilskij SA, Zaldivar-Riverón A, Quicke DLJ. 2004. Phylogeny of the genera of the parasitic wasps subfamily Doryctinae (Hymenoptera: Braconidae) based on morphological evidence. Zool J Linn Soc. 142:369–404.
- Belokobylskij SA. 2006. Neoheterospilus gen. n., a new genus of the tribe Heterospilini (Hymenoptera: Braconidae: Doryctinae) with highly modified ovipositor and a worldwide distribution. Insect Syst Evol. 37:149–178.
- Braet Y, Barbalho SM, van Achterberg C. 2003. Description of four new genera and nine new species of Doryctinae (Hymenoptera: Braconidae) from French Guyana. Zool Meded. 77:93–125.
- Desneux N, Stary P, Delebecque CJ, Gariepy TD, Barta RJ, Hoelmer KA, Heimpel GE. 2009. Cryptic species of parasitoids attacking the soybean aphid (Hemiptera: Aphididae) in Asia: Binodoxys communis and Binodoxys koreanus (Hymenoptera: Braconidae: Aphidiinae). Ann Entomol Soc Am. 102:925–936.
- Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 7:214.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 3:294–299.
- Fontaneto D, Herniou EA, Boschetti C, Caprioli M, Melone G, Ricci C, Barraclough TG. 2007. Independently evolving species in asexual bdelloid rotifers. PLoS Biol. 5:914–921.
- Gomes SAG, Penteado-Dias AM. 2007. Description of a new genus of Doryctinae wasps (Hymenoptera: Braconidae) from Brazil. Zool Meded. 81:115–119.
- Gossner MM, Hausmann A. 2009. DNA barcoding enables the identification of caterpillars feeding on native and alien oak. Mitt Münch Entomol Ges. 99:135–140.
- Hajibabaei M, Singer GAC, Hebert PDN, Hickey DA. 2007. DNA barcoding: How it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 23:167–172.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identification through DNA barcodes. Proc R Soc B Biol Sci. 270:S96–S99.
- Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. 2004. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 101:14812–14817.
- Lanave CG, Preparata C, Saccone C, Serio G. 1984. A new method for calculating evolutionary substitution rates. J Mol Evol. 20:86–93.
- Li YW, Zhou X, Feng G, Hu HY, Niu LM, Hebert PDN, Huang DW. 2010. COI and ITS2 sequences delimit species, reveal cryptic taxa and host specificity of fig-associated Sycophila (Hymenoptera, Eurytomidae). Mol Ecol Res. 10:31–40.
- Marsh PM. 1997. Doryctinae. In: Wharton RA, Marsh PM, Sharkey MJ. editors. Manual of the New World Genera of the family Braconidae (Hymenoptera). International Society of Hymenopterists, Special publication. Washington D.C.: Vol. 1. p 207–233.
- Marsh PM. 2002. The Doryctinae of Costa Rica (excluding the genus Heterospilus). Mem Am Entomol Inst. 70:1–319.
- Martínez JJ, Zaldívar-Riverón A. 2010. A new species of Neoheterospilus (Braconidae: Doryctinae) from Chamela, Jalisco, Mexico. J Hymenopt Res. 19:217–222.
- Monaghan MT, Wild R, Elliot M, Fujisawa T, Balke M, Inward DJG, Lees DC, Ranaivosolo R, Eggleton P, Barraclough TG, Vogler AP. 2009. Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Syst Biol. 58:298–311.
- Pons J, Barraclough TG, Gómez-Zurita J, Cardoso A, Duran DP, Hazell S, Kamoun S, Sumlin WD, Vogler AP. 2006. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol. 55:595–609.
- Posada D. 2004. Collapse: Describing haplotypes from sequence alignments. Vigo, Spain: University of Vigo. [Online] Available at: http://darwin.uvigo.es/software/collapse.html.
- Posada D. 2008. jModelTest: Phylogenetic model averaging. Mol Biol Evol. 25:1253–1256.
- Rambaut A, Drummond AJ. 2007. Tracer v1.4. [Online] Available at: http://beast.bio.ed.ac.uk/Tracer.
- Rambaut A, Drummond AJ. 2008. Tree annotator: MCMC output analysis. [Online] Available at: http://beast.bio.ed.ac.uk.
- Sheffield CS, Hebert PDN, Kevan PG, Packer L. 2009. DNA barcoding a regional bee (Hymenoptera: Apoidea) fauna and its potential for ecological studies. Mol Ecol Res. 9:196–207.
- Smith MA, Rodríguez JJ, Whitfield JB, Deans AR, Janzen DH, Hallwachs W, Hebert PDN. 2008. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc Natl Acad Sci USA. 105:12359–12364.
- Smith MA, Fernández-Triana J, Roughley R, Hebert PDN. 2009. DNA barcode accumulation curves for understudied taxa and areas. Mol Ecol Notes. 9:208–216.
- Wharton RA, Hanson PE. 2005. Gall wasps in the family Braconidae (Hymenoptera). In: Raman A, Schaefer WC, Withers TM. editors. Biology, ecology, and evolution of gall-inducing arthropods. Enfield, NH: Science Publishers. p 495–505.
- Yang Z. 1994. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. J Mol Evol. 39:306–314.
- Yu DS, van Achterberg C, Horstman K. 2005. World Ichneumonoidea 2004. Taxonomy, Biology, Morphology and Distribution. CD/DVD. Vancouver, Canada: Taxapad. [Online] Available at: http://www.taxapad.com.
- Zaldivar-Riverón A, Belokobylskij SA, León-Regagnon V, Martínez JJ, Briceño R, Quicke DLJ. 2007. A single origin of gall association in a group of parasitic wasps with disparate morphologies. Mol Phylogenet Evol. 44:981–992.
- Zaldivar-Riverón A, Belokobyslkij SA, León-Regagnon V, Briceño GR, Quicke DLJ. 2008. Molecular phylogeny and historical biogeography of the cosmopolitan parasitic wasp subfamily Doryctinae (Hymenoptera: Braconidae). Invertebr Syst. 22:345–363.