Abstract
This study presents the Edinburgh Cognitive and Behavioural ALS Screen (ECAS), developed for ALS patients with physical disability for use by health care professionals. The screen is designed to detect the specific profile of cognition and behaviour changes in ALS and to differentiate it from other disorders. Forty-eight ALS patients (none with evident dementia), 40 healthy controls and 20 carers were recruited. The ECAS, a 15–20-min screen, includes an ALS-Specific score (executive functions and social cognition; fluency; language); an ALS Non-specific score (memory; visuospatial functions); and a carer behaviour screen of five domains characteristic of frontotemporal dementia (FTD). Data from healthy controls produced abnormality cut-offs of 77/100 ALS-Specific score; 24/36 ALS Non-specific score; 105/136 ECAS Total. Twenty-nine percent of patients showed abnormal ALS-Specific scores, and 6% also showed abnormal ALS Non-specific scores. The most prevalent deficit occurred in language functions (35%) followed by executive functions and fluency (23% each). Forty percent of carers reported behaviour change in at least one domain, while 15% met criteria for possible FTD. In conclusion, the ECAS is an effective within-clinic assessment for ALS that determines the presence, severity and type of cognitive and/or behavioural changes, an essential first step to managing these symptoms.
Introduction
Cognitive and behavioural changes in ALS are now recognized as an integral feature of the disease (Citation1). A proportion of patients present with a full-blown frontotemporal dementia syndrome (typically a behavioural variant), while specific and selective cognitive changes are more commonly found with characteristic deficits on tests of executive functions (Citation2,Citation3). Moreover, recent studies have also highlighted prominent changes in language (Citation4,Citation5) and social cognition (Citation6−8) (see (Citation1) for review). Despite the increased awareness of ALS as a multi-system disorder, the cognitive status of most ALS patients attending clinics remains unknown and only those with a clear and evident dementia syndrome are referred to scarce clinical neuropsychology services (Citation9). This neglect of cognitive presentations has significant health care implications: inappropriate interventions may be offered, and there is no adjustment to ensure effective decision making and informed consent to treatment by the patient.
Determining the cognitive status of ALS patients can be best achieved through the implementation of clinical screening tools specifically designed for ALS patients. Standard assessments for the detection of dementia are not appropriate in this patient group due to the range of physical problems, including difficulties with speech, writing and drawing, all of which form an important part of most cognitive screening batteries. Current ALS screening measures either do not sufficiently accommodate for motor disability in ALS, which may exaggerate performance decrements (Citation3), or assess only one cognitive domain (executive dysfunction) and therefore will not be sensitive to the whole range of cognitive and behavioural changes that can occur in ALS patients (Citation10−12).
Effective screening should address, therefore, the following questions: 1) Which patients have a cognitive impairment? The test must differentiate the patients with cognitive and/or behavioural changes from those with pure motor system involvement (ALS-motor); 2) How severe is that impairment? The test must be sensitive to both dementia but also to the more specific and selective cognitive changes; 3) What is the type of cognitive impairment? The test must differentiate cognitive and behaviour changes specific to ALS from those associated with other disorders (Citation9).
Here we present the Edinburgh Cognitive and Behavioural ALS Screen (ECAS), a brief assessment designed specifically for ALS patients − which is available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/doi/abs/10.3109/201678421.2013.805784 (The ECAS is kindly reproduced with permission from the authors. The copyright of this material remain with Abrahams, S. and Bak, T.H.). It can be implemented by doctors, nurse specialists and other medical professionals in the clinic or on home visits. This multi-domain assessment was designed to be sensitive to the range of cognitive and behaviour change in ALS, including not only measures of executive function and fluency but also language (ALS-Specific functions). A comprehensive executive functions assessment is core to the test, the most commonly affected domain in ALS, (Citation1,Citation3,Citation13−15) and includes assessment of functions recently shown to be affected in ALS − social cognition (Citation8) and inhibitory control (Citation16). Although fluency is dependent on both executive and language functions, a separate fluency score is produced due to the particular sensitivity of this test to ALS (Citation2,Citation3,Citation17). The predominance of language impairment in ALS has been recently highlighted (Citation4,Citation5) and the screen includes object naming previously found to be affected in ALS (Citation19,Citation20), comprehension including use of verbs in line with the previous finding of specific verb deficits in some ALS patients (Citation21) and spelling, following recent reports of impairment in ALS (Citation22). Furthermore, the screen includes assessment of ALS Non-specific functions (recall and recognition memory and visuospatial functions) to differentiate cognitive change characteristic of ALS from other disorders common in older adults, such as Alzheimer's disease (AD). In accordance with the cognitive profile characteristic of AD (Citation23), the memory score is weighted to processes of retention and recognition. The ECAS was also specifically developed for ALS patients to minimize the effect of physical disability on performance measures with interchangeable tests for those with bulbar or limb involvement. A separate brief carer interview also provided an assessment of behaviour change in the patient, based on the recent guidelines for diagnosing behavioural variant FTD (Citation24), with items once again adapted for ALS. Further questions are included on the presence of psychotic symptoms in light of recent findings of the prevalence of such symptoms in ALS patients with frontotemporal dementia (Citation25).
Materials and methods
Participants
Healthy controls (18 males, 22 females) were recruited from the University of Edinburgh Psychology Department's Volunteer Participant Panel, or from spouses or friends of ALS patients. No participant had significant neurological or psychiatric history and English was their first language. The age of healthy controls was selected to match a typical ALS group (see ).
ALS patients (33 males, 15 females) with ALS (46 sporadic, two familial – patients with a history of suspected ALS in a first degree relative) were recruited through the MND Register for Scotland, University of Edinburgh. All had clinical and electrophysiological evidence of combined upper and lower motor neuron involvement and fulfilled the revised El Escorial criteria for clinically definite or probable ALS (Brooks et al., 1998). Fourteen patients had bulbar onset of symptoms. Patients were excluded if they were in terminal stages of disease or had major comorbid medical, neurological or psychiatric history such as severe diabetes, epilepsy, alcohol/substance-related disorders, severe head injury that had required hospitalization in an intensive care setting, traumatic brain injury including subarachnoid haemorrhage, and any other significant illness such as cerebrovascular disease or stroke. Disease status was assessed with ALS Functional Rating Scale-Revised (ALSFRS-R; Cedarbaum et al., 1999). Respiratory functioning was assessed with Sniff Nasal Inspiratory Pressure (SNIP). Thirty of the 48 ALS patients had a SNIP score of lower than 40 cm (mean 30.13, 17.78 SD, range 2−78). Ten patients were receiving non-invasive ventilation and seven patients had a radiologically inserted gastrostomy. Dementia was not noted in the clinical files of any patient. This study was reviewed by the South-East Scotland Research Ethics Committee and Department of Psychology Ethics Committee, University of Edinburgh, and procedures were followed in accordance with ethics standards of these committees in addition to the Helsinki Declaration of 1975, as revised in 1983.
Methods
ECAS: The ECAS (http://www.era.lib.ed.ac.uk/handle/1842/6592) is a 15−20-min screen that includes assessment of the following domains: Executive Functions (Reverse Digit Span, Alternation, Inhibitory Sentence Completion, Social Cognition); Fluency (Free-words beginning with the letter S and Restrained-words beginning with the letter T but with only four letters); Language (Naming, Comprehension, Spelling); Memory (Immediate Recall, Delayed Percentage Retention, Delayed Recognition); Visuospatial Functions (Dot Counting, Cube Counting, Number Location). Wherever possible, participants were encouraged to respond using spoken responses to minimize testing time. In patients with marked dysarthria participants were allowed to write responses. For fluency the fluency index is calculated that accommodates for slowed speech or writing, in which the average time to think of each word is estimated, as described previously (Citation3,Citation18). Twenty healthy control participants undertook the spoken version of the fluency tasks, while 20 undertook the written version to produce separate normative datasets. Fluency indices were converted to a scaled score as shown in the ECAS. Object naming includes both living and non-living things, manipulable and non-manipulable objects, while comprehension items include different grammatical constructions and the use of verbs. Spelling words were of low to medium frequency including nouns, verbs and compound words. An ALS-Specific score was calculated as the total of Executive Functions, Fluency and Language (max 100), while an ALS Non-specific score was calculated as the total of Memory and Visuospatial Functions (max 36). Finally, a total ECAS score was calculated (max 136).
The separate carer interview consists of a checklist of 10 behaviours across five domains (Behavioural Disinhibition, Apathy, Loss of Sympathy/Empathy, Perseverative/Stereotyped behaviour, Hyperorality/Altered Eating Behaviour) followed by a three questions on the presence of psychotic symptoms. Guidance notes for administration of the ECAS and behaviour screen are available from the author.
Results
Healthy control: normative data
Characteristics of healthy controls are presented in and data on the ECAS in , with suggested cut-off levels for abnormality based on values > 2 SD from the mean.
Table I. Normative data on the ECAS.
Table II. Characteristics of ALS patients and healthy controls.
Patient data
Clinical characteristics of the patient sample are presented in . Performance on the ECAS Scores is presented in with a breakdown of performance in the cognitive sub-domains in . Ten patients were impaired on the ALS-Specific score and the ECAS Total score but not the ALS Non-specific score, while one patient was impaired on the ALS-Specific score only. Three patients were in the abnormal range on the ALS-Specific score, ALS Non-specific score and ECAS Total score. Furthermore, one patient was impaired on the ECAS Total score only and did not fall below criteria on either subscore, although was close to criteria on the ALS-Specific score. ECAS Total scores did not correlate with duration of illness. Reliability of the ECAS, measured in terms of internal consistency using the Cronbach's alpha coefficient, was 0.75 for ECAS Total score for the combined dataset (patients and controls) and 0.77 for the ALS patient group only (a coefficient of 0.7−0.8 is considered good to excellent).
Figure 1. ECAS subdomains: frequency of abnormal performance in ALS patients across each cognitive domain.
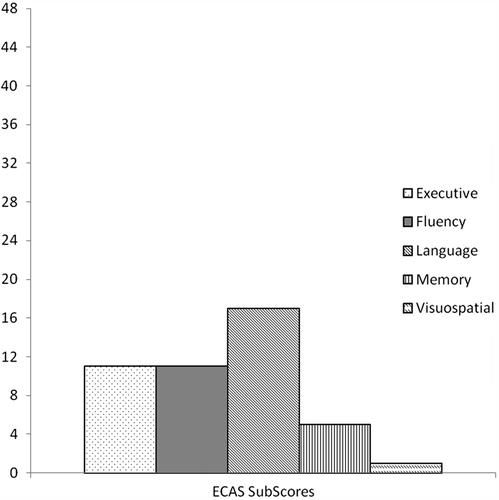
Table III. ECAS scores: frequency of abnormal performance in ALS group.
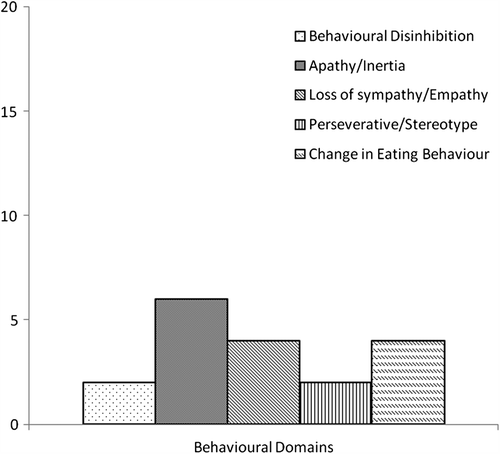
Twenty carers completed the behaviour screen. The frequency of symptoms across five domains characteristic of FTD is displayed in . Eight of 20 patients showed evidence of behaviour change in at least one of the five behavioural domains. Three patients met criteria for possible FTD with evidence of behaviour change in at least three of the five domains. Six patients showed evidence of behaviour change as rated by their carer but were above abnormality cut-offs on the ECAS. Two of the three patients who met criteria for possible FTD also showed abnormal cognitive performance on the ECAS with below cut-off scores for the ECAS Total and ALS-Specific scores. Only one of these two patients showed abnormal performance on the ALS Non-specific score. The carer of this patient also described evidence of psychotic symptoms with the presence of strange or bizarre beliefs and hearing or seeing things that are not there. No other carer reported evidence of psychotic symptoms.
Discussion
This paper presents the first dataset on a new multi-domain cognitive and behaviour screen specifically designed for the assessment of ALS patients with and without FTD. Of a group of 48 patients on a national disease register, none of whom were noted to have dementia in clinical presentation, 29% showed abnormal performance on the cognitive screen of the ECAS. This finding is in accordance with prevalence rates of cognitive impairment previously reported (Citation13,Citation14,Citation17). Furthermore, the ECAS demonstrated high internal consistency indicating that the component scores correlate well with total score. Twenty-nine percent also showed evidence of a deficit on functions that were specific to the known cognitive profile of ALS (composite of executive and language functions and fluency). This finding indicates that the ECAS has been successful in detecting cognitive impairment characteristic for ALS. Within each cognitive domain the most frequent deficit occurred in language functions, which was present in 17/48 (35%) of the patient sample. This impairment was primarily driven by spelling difficulties. The next most prevalent deficit was in executive functions 11/48 (23%) of patient sample. The finding of particularly prevalent language deficits is consistent with the recent results of Taylor et al. (Citation4) (see (Citation5) for comment) who demonstrated that language deficits were more common than executive dysfunction in a prevalent population. Together these findings highlight the importance of including this domain within assessment. A deficit in ALS Non-specific functions was found in ˜6% of cases. Of note, all of these cases also had deficits in ALS-Specific functions. This may indicate spread of disease in these patients, or that severe executive impairments including attentional dysfunction may impact on other cognitive domains such as memory processes. It should be noted that these data were from a prevalent rather than an incident population with a high proportion of patients with respiratory dysfunction. However, rates of impairment appear consistent across studies.
Of the 20 carers who completed the behaviour screen, evidence of behaviour change was found in 8/20 (40%) of cases. Three of the 20 (15%) met criteria for behavioural variant FTD, which again is in accordance with previous prevalence estimates (Citation13,Citation14,Citation17). It is of note that in none of these three patients was dementia noted in their clinical files prior to assessment with the ECAS. Six of the eight patients did not show abnormal scores on the cognitive section of the ECAS indicating that the behaviour questionnaire can detect the presentation of ALS with behavioural impairment, as described by Strong et al. (Citation15). Consistent with current literature, apathy was the most prevalent symptom (Citation26−28). Previous assessments of apathy have been confounded by methodological difficulty due to questions being dependent on motor ability. Here the symptom has remained prevalent despite the wording of the assessment having been specifically designed for ALS, not to be weighted towards effective motor functions. Furthermore, behaviour change was noted across all domains with particularly prevalent findings in Loss of Sympathy/Empathy and Change in Eating Behaviour. The presence of psychotic symptoms was not a common feature, having been found in only one patient who met criteria for FTD.
In summary, the ECAS provides an effective measure of cognitive and behaviour assessment of ALS for use within the clinic. The assessment determines not only the presence of cognitive impairment, but also its severity and nature. This level of identification is essential as the first step to managing cognitive and behavioural presentations in ALS. Assessment at the earliest point of contact will help to streamline care into the correct clinical pathway, provide a baseline to assess progression and also be soon essential for epidemiological and clinical trials. The screen provides a profile of impairment that will help to tailor intervention to the individual needs, e.g. spelling impairments affecting communication and implementation of speech aids, or executive dysfunction prompting the need for help with decision making. It should be noted that caution is recommended when interpreting scores in individual patients with low premorbid IQ or who have a history of reading/writing difficulties and full neuropsychological evaluation may be warranted. Future studies will further validate the assessment against detailed neuropsychology and determine specificity to ALS compared to other patient groups.
Edinburgh Cognitive and Behavioural ALS Screen – ECAS English Version (2013)
Download PDF (1.6 MB)Acknowledgements
This study was funded by an award from the Motor Neurone Disease Association. The authors would like to thank all the people with MND and their carers for participating in this research. The authors also thank Shuna Colville, Siddharthan Chandran and Richard Davenport for their support and help in recruiting patients with MND.
Disclosure of interest: The authors have no known conflict of interest in relation to publication of this paper. Three of the authors (JN, EN, JF) were funded by an award from the Motor Neurone Disease Association when undertaking this research. The remaining two authors were funded by the University of Edinburgh. The authors alone are responsible for the content and writing of the paper.
References
- Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral scleroris: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–80.
- Raaphorst J, de Visser M, Linssen WH, de Haan RJ, Schmand B, Raaphorst J, et al. The cognitive profile of amyotrophic lateral sclerosis: A meta-analysis. Amyotroph Lateral Scler. 2010;11:27–37.
- Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia. 2000;38:734–47.
- Taylor LJ, Tsermentseli S, Al-Chalabi A, Shaw CE, Leigh PN, Goldstein LH. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis?. J Neurol Neurosurg Psychiatry. 2013;84:494–8.
- Abrahams S. Executive dysfunction in ALS is not the whole story. J Neurol Neurosurg Psychiatry. (Editorial Commentary). 2013;84:474–5.
- Abrahams S. Social Cognition in Amyotrophic Lateral Sclerosis. Neurodegenerative Disease Management. 2012; 1:397–405.
- Cavallo M, Adenzato M, Macpherson SE, Karwig G, Enrici I, Abrahams S. Evidence of social understanding impairment in patients with amyotrophic lateral sclerosis. PLoS One. 2011;6:e25948.
- Girardi A, Macpherson SE, Abrahams S, Girardi A, Macpherson SE, Abrahams S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology. 2011;25:53–65.
- Abrahams S. ALS, cognition and the clinic. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2013;14:3–5.
- Flaherty-Craig C, Eslinger P, Stephens B, Simmons Z, Flaherty-Craig C, Eslinger P, et al. A rapid screening battery to identify frontal dysfunction in patients with ALS. Neurology. 2006;12;2070–2.
- Gordon PH, Wang Y, Doorish C, Lewis M, Battista V, Mitsumoto H, et al. A screening assessment of cognitive impairment in patients with ALS. Amyotroph Lateral Scler. 2007;8:362–5.
- Woolley SC, York MK, Moore DH, Strutt AM, Murphy J, Schulz PE, et al. Detecting frontotemporal dysfunction in ALS: utility of the ALS Cognitive Behavioural Screen (ALS-CBS). Amyotroph Lateral Scler. 2010;11:303–11.
- Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2012;83:102–8.
- Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65: 586–90.
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis.(Erratum appears in Amyotroph Lateral Scler. 2009;10:252). Amyotroph Lateral Scler. 2009;10:131–46.
- Lillo P, Savage S, Mioshi E, Kiernan MC, Hodges JR. Amyotrophic lateral sclerosis and frontotemporal dementia: a behavioural and cognitive continuum. Amyotroph Lateral Scler. 2012;13:102–9.
- Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal?Neurology. 2003;60:1094–7.
- Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. 1996;119: 2105–20.
- Rakowicz WP, Hodges JR. Dementia and aphasia in motor neuron disease: an under-recognized association?J Neurol Neurosurg Psychiatry. 1998;65:881–9.
- Abrahams S, Goldstein LH, Simmons A, Brammer M, Williams SC, Giampietro V, et al. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain. 2004;127:1507–17.
- Bak T, O’Donovan DG, Xuereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neuron disease-dementia-aphasia syndrome. Brain. 2001;124:103–20.
- Ichikawa H, Hieda S, Ohno H, Ohnaka Y, Shimizu Y, Nakajima M, et al. Kana versus kanji in amyotrophic lateral sclerosis: a clinicoradiological study of writing errors. Eur Neurol. 2010;64:148–55.
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46.
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77.
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AMT, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9orf72 mutations. Brain. 2012;135:693–708.
- Girardi A, Macpherson SE, Abrahams S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology. 2011;25:53–65.
- Grossman AB, Woolley-Levine S, Bradley WG, Miller RG, Grossman AB, Woolley-Levine S, et al. Detecting neurobehavioural changes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2007;8:56–61.
- Woolley SC, Zhang Y, Schuff N, Weiner MW, Katz JS, Woolley SC, et al. Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph Lateral Scler. 2011;12:52–8.